
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sirius Huang | -- | 1875 | 2022-11-30 01:42:32 |
Video Upload Options
A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions. Unlike 2D environments (e.g. a Petri dish), a 3D cell culture allows cells in vitro to grow in all directions, similar to how they would in vivo. These three-dimensional cultures are usually grown in bioreactors, small capsules in which the cells can grow into spheroids, or 3D cell colonies. Approximately 300 spheroids are usually cultured per bioreactor.
1. Background
3D cell cultures have been used in research for several decades.[1] One of the first recorded approaches for their development was at the beginning of the 20th century, with the efforts of Alexis Carrel to develop methods for prolonged in vitro tissue cultures.[2] Early studies in the 80's, led by Mina Bissell from the Lawrence Berkeley National Laboratory, highlighted the importance of 3D techniques for creating accurate in vitro culturing models. This work focused on the importance of the extracellular matrix and the ability of cultures in artificial 3D matrices to produce physiologically relevant multicellular structures, such as acinar structures in healthy and cancerous breast tissue models. These techniques have been applied to for in vitro disease models used to evaluate cellular responses to pharmaceutical compounds.[3]
Eric Simon, in a 1988 NIH SBIR grant report, showed that Electrospinning could be used to produced nano- and submicron-scale polystyrene and polycarbonate fibrous mats (now known as scaffolds) specifically intended for use as in vitro cell substrates. This early use of electrospun fibrous lattices for cell culture and tissue engineering showed that various cell types including Human Foreskin Fibroblasts (HFF), transformed Human Carcinoma (HEp-2), and Mink Lung Epithelium (MLE) would adhere to and proliferate upon the fibers. It was noted that as opposed to the flattened morphology typically seen in 2D culture, cells grown on the electrospun fibers exhibited a more histotypic rounded 3-dimensional morphology generally observed in vivo.[4]
2. Properties
In living tissue, cells exist in 3D microenvironments with intricate cell-cell and cell-matrix interactions and complex transport dynamics for nutrients and cells.[5][6][7][8][9][10][11][12][13] Standard 2D, or monolayer, cell cultures are inadequate representations of this environment, which often makes them unreliable predictors of in vivo drug efficacy and toxicity.[11][14] 3D spheroids more closely resemble in vivo tissue in terms of cellular communication and the development of extracellular matrices.[15] These matrices help the cells to be able to move within their spheroid similar to the way cells would move in living tissue.[7] The spheroids are thus improved models for cell migration, differentiation, survival, and growth.[12] Furthermore, 3D cell cultures provide more accurate depiction of cell polarization, since in 2D, the cells can only be partially polarized.[7] Moreover, cells grown in 3D exhibit different gene expression than those grown in 2D.[7]
The third dimension of cell growth provides more contact space for mechanical inputs and for cell adhesion, which is necessary for integrin ligation, cell contraction and even intracellular signalling.[16][17] Normal solute diffusion and binding to effector proteins (like growth factors and enzymes) is also reliant on the 3D cellular matrix, so it is critical for the establishment of tissue scale solute concentration gradients [18][19]
For the purposes of drug toxicology screening, it is much more useful to test gene expression of in vitro cells grown in 3D than 2D, since the gene expression of the 3D spheroids will more closely resemble gene expression in vivo. Lastly, 3D cell cultures have greater stability and longer lifespans than cell cultures in 2D.[20] This means that they are more suitable for long-term studies and for demonstrating long-term effects of the drug. 3D environments also allow the cells to grow undisturbed. In 2D, the cells must undergo regular trypsinization in order to provide them with sufficient nutrients for normal cell growth.[21] 3D spheroids have been cultured in a lab setting for up to 302 days while still maintaining healthy, non-cancerous growth.[20]
3. Classification of 3D Culture Methods
There are a large number of commercially available culturing tools that claim to provide the advantages of 3D cell culture. In general, the platforms can be classified in two types of 3D culturing methods: scaffold techniques and scaffold-free techniques.
