Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tatiana Ivanovna Moiseenko | -- | 3137 | 2022-11-30 10:03:23 | | | |
| 2 | Catherine Yang | Meta information modification | 3137 | 2022-12-01 02:11:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moiseenko, T.I. Surface Water under Growing Anthropogenic Loads. Encyclopedia. Available online: https://encyclopedia.pub/entry/37320 (accessed on 08 February 2026).
Moiseenko TI. Surface Water under Growing Anthropogenic Loads. Encyclopedia. Available at: https://encyclopedia.pub/entry/37320. Accessed February 08, 2026.
Moiseenko, Tatyana I.. "Surface Water under Growing Anthropogenic Loads" Encyclopedia, https://encyclopedia.pub/entry/37320 (accessed February 08, 2026).
Moiseenko, T.I. (2022, November 30). Surface Water under Growing Anthropogenic Loads. In Encyclopedia. https://encyclopedia.pub/entry/37320
Moiseenko, Tatyana I.. "Surface Water under Growing Anthropogenic Loads." Encyclopedia. Web. 30 November, 2022.
Copy Citation
The increase in the fluxes of elements and compounds into the environment, such as the emission of greenhouse gases and dispersion of nutrients (nitrogen and phosphorus), acidifying gases, and toxic elements and compounds that adversely affect water quality, are considered.
surface water
anthropogenic loads
warming climate
1. The Importance of Freshwater in the Life Support
The worldwide utilization of freshwaters is evaluated at 2600 km3 per year and was 415 km3 per year in the preindustrial epoch [1]. Nowadays, approximately 70% of the world’s freshwater resources are used for agricultural needs, industries utilize 20%, and 10% is consumed for domestic purposes. According to global evaluations [2], the allowable global water withdrawal threshold has not been exceeded as of yet. The global consumption of freshwater by humankind amounts to 4000 km3 per year.
It should be mentioned that water resources are unevenly distributed over the planet’s surface, and many of these resources occur in northern areas that are sparsely populated. For example, the average long-term total runoff of northern rivers is estimated at 4300 km3, which is commensurable with the allowable freshwater consumption by the whole world’s human population. The northern areas are populated very sparsely. The total volume of secular freshwater resources stored in Russia’s lakes is 26,500 km3. (including 23,000 km3 in Lake Baikal, 903 km3 in Ladoga Lake, and 295 km3 in Onega Lake). First, the runoff of large Siberian rivers, Ob, Lena, and Yenisei, amounts to 1600 km3 per year, i.e., one-third of the world’s riverine runoff [3].
The greatest deficit of water resources (not only in quantity but also in the quality of the waters) occurs in densely populated steppe and arid parts of our planet, where more than 40% of the world’s human population currently lives. Statistical data indicate that practically one-fifth of the global population suffers from an acute shortage of drinkable water [4]. According to scientific estimates, more than half of countries worldwide will suffer from serious water shortage or insufficient supply by 2025 and three-quarters of the Earth’s population will have been critically short in freshwater by the mid-21st century. According to estimates, 47% of the world’s population will have been seriously threatened by water shortage by 2030. The population will significantly grow by 2050 in rapidly developing countries, which are already short of water [5].
Along with the water deficit, an important problem is the contamination of freshwaters with industrial emissions and discharges, the runoff of fertilizer-contaminated waters from urbanized territories, atmospheric fallouts, and the penetration of salty waters into fresh aquifers in coastal areas because of groundwater withdrawal. Operating along with such global processes as climate warming, anthropogenic loads result in both quantitative and qualitative depletion of water resources.
2. Effect of Climate Warming on Aquatic Systems
Lately, many researchers worldwide have focused on the effects of climate warming. The planet’s average air temperature has increased by 1.5 °C since 1980, and the Earth’s surface continues to warm at a rate of approximately 0.16 °C per decade, varying from one region to another. It has been proved that the warming of the biosphere brings about weather instability, along with changes in atmospheric precipitation and disturbances in hydrological cycles (longer arid periods and expansion of deserts in a warm climate with a simultaneous increase in precipitation and flooding in humid zones). The principal reason for climate warming is the ever-growing emission of greenhouse gases, first of all, CO2. The atmosphere now contains 42% more CO2 than at the beginning of the industrial era [6]. The latest IPCC report shows that emissions of greenhouse gases continue to rise, and current plans to address climate change are not ambitious enough to limit warming to 1.5 °C above the preindustrial level [7].