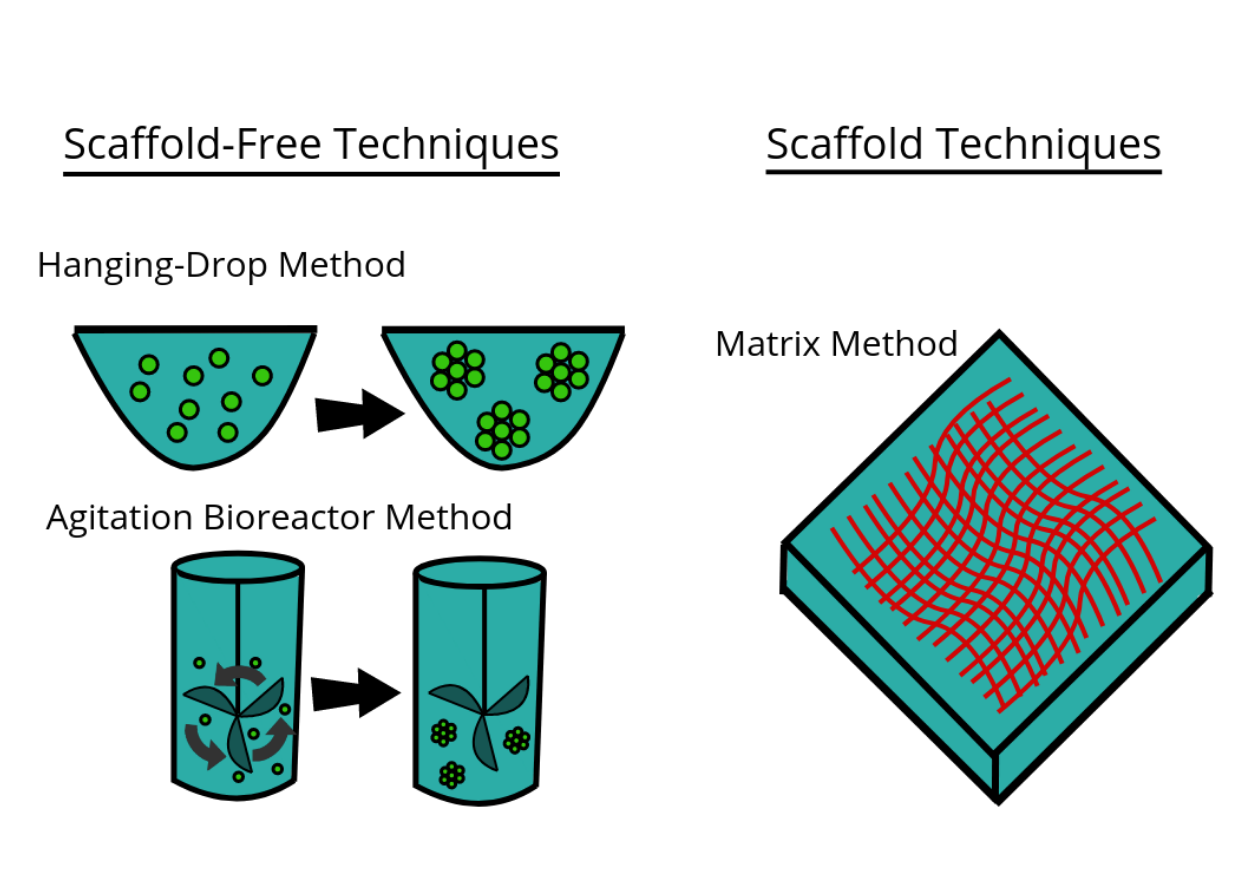
3.1. Scaffold Techniques
Scaffold techniques include the use of solid scaffolds, hydrogels and other materials. In a recent study potentiality of human CD34+ stem cells explored by generating in vitro agarose gel 3D model to understand the bone ossification process.[22]
Hydrogels
As the natural extracellular matrix (ECM) is important in the survival, proliferation, differentiation and migration of the cells, different hydrogel matrices mimicking natural ECM structure are considered as potential approaches towards in vivo –like cell culturing.[23][24][25] Hydrogels are composed of interconnected pores with high water retention, which enables efficient transport of e.g. nutrients and gases. Several different types of hydrogels from natural and synthetic materials are available for 3D cell culture, including e.g. animal ECM extract hydrogels, protein hydrogels, peptide hydrogels, polymer hydrogels, and wood-based nanocellulose hydrogel.