Global warming intensifies bio-cycling in the freshwater system and facilitates the establishment of feedback and changes in the environment, landscapes, and human society. An increase in air temperature, particularly in summertime and early autumn, means that the atmosphere can retain more water [8]. Climate changes also brings more moisture from lower latitudes to the pole. This increases the amount of precipitation in the Arctic, with this precipitation falling off in the form of either rain or snowfalls. In many parts of the Arctic, the amount of precipitation in the form of rain (but not snow) has increased, and the snow-cover period has become shorter [9].
Climate warming also affects runoff [10]. An increase in the precipitation, runoff and ice melting in ice covers results in a greater freshwater flow into the Arctic Ocean. For example, the riverine runoff was estimated to increase to 4200 km3 (±420 km3) in 2000–2010 compared to 3900 km3 (±390 km3) in 1980–2000. These changes are predicted to continue, and numerical simulations indicate that the riverine runoff will perhaps increase by 25 to 50% over most of the Arctic [9]. The example of large Siberian rivers, such as Ob (at the gauging section in Salekhard), Yenisei (Igarka), and Lena (Kyusyur), indicates that an increase in the runoff of these rivers was simultaneous with the onset of the modern air warming. It has been demonstrated that the long-lasting phases of runoff changes are synchronous with phases of changes in the air temperature and large-scale atmospheric circulation [11].
The most hazardous phenomenon is that warming impacts permafrost rocks (PFR), widespread in continental West Siberia over an integral area of about 700 thousand km2, i.e., more than one-fifth of the area. The thawing of permafrost peatlands in northern West Siberia may increase the release of methane and other greenhouse gases and augment water volumes in the rivers and lakes. The intensification of the thermokarst process should increase the number of lakes and their surface area [12][13][14][15].
The snow cover is the main source of annual runoff water in the summertime in northern territories and controls the export dynamics of nutrients and dissolved organic carbon (DOC). Changes in the hydrological cycles should inevitably modify concentrations and remove chemical elements in the waters, including suspending matter, DOC, and nutrients [16]. Climate warming should change (with regard to the predictions) the time and intensity of snow melting, which in turn, should change the runoff to the lakes and marginal seas. In addition, the frequency of autumn storms and floods may increase [17]. It is still largely uncertain how these factors may correlate with changes in the transfer of dissolved compounds from the catchments, but it is evident that the biogeochemical cycles in the catchment–water body system should thereby change.
The literature presents ample evidence that climate changes result in changes in the chemical composition of the waters [18][19][20]. D. Houle et al. [21] mention that higher annual average air temperatures correlate with the pH of the lake waters in long time series of monitoring data. The increase in organic matter content may be explained by the increase in the influx of nutrients (nitrates and phosphates) from the catchments as a consequence of climate warming [22][23].
The influence of temperature on water resources is controlled primarily through changes in the hydrological conditions under which the waters are formed and through biogeochemical cycles, i.e., the amount of precipitation, the occurrence of a snow cover, the conditions of the rocks underlying soils at the catchments, the saturation or depletion of the waters with exchange bases and accumulation at the catchment over the historical period, microbiological activity, the acceleration of vegetation growth, and perhaps, also the runoff of nutrients.
It has been demonstrated [24] how an elevated temperature affects the chemical composition of waters in various natural climatic zones (from the Arctic to steppes). These data indicate that temperature most strongly affects water eutrophication. Three-parameter dependencies were derived for parameters of the chemical composition of the waters on climatic parameters at the catchments. These dependencies provided a basis for prognostic models that enabled predicting the probable changes in salt and phosphorus concentrations in surface waters at warming for 0.5, 1.0, 1.5, and 2.0 °C (Figure 1).
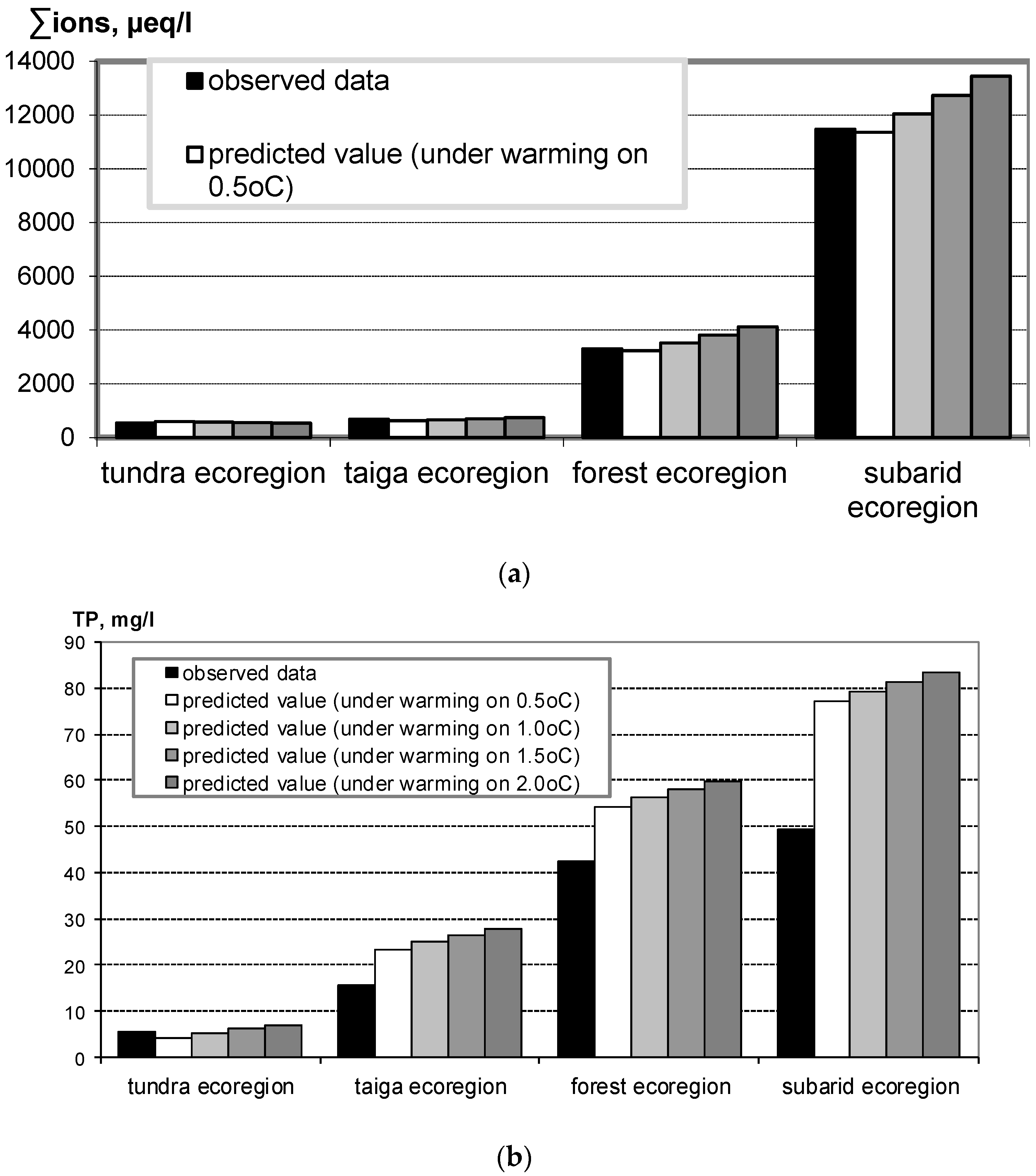
Figure 1. The observed and predicted values of sum ions (a) and total phosphorus (b) for the climatic ecoregions within European Russia along a climatic transect from northern tundra to the southern arid zone [24].
According to the calculated dependencies, the intensity of chemical weathering at a temperature increase of 0.5 and 1 °C should not result in any significant increase in the total dissolved salts (TDS) of waters in northern areas. However, a notable increase ( about 25%) in the total salt concentrations should occur in central and southern regions of the European part of Russia at a temperature increase of 2 °C. A significant increase (by approximately 50%) in the total phosphorus concentration in lake waters should occur practically everywhere (except only tundra and forested tundra territories), already at an increase in the average daily temperatures by 0.5 °C. Phosphorus concentrations should particularly increase in arid zones, in which climate warming should result in significant eutrophication of the waters.
Lately acquired data indicate that the temperature in the Arctic has significantly increased, and this resulted in a notable increase in concentrations of nutrients in the waters [22], which will be discussed below. Climate warming thus changes not only hydrological conditions under which the waters are formed but also modifies the chemical composition of the waters: the salt concentrations increase, as also do the concentrations of nutrients, and this should be most clearly seen in southern regions and also be discernible in the Arctic (Figure 2).