3.2. Scaffold-Free Techniques
Scaffold free techniques employ another approach independent from the use scaffold. Scaffold-free methods include e.g. the use of low adhesion plates, hanging drop plates, micropatterned surfaces, and rotating bioreactors, magnetic levitation, and magnetic 3D bioprinting.
Spheroids
File:Electron microscopy (Ho).tif Spheroids are a type of three-dimensional cell modeling that better simulate a live cell's environmental conditions compared to a two-dimensional cell model, specifically with the reactions between cells and the reactions between cells and the matrix.[26] Spheroids are useful in the study of changing physiological characteristics of cells,[27] the difference in the structure of healthy cells and tumor cells, and the changes cells undergo when forming a tumor.[28] Spheroids co-cultured with tumor and healthy cells were used to simulate how cancerous cells interact with normal cells.[29] Spheroids can be grown with a few different methods. One common method is to use low cell adhesion plates, typically a 96 well plate, to mass-produce spheroid cultures, where the aggregates form in the rounded bottom of the cell plates.[30][31] Spheroids can also be cultured using the hanging drop method[32] involving forming cell aggregates in drops that hang from the surface of a cell plate.[26] Other methods under investigation include the use of rotating wall vessel bioreactors, which spins and cultures the cells when they are constantly in free fall and forms aggregates in layers [33] Recently, some protocols have been standardized in order to produce uniform and reliable spheroids.[34] Researchers had also explored standardized, economical and reproducible methods for 3D cell culture. [35]
Bioreactors
The bioreactors used for 3D cell cultures are small plastic cylindrical chambers that are specifically engineered for the purpose of growing cells in three dimensions. The bioreactor uses bioactive synthetic materials such as polyethylene terephthalate membranes to surround the spheroid cells in an environment that maintains high levels of nutrients.[36][37] They are easy to open and close, so that cell spheroids can be removed for testing, yet the chamber is able to maintain 100% humidity throughout.[15] This humidity is important to achieve maximum cell growth and function. The bioreactor chamber is part of a larger device that rotates to ensure equal cell growth in each direction across three dimensions.[15]
MC2 Biotek has developed a bioreactor to incubate proto-tissue that uses gas exchange to maintain high oxygen levels within the cell chamber.[38] This is an improvement over previous bioreactors because the higher oxygen levels help the cell grow and undergo normal cell respiration.[12]
4. Microfluidics
The various cell structures in the human body must be vascularized to receive the nutrients and gas exchange help that they need to survive. Similarly, 3D cell cultures in vitro require certain levels of fluid circulation, which can be problematic for dense, 3D cultures where cells may not all have adequate exposure to nutrients. This is particularly important in hepatocyte cultures because the liver is a highly vascularized organ. One study cultured hepatocytes and vascular cells together on a collagen gel scaffold between microfluidic channels, and compared growth of cells in static and flowing environments, and showed the need for models with tissues and a microvascular network.[39]
5. High-Throughput Screening
Advanced development of 3D models for high-throughput screening in high density formats has recently been achievable due to technological achievements related to increased microplate density. These can be found in 384 and 1536-well formats that are cell repellent, cost effective, and amenable to fully automated screening platforms.[40] Two options that afford 1536-well formats are available from either Greiner Bio-One utilizing the m3D Magnetic 3D bioprinting[41] and Corning Life Sciences which incorporates a ultra-low attachment surface coating, along with a microcavity geometry and gravity to create 3D models.[42][43] Due to the rapid and affordable methods and technologies that have been developed for 3D screening, parallel high-throughput screening approaches to test isogenic pairs of oncogene related mutants versus wildtype have been enabled.[44]
6. Pharmacology and Toxicology
A primary purpose of growing cells in 3D scaffolds and as 3D cell spheroids in vitro is to test pharmacokinetic and pharmacodynamic effects of drugs and nanomaterials in preclinical trials.[12][45][46][47][48] Toxicology studies have shown 3D cell cultures to be nearly on par with in vivo studies for the purposes of testing toxicity of drug compounds. When comparing LD50 values for 6 common drugs: acetaminophen, amiodarone, diclofenac, metformin, phenformin, and valproic acid, the 3D spheroid values correlated directly with those from in vivo studies.[49] Although 2D cell cultures have previously been used to test for toxicity along with in vivo studies, the 3D spheroids are better at testing chronic exposure toxicity because of their longer life spans.[50] The matrix in 3D Spheroids causes cells to maintain actin filaments and is more relevant physiologically in cytoskeletal organization and cell polarity and shape of human cells.[51] The three-dimensional arrangement allows the cultures to provide a model that more accurately resembles human tissue in vivo without utilizing animal test subjects.[52]
7. Criticisms
Existing 3D methods are not without limitations, including scalability, reproducibility, sensitivity, and compatibility with high-throughput screening (HTS) instruments. Cell-based HTS relies on rapid determination of cellular response to drug interaction, such as dose dependent cell viability, cell-cell/cell-matrix interaction, and/or cell migration, but the available assays are not optimized for 3D cell culturing. Another challenge faced by 3D cell culturing is the limited amount of data and publications that address mechanisms and correlations of drug interaction, cell differentiation, and cell-signalling in these 3D environments. None of the 3D methods have yet replaced 2D culturing on a large scale, including in the drug development process; although the number of 3D cell culturing publications is increasing rapidly, the current limited biochemical characterization of 3D tissue diminishes the adoption of new methods.
There are also problems using spheroids as a model for cancerous tissue. Although beneficial for 3D tissue culture, tumor spheroids have been criticized for being challenging or impossible to “manipulate gradients of soluble molecules in [3D spheroid] constructs, and to characterize cells in these complex gradients”, unlike the paper-supported 3D cell culture for tissue-based bioassays explored by Ratmir et al.[37] Further challenges associated with complex 3D cell culture techniques include: imaging due to large scaffold sizes and incompatibility with many fluorescence microscopes, flow cytometry because it requires the dissociation of spheroids into a single-cell suspension, and the automation of liquid handling.[53]
References
- Mapanao, Ana Katrina; Voliani, Valerio (June 2020). "Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research". Applied Materials Today 19: 100552. doi:10.1016/j.apmt.2019.100552. https://dx.doi.org/10.1016%2Fj.apmt.2019.100552
- "On the Permannet Life of Tissues Outside of the Organisms". The Journal of Experimental Medicine 15 (5): 516–28. May 1912. doi:10.1084/jem.15.5.516. PMID 19867545. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2124948
- MERIT Award Recipient: Mina J. Bissell, Ph.D. (n.d.). Retrieved June 16, 2016, from http://www.cancer.gov/research/nci-role/spotlight/merit/Bissell
- Simon, Eric M. (1988). "NIH PHASE I FINAL REPORT: FIBROUS SUBSTRATES FOR CELL CULTURE (R3RR03544A) (PDF Download Available)" (in en). https://www.researchgate.net/publication/317053872.
- Marx, Vivien (11 April 2013). "A Better Brew". Nature. http://brown.edu/academics/molecular-pharmacology-physiology-and-biotechnology/sites/brown.edu.academics.molecular-pharmacology-physiology-and-biotechnology/files/uploads/Nature%20apr%2011%20a%20better%20brew.pdf.
- "Three-dimensional tissue culture based on magnetic cell levitation". Nature Nanotechnology 5 (4): 291–6. April 2010. doi:10.1038/nnano.2010.23. PMID 20228788. Bibcode: 2010NatNa...5..291S. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4487889
- "The third dimension bridges the gap between cell culture and live tissue". Nature Reviews Molecular Cell Biology 8 (10): 839–45. October 2007. doi:10.1038/nrm2236. PMID 17684528. https://dx.doi.org/10.1038%2Fnrm2236
- "A pericellular collagenase directs the 3-dimensional development of white adipose tissue". Cell 125 (3): 577–91. May 2006. doi:10.1016/j.cell.2006.02.050. PMID 16678100. https://dx.doi.org/10.1016%2Fj.cell.2006.02.050
- "Modeling tissue morphogenesis and cancer in 3D". Cell 130 (4): 601–10. August 2007. doi:10.1016/j.cell.2007.08.006. PMID 17719539. https://dx.doi.org/10.1016%2Fj.cell.2007.08.006
- "Spheroid-based drug screen: considerations and practical approach". Nature Protocols 4 (3): 309–24. 12 February 2009. doi:10.1038/nprot.2008.226. PMID 19214182. https://dx.doi.org/10.1038%2Fnprot.2008.226
- "Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach". Journal of Cellular Biochemistry 101 (6): 1370–83. August 2007. doi:10.1002/jcb.21386. PMID 17492655. https://dx.doi.org/10.1002%2Fjcb.21386
- "Capturing complex 3D tissue physiology in vitro". Nature Reviews Molecular Cell Biology 7 (3): 211–24. March 2006. doi:10.1038/nrm1858. PMID 16496023. https://dx.doi.org/10.1038%2Fnrm1858
- "Three-dimensional cell culture matrices: state of the art". Tissue Engineering. Part B, Reviews 14 (1): 61–86. March 2008. doi:10.1089/teb.2007.0150. PMID 18454635. https://deepblue.lib.umich.edu/bitstream/2027.42/63369/1/teb.2007.0150.pdf.