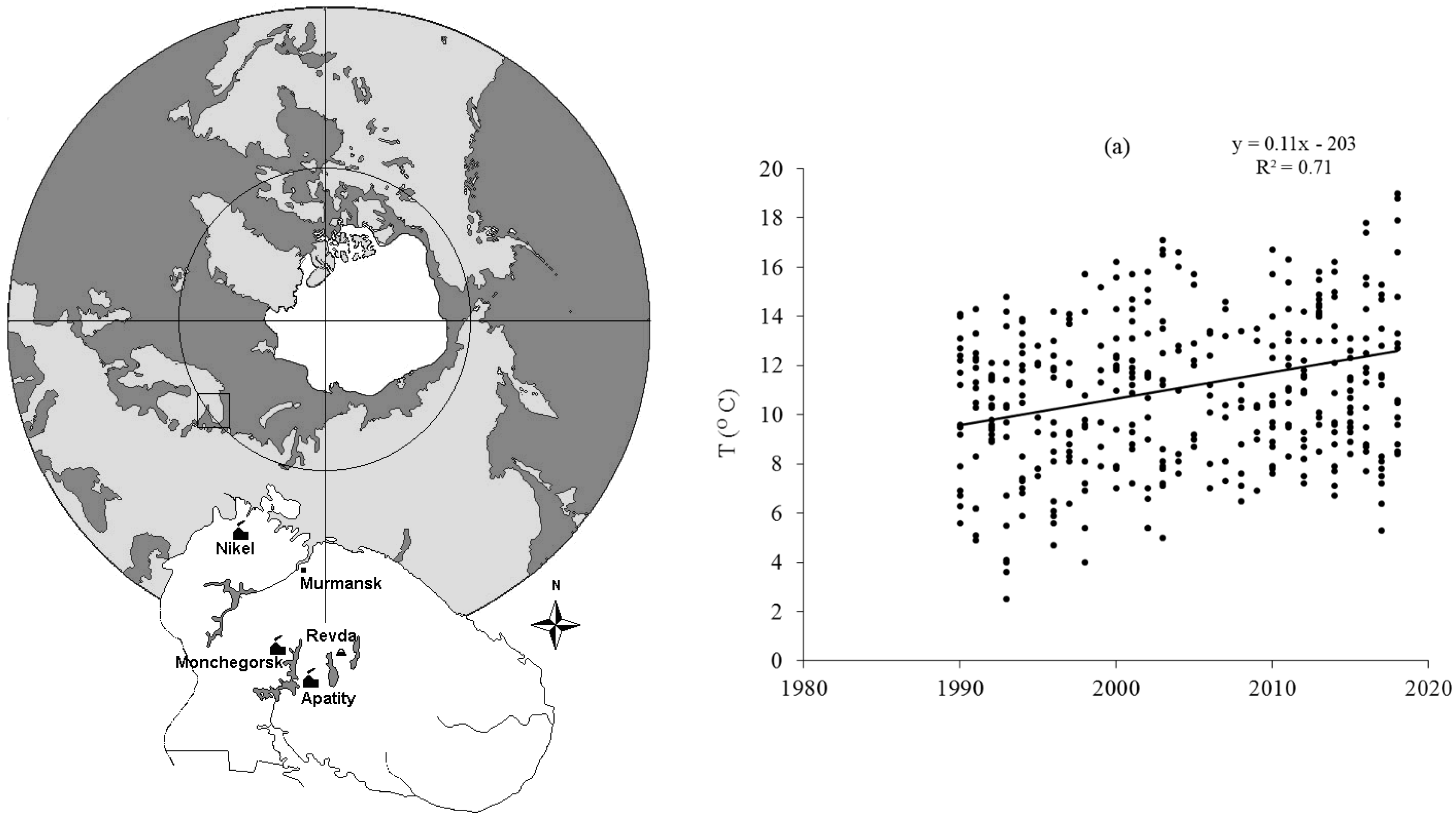
Figure 2. An example of an increase in mean monthly surface air temperature T (C) a 28 year period (1990–2018) on the territory of the Kola North. Significant positive slope of the temperature (T) SKT = (+4.71), p < 0.001.