- 3D cell culture: a review of current approaches and techniques.. Methods in Molecular Biology. 695. 2011. pp. 1–15. doi:10.1007/978-1-60761-984-0_1. ISBN 978-1-60761-983-3. https://dx.doi.org/10.1007%2F978-1-60761-984-0_1
- Fey, Stephen; Wrzesinski, Krzysztof (2013). "Determination of Acute Lethal and Chronic Lethal Thresholds of Valproic Acid Using 3D Spheroids Constructed From the Immortal Human Hepatocyte Cell Line HEPG2/C3A". Valproic Acid. Nova Science Publishers, Inc.. pp. 141–165. ISBN 978-1-62417-952-5. http://www.mc2biotek.com/media/7655/fey_wrzesinski_2013_valproic_acid_978-1-62417-952-5_ch5.pdf.
- "Building in vitro models of organs". A Survey of Cell Biology. International Review of Cytology. 244. 2005. pp. 137–73. doi:10.1016/s0074-7696(05)44004-8. ISBN 9780123646484. https://dx.doi.org/10.1016%2Fs0074-7696%2805%2944004-8
- "Status and prospects of in vitro tests in risk assessment". Alternatives to Laboratory Animals 32 (4): 431–5. October 2004. doi:10.1177/026119290403200416. PMID 15651929. https://dx.doi.org/10.1177%2F026119290403200416
- "Visualizing muscle cell migration in situ". Current Biology 10 (10): 576–85. May 2000. doi:10.1016/s0960-9822(00)00486-3. PMID 10837222. https://dx.doi.org/10.1016%2Fs0960-9822%2800%2900486-3
- "Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction". Proceedings of the National Academy of Sciences of the United States of America 91 (26): 12378–82. December 1994. doi:10.1073/pnas.91.26.12378. PMID 7528920. Bibcode: 1994PNAS...9112378R. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=45441
- "HepG2/C3A spheroids exhibit stable physiological functionality for at least 24 days after recovering from trypsinisation". Toxicol. Res. 2 (3): 163–172. 2013. doi:10.1039/C3TX20086H. http://findresearcher.sdu.dk/portal/da/publications/hepg2c3a-3d-spheroids-exhibit-stable-physiological-functionality-for-at-least-24-days-after-recovering-from-trypsination(57ab9c9e-1777-41e7-9247-47b9abeb56aa).html.
- "After trypsinisation, 3D spheroids of C3A hepatocytes need 18 days to re-establish similar levels of key physiological functions to those seen in the liver". http://www.mc2biotek.com/media/7075/121217_toxres_published_manus_c2tx20060k.pdf.
- "+ stem cells". Molecular Biology Reports 43 (11): 1233–1242. November 2016. doi:10.1007/s11033-016-4053-4. PMID 27497820. https://dx.doi.org/10.1007%2Fs11033-016-4053-4
- "Controlled pattern of cell growth in modulated protein nanocomplexes: Regulating cells spreading in three dimensions". Materials Today 21 (6): 686–688. 2018. doi:10.1016/j.mattod.2018.06.003. https://dx.doi.org/10.1016%2Fj.mattod.2018.06.003
- "Hydrogels as extracellular matrix mimics for 3D cell culture". Biotechnology and Bioengineering 103 (4): 655–63. July 2009. doi:10.1002/bit.22361. PMID 19472329. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2997742
- "Engineering hydrogels as extracellular matrix mimics". Nanomedicine (London, England) 5 (3): 469–84. April 2010. doi:10.2217/nnm.10.12. PMID 20394538. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2892416
- "Spheroid culture as a tool for creating 3D complex tissues". Trends in Biotechnology 31 (2): 108–15. February 2013. doi:10.1016/j.tibtech.2012.12.003. PMID 23336996. https://dx.doi.org/10.1016%2Fj.tibtech.2012.12.003
- "A multiscale model for avascular tumor growth". Biophysical Journal 89 (6): 3884–94. December 2005. doi:10.1529/biophysj.105.060640. PMID 16199495. Bibcode: 2005BpJ....89.3884J. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1366955
- "Prolonged mammosphere culture of MCF-7 cells induces an EMT and repression of the estrogen receptor by microRNAs". Breast Cancer Research and Treatment 132 (1): 75–85. February 2012. doi:10.1007/s10549-011-1534-y. PMID 21553120. https://dx.doi.org/10.1007%2Fs10549-011-1534-y
- "A heterologous 3-D coculture model of breast tumor cells and fibroblasts to study tumor-associated fibroblast differentiation". Experimental Cell Research 266 (1): 74–86. May 2001. doi:10.1006/excr.2001.5210. PMID 11339826. https://dx.doi.org/10.1006%2Fexcr.2001.5210
- "The development and characterization of a human mesothelioma in vitro 3D model to investigate immunotoxin therapy". PLOS ONE 6 (1): e14640. January 2011. doi:10.1371/journal.pone.0014640. PMID 21305058. Bibcode: 2011PLoSO...614640X. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3031536
- "Rapid generation of in vitro multicellular spheroids for the study of monoclonal antibody therapy". Journal of Cancer 2: 507–14. 2011. doi:10.7150/jca.2.507. PMID 22043235. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3204399
- "High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array". The Analyst 136 (3): 473–8. February 2011. doi:10.1039/c0an00609b. PMID 20967331. Bibcode: 2011Ana...136..473T. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=7454010
- "Three-dimensional in vitro tumor models for cancer research and drug evaluation". Biotechnology Advances 32 (7): 1256–1268. November 2014. doi:10.1016/j.biotechadv.2014.07.009. PMID 25116894. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4171250
- Santi, Melissa; Mapanao, Ana Katrina; Cappello, Valentina; Voliani, Valerio (2020-07-01). "Production of 3D tumor models of head and neck squamous cell carcinomas for nanotheranostics assessment" (in en). ACS Biomaterials Science & Engineering 6 (9): 4862–4869. doi:10.1021/acsbiomaterials.0c00617. ISSN 2373-9878. PMID 33395269. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=7735655
- Tan, Loh Teng Hern; Low, Liang Ee; Tang, Siah Ying; Yap, Wei Hsum; Chuah, Lay Hong; Chan, Chim Kei; Lee, Learn Han; Goh, Bey Hing (2019). "A reliable and affordable 3D tumor spheroid model for natural product drug discovery: A case study of curcumin". Progress in Drug Discovery & Biomedical Science 2. doi:10.36877/pddbs.a0000017. https://doi.org/10.36877/pddbs.a0000017.
- "Synthetic sandwich culture of 3D hepatocyte monolayer". Biomaterials 29 (3): 290–301. January 2008. doi:10.1016/j.biomaterials.2007.09.016. PMID 17964646. https://dx.doi.org/10.1016%2Fj.biomaterials.2007.09.016
- "Paper-supported 3D cell culture for tissue-based bioassays". Proceedings of the National Academy of Sciences of the United States of America 106 (44): 18457–62. November 2009. doi:10.1073/pnas.0910666106. PMID 19846768. Bibcode: 2009PNAS..10618457D. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2773961
- Fey, Stephen J. WO2012022351. European Patent Register.