Climate warming can stimulate the processes of salinization of lakes in arid and semi-arid regions due to an increase in evaporative concentration. This phenomenon has been established for a number of lakes in arid zones in North America, Kazakhstan, South Russia, Iran (Lake Urmia), and other water bodies [25][26][27][28][29]. The Crimean lakes of Russia on the western coast of the peninsula are located in the arid climate zone, they are typical hypersaline reservoirs, and in summer, they dry out at high air and water temperatures. The dynamics of changes in their ecological state during the year season may reflect the process of ecosystem transformation during the period of extremely hot conditions in the summer period, followed by restoration to normal functioning in the autumn-winter period [28]. From the cited works, it is concluded that climate warming consequences are most dramatic in the land waters of the southern regions. At the same time, there are changes in the water of the northern regions in the direction of increasing their trophic status.
3. Contamination of the Waters with Toxic Compounds
Toxic contamination of water is one of the most hazardous processes. Toxic properties of chemical elements and compounds are understood as their ability to adversely affect living organisms. It is widely known that many newly synthesized compounds are highly toxic to living organisms. It is also known that industrial byproducts and many naturally occurring elements in atypically high concentrations, for example, the essential metals, such as Cu and Zn, are also toxic to living organisms [30][31][32][33]. Aquatic systems are collectors of all toxic elements and compounds known to occur in the environment, and the implications and consequences of this phenomenon are still not fully understood because they may manifest their hazardous properties even if occurring in low concentrations. Coming into aquatic systems, toxic elements and compounds may circulate and be accumulated when moving along the food chains.
All ecologically toxic compounds can be grouped into the following classes:
-
Metals and metalloids, which are elements occurring in nature and toxically affecting living organisms when in high concentrations or certain speciation;
-
Persistent Organic Pollutants (POPs), are industrially synthesized compounds or byproducts of some technological processes involving naturally occurring compounds. This group comprises a broad class of organic xenobiotics: herbicides, insecticides, dioxins, furans, phthalates, etc.
Some contaminants are brought to the environment by technological disasters, such as oil spills, massive discharges (and/or leaks) of toxic compounds, and protection-dam breaks at tailing dumps. Natural disastrous events, such as volcanic activity and fires, can also release toxic compounds in concentrations harmful to living organisms; one example is mercury [34].
Metals and metalloids. Metals are brought to water arteries with the runoff from various industrial facilities, diffuse sources, and are leached from rocks by acidic precipitation. Mining operations and smelting facilitate the dispersion of chemical elements. Anthropogenic activities result in the volume of metals coming to water bodies due to anthropogenic processes comparable to the naturally occurring fluxes of these metals (Table 1). The number of publications on the levels of metal concentrations in water, sediments, and biota, as well as their toxic properties (experimental data), is high and continues to increase [35][36].
Table 1. Natural runoff of metals with rivers and anthropogenic input of elements into the environment and lakes, including metals (according to estimates by various authors: (1)—Bryan [37]; (2)—Moore, Ramamurth [38]; (3)—Venitsianov, Lepikhin [39]; (4)—Heath [40].
| Metals | River Runoff by Chemical Weathering | Inflow to Lakes (According to the Calculations) | Dispersion Into the Environment |
|---|---|---|---|
| Cr | 60.0 (3) | 63 | 54–130 (3) |
| Mn | 440 (1) | 2903 | - |
| Fe | 25,000 (1) | 31,925 | - |
| Ni | 300 (1) | 161 | 47.4 (2); 43–98 (3) |
| Cu | 375 (1) | 229 | 56 (2); 56–263 (3) |
| Zn | 370 (1) | 693 | 314 (2); 315–840 (3) |
| Mo | 13 (1) | 92 | - |
| Ag | 5 (1) | 3.9 | - |
| Cd | 4.65 (3) | 43 | 7–11 (3) |
| Sn | 1.5 (1) | 26 | - |
| Sb | 1.3 (1) | 22.2 | - |
| Pb | 180 (1) | 119 | 449 (2); 360–440 (3) |
| Hg | 3 (4) | - | 5–10 (3) |
Two regions north of the Arctic Circle, the Kola Peninsula and the Norilsk area, are the most ecologically alarming regarding water contamination with heavy metals (2020). It has been proved that a broad spectrum of metals (Ni, Cu, Mn, Sr, Fe, Al, Co, Cr, Cd, and Pb) migrates in Arctic water bodies dominantly in the form of ions, which are the most toxic. The toxic properties of the metals are enhanced by the associated eutrophication and acidification of the waters. The acidic atmospheric precipitation intensifies the metals leaching form rocks, further enhancing the hazardousness of the metals in acidic waters [41].
At accompanying eutrophication and the development of oxygen deficit in Arctic regions, the desorption of metals from bottom sediments is responsible for high gradients of the toxic effects in the bottom water layers for the fauna during long wintertime in the Arctic. The mechanism of the redox cycle has been thoroughly studied for Mn and Fe. These elements ascend in the form of dissolved reduced species to the oxicline boundary, into oxygen-enriched water layers, where these metals are oxidized again, form low-solubility compounds, and precipitate to the bottom, where the aforementioned cycle is restarted under reduced conditions. Data on the layer-by-layer distribution of elements in the water column of an Arctic lake indicate that a concentration gradient is thus produced not only for Fe and Mn but also for a large group of other elements, such as Cd, Hg, Cu, Mo, Ni, Pb, Zn, Cr, Co, Ba, Ga, and U. A generalized scheme of the metal cycle in the Arctic waters is presented in previous work [42].
The world’s scientific community currently pays much attention to such hazardous elements as Hg, Cd, and Pb. In water bodies in the studied areas of the Arctic, Hg concentrations were very low, whereas the accumulation of these elements in various organs and tissues of the fish indicates that the regional waters are ubiquitously contaminated with Hg. The lakes mostly contain Pb (>70%) in the form of its ionic species, which can readily penetrate into the fish organism. This element is accumulated in all systems of the fish organism depending on Pb concentration in the water. Cd is accumulated in all systems of the fish organism, and its highest concentrations were found in the kidneys [43].
Nowadays, environment-protection administrating bodies declare that methods for evaluating the biological availability of elements should be incorporated into procedures for assessing risks of water pollution with metals and into ecological management of water quality [44][45][46][47]. For example, the Water Framework Directive assumed in the European Community stresses that standards for water quality should include bioavailable Ni and Pb species [48]. The United States Environmental Protection Agency (EPA) has introduced a tool for assessing the bioavailability of Cu in standards for water contamination with this metal [49]. The models rely on determining the amounts of metal ions of the greatest penetrating capacity, i.e., bioavailability. While the standards assumed for Hg in Russia are comparable to those in the West, those for Pb and Cd are much more strict in Europe and the United States.
Persistent organic pollutants (POPs). These are industrially produced compounds that can be retained for years in the environment and be accumulated in fatty tissues. POPs include pesticides, such as aldrin, chlordan, DDT, dieldrin, endrin, heptachlor, hexachlorbenzene, mirex, α- and β-hexachlorocyclohexane, chlordecone, and lindane, as well as industrially manufactured chemical compounds, such as polychlorobiphenyls, hexachlorobenzene (which is also a pesticide), hexabromdiphenyl, hexa- and- heptabromdiphenyl ether (commercial octabromdiphenyl ether), pentachlorbenzene, perfluoroctane sulphacid, and its salts, perfluoroctane sulfonyl fluoride, tetra- and pentabromdiphenyl ether (commercial pentabromdiphenyl ether), and such unintentional byproducts as polychlorinated dioxins and furans [50]. These highly toxic compounds can come to the environment due to various anthropogenic activities, can be transported for long distances by air and water, and can make up hazardous concentrations in waters [36][51][52]. In 2021, the European Commission adopted a proposal to protect human health and the environment from some of the most harmful chemicals in waste: POPs. The convention provided for the possibility of appending the current list of compounds with other ones with the accumulation of necessary information if the newly introduced compounds have principal characteristics of POPs: high stability in the environment, resistance to degradation, acute and chronic toxicity, bioaccumulation, and the ability to be transferred across boundaries for great distances in the environment [51][52]. Table 2 lists the time periods needed for the decomposition of these compounds. These data show how long these compounds can be retained at a catchment and/or in a water body.
Table 2. Half-life of some xenobiotics in the environment [53].
| Pollutant | Half-Life in Temperate Climates | |||
|---|---|---|---|---|
| Air | Water | Bottom Sediments | Soil | |
| DDT | 2 days | >1 year | ||
| TCDD | 9 days | >5 years | >1 year | 10 years |
| Aldrin | <9 h | <590 days | no data | 5 years |
| Dieldrin | <40.5 h | >2 years | no data | >2 years |
| Endrin | 1.45 h | >112 days | no data | <12 years |
| Chlordane | <52 h | >4 years | no data | 1 year |
| Heptachlor | no data | <1 day | no data | 120–240 days |
| Hexachlorobenzene | <4.3 years | >100 years | no data | >3 years |
| Mirex | no data | >10 h | >600 years | >600 years |
| Toxaphene | <5 days | 20 years | no data | 10 years |
| PCB | 3–21 days | >5 days | no data | >40 days |
The behavior of these compounds in aquatic ecosystems is characterized by the fact that the compounds can be strongly accumulated in aquatic systems because of gradual enrichment in the food chains.