- "Transport-mediated angiogenesis in 3D epithelial coculture". FASEB Journal 23 (7): 2155–64. July 2009. doi:10.1096/fj.08-122820. PMID 19246488. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2718841
- Baillargeon P, Shumate J, Hou S, Fernandez-Vega V, Marques N, Souza G (2019). "Automating a Magnetic 3D Spheroid Model Technology for High-Throughput Screening.". SLAS Technol 24 (4): 420–428. doi:10.1177/2472630319854337. PMID 31225974. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=7704036
- Hou S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J (2018). "Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening.". SLAS Discov 23 (6): 574–584. doi:10.1177/2472555218766842. PMID 29673279. PMC 6013403. https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29673279.
- Madoux F, Tanner A, Vessels M, Willetts L, Hou S, Scampavia L (2017). "A 1536-Well 3D Viability Assay to Assess the Cytotoxic Effect of Drugs on Spheroids.". SLAS Discov 22 (5): 516–524. doi:10.1177/2472555216686308. PMID 28346088. https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28346088.
- Quereda V, Hou S, Madoux F, Scampavia L, Spicer TP, Duckett D (2018). "A Cytotoxic Three-Dimensional-Spheroid, High-Throughput Assay Using Patient-Derived Glioma Stem Cells.". SLAS Discov 23 (8): 842–849. doi:10.1177/2472555218775055. PMID 29750582. PMC 6102052. https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29750582.
- Kota S, Hou S, Guerrant W, Madoux F, Troutman S, Fernandez-Vega V (2018). "A novel three-dimensional high-throughput screening approach identifies inducers of a mutant KRAS selective lethal phenotype.". Oncogene 37 (32): 4372–4384. doi:10.1038/s41388-018-0257-5. PMID 29743592. PMC 6138545. https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29743592.
- "Photothermal effect by NIR-responsive excretable ultrasmall-in-nano architectures" (in en). Materials Horizons 6 (3): 531–537. 2019. doi:10.1039/C9MH00096H. ISSN 2051-6347. https://dx.doi.org/10.1039%2FC9MH00096H
- "Endogenously Triggerable Ultrasmall-in-Nano Architectures: Targeting Assessment on 3D Pancreatic Carcinoma Spheroids". ACS Omega 3 (9): 11796–11801. September 2018. doi:10.1021/acsomega.8b01719. PMID 30320273. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6173554
- Zustiak, Silviya Petrova; Dadhwal, Smritee; Medina, Carlos; Steczina, Sonette; Chehreghanianzabi, Yasaman; Ashraf, Anisa; Asuri, Prashanth (February 2016). "Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins". Biotechnology and Bioengineering 113 (2): 443–452. doi:10.1002/bit.25709. ISSN 1097-0290. PMID 26184715. https://dx.doi.org/10.1002%2Fbit.25709
- Otieno, Monicah A.; Gan, Jinping; Proctor, William (2018), Chen, Minjun; Will, Yvonne, eds., "Status and Future of 3D Cell Culture in Toxicity Testing" (in en), Drug-Induced Liver Toxicity, Methods in Pharmacology and Toxicology (New York, NY: Springer): pp. 249–261, doi:10.1007/978-1-4939-7677-5_12, ISBN 978-1-4939-7677-5 https://dx.doi.org/10.1007%2F978-1-4939-7677-5_12
- "Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line". Toxicological Sciences 127 (2): 403–11. June 2012. doi:10.1093/toxsci/kfs122. PMID 22454432. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3355318
- "Multi-cell type human liver microtissues for hepatotoxicity testing". Archives of Toxicology 87 (1): 209–13. January 2013. doi:10.1007/s00204-012-0968-2. PMID 23143619. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3535351
- "Human embryonic stem cell technologies and drug discovery". Journal of Cellular Physiology 219 (3): 513–9. June 2009. doi:10.1002/jcp.21732. PMID 19277978. https://dx.doi.org/10.1002%2Fjcp.21732
- "A novel lab-on-a-chip platform for spheroid metabolism monitoring". Cytotechnology 70 (1): 375–386. February 2018. doi:10.1007/s10616-017-0152-x. PMID 29032507. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5809666
- "Is It Time to Start Transitioning From 2D to 3D Cell Culture?" (in en). Frontiers in Molecular Biosciences 7: 33. 2020. doi:10.3389/fmolb.2020.00033. PMID 32211418. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=7067892