The adverse effects of toxic compounds at their direct or diffuse introduction into lakes and rivers commonly manifest themselves through the chronic effects of low concentrations. In these situations, disturbances are slowly (and often imperceptibly) accumulated in the aquatic organisms and may manifest themselves with the passage of time or in some critical situations, for example, when the temperature is anomalously high, during rain flooding, at stormy weather, etc. [31]. Chronic effects are more difficult to identify in spatiotemporal sections, and their identification requires long-term observations of the organisms, populations (during a few iterations), and communities. Despite great progress in studying the consequence of toxic contaminations, it is still not possible to predict all remote aftermaths of small doses of chronic contamination [32][54][55][56][57].
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S., 3rd; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475.
- Zhang, X.; Davidson, E.A.; Zou, T.; Lassaletta, L.; Quan, Z.; Li, T.; Zhang, W. Quantifying nutrient budgets for sustainable nutrient management. Glob. Biogeochem. Cycles 2020, 34, e2018GB006060.
- Edelstein, K.K. Continental Hydrology; Publishing Center “Academy”: Moscow, Russia, 2011; p. 304.
- Danilov-Danilyan, V.I.; Khranovich, I.L. Water Resources Management. Coordination of Water Use Strategies; Nauchny mir: Moscow, Russia, 2010; p. 233.
- UN World Water Development Report: Valuing Water. 2021. Available online: https://www.unwater.org/publications/un-world-water-development-report-2021 (accessed on 27 September 2022).
- AR5 Synthesis Report: Climate Change. 2014. Available online: https://www.ipcc.ch/report/ar5/syr/Ar5/ar5.html (accessed on 19 January 2022).
- AR6 Climate Change 2022: Mitigation of Climate Change—IPCC. Sixth Assessment Report. 2022. Available online: https://www.ipcc.ch/report/sixth-assessment-report (accessed on 9 July 2021).
- Biswas, A.K. Water Security Under Climate Change; Asit, K., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2022; p. 830.
- Arctic Freshwater System in a Changing Climate, WCRP CliC Project. AMAP. IASC CliC/AMAP/IASC. 2016. Available online: http://www.amap.no/documents/doc/The-Arctic-Freshwater-System-in-a-Changing-Climate/1375.AMAП (accessed on 1 January 2020).
- Hayhoe, K.; Wake, C.P.; Huntington, T.G.; Luo, L.; Schwartz, M.D.; Sheffield, J.; Wood, E.; Anderson, B.; Bradbury, J.; DeGaetano, A. Past and future hanges in climate and hydrological indicators in the US Northeast. Clim. Dyn. 2007, 28, 381–407.
- Georgiadi, A.G.; Kashutina, E.A. Long-term runoff changes of the largest siberian rivers. Izv. Ross. Akad. Nauk. Seriya Geogr. 2016, 5, 70–81. (In Russian)
- Jin, H.; Huang, Y.; Bense, V.F.; Ma, Q.; Marchenko, S.S.; Shepelev, V.V.; Hu, Y.; Liang, S.; Spektor, V.V.; Jin, X.; et al. Permafrost Degradation and Its Hydrogeological Impacts. Water 2022, 14, 372.
- Pavlov, A.V.; Malkova, G.D. Small-scale mapping of trends of the contemporary ground temperature changes in the Russian north. Earth Cryosphere 2009, 103, 32–39.
- Grosse, G.; Goetz, S.; McGuire, D.; Romanovsky, V.E.; Schuur, E.A. Changing permafrost in a warming world and feedbacks to the Earth system. Environ. Res. Lett. 2016, 11, 040201.
- Gao, X.; Schlosser, C.A.; Sokolov, A.; Anthony, K.W.; Zhuang, Q.; Kicklighter, D. Permafrost degradation and methane: Low risk of biogeochemical climate-warming feedback. Environ. Res. Lett. 2013, 8, 035014.
- Meingast, K.M.; Kane, E.; Coble, A.A.; Marcarelli, A.M.; Toczydlowski, D. Climate, snowmelt dynamics and atmospheric deposition interact to control dissolved organic carbon export from a northern forest stream over 26 years. Environ. Res. Lett. 2020, 15, 104034.
- Campbell, J.L.; Rustad, L.E.; Boyer, E.W.; Christopher, S.F.; Driscoll, C.T.; Fernandez, I.J.; Groffman, P.M.; Houle, D.; Kiekbusch, J.; Magill, A.H.; et al. Consequences of climate change for biogeochemical cycling in forests of northeastern North America Can. J. For. Res. 2009, 39, 264–284.
- De Wit, H.A.; Stoddard, J.L.; Monteith, D.T.; Sample, J.E.; Austnes, K.; Couture, S.; Fölster, J.; Higgins, S.N.; Houle, D.; Hruška, J.; et al. Cleaner air reveals growing influence of climate on dissolved organic carbon trends in northern headwaters. Environ. Res. Lett. 2021, 16, 104009.
- Watmough, S.A.; Eimers, C.; Baker, S. Impediments to recovery from acid deposition. Atmos. Environ. 2016, 146, 15–27.
- Gavin, A.L.; Nelson, S.J.; Klemmer, A.J.; Fernandez, I.J.; Strock, K.E.; McDowell, W.H. Acidification and climate linkages to increased dissolved organic carbon in high elevation lakes. Water Resour. Res. 2018, 54, 5187–5877.
- Houle, D.; Couture, S.; Gagnon, C. Relative role of decreasing precipitation sulfate and climate on recent lake recovery. Glob. Biogeochem. Cycles 2010, 24, 4029.
- Moiseenko, T.I.; Bazova, M.M.; Dinu, M.I.; Gashkina, N.A.; Kudryavtseva, L.P. Changes in the Geochemistry of Land Waters at Climate Warming and a Decrease in Acid Deposition: Recovery of the Lakes or Their Evolution? Geochem. Int. 2022, 60, 685–701.
- Corman, J.R.; Bertolet, B.L.; Casson, N.J.; Sebestyen, S.D.; Kolka, R.K.; Stanley, E.H. Nitrogen and phosphorus loads to temperate seepage lakes associated with allochthonous dissolved organic carbon loads. Geophys. Res. Lett. 2018, 45, 5481–5490.
- Moiseenko, T.I.; Skjelkvåle, B.L.; Gashkina, N.A.; Shalabodov, A.D.; Khoroshavin, V.Y. Water chemistry in small lakes along a transect from boreal to arid ecoregions in European Russia: Effects of air pollution and climate change. Appl. Geochem. 2013, 28, 69–79.
- Jellison, R.; Williams, W.D.; Timms, B.; Aladin, N.V. Salt lakes: Values and future. In Aquatic Ecosys-Tems: Trends and Global Prospects; Cambridge University Press: Cambridge, UK, 2008; pp. 92–110.
- Pouladi, P.; Mohammad, A.; Afshar, H.; Molajou, A.; Farahmand, H. Agent-based socio-hydrological modeling for restoration of Urmia Lake: Application of theory of planned behavior. J. Hydrol. 2019, 576, 736–748.
- Tussupova, K.; Hjorth, A.P.; Morave, M. Drying lakes: A review on the applied restoration strategies and health conditions in contiguous areas. Water 2020, 12, 749.
- Rudneva, I.I.; Shaidaa, V.G.; Shcherba, A.V.; Zavyalova, A.V. Influence of Climatic Factors on Interan-nual and Seasonal Dynamics of the Environmental State of the Salt Lake Adzhi-Baichi (Crimea). Arid. Ecosys-Tems 2021, 11, 434–442.
- Mojtahedi, A.; Dadashzadeh, M.; Azizkhani, M.; Mohammadian, A.; Almasi, R. Assessing climate and human activity effects on lake characteristics using spatio-temporal satellite data and an emotional neural network. Environ. Earth Sci. 2022, 81, 1–20.
- Chapman, P.M. Integrating toxicology and ecology: Putting the “eco” into ecotoxicology. Mar. Pollut. Bull. 2002, 44, 7–15.
- Newman, M.C.; Clements, W.H. Aquatic Toxicology: Concepts, Practice, New Directions. In General, Applied and Systems Toxicology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9780470744307 (accessed on 27 September 2022).
- Boudou, A. Aquatic Ecotoxicology. Volume 1: Fundamental Concepts and Methodologies; Taylor & Francis eBooks: College Park, MD, USA, 2018; p. 328. Available online: https://www.routledge.com/Aquatic-Ecotoxicology-Volume-1-Fundamental-Concepts-and-Methodologies/Boudou/p/book/9781315890753 (accessed on 27 September 2022).
- Vasseur, P.; Masfaraud, J.-F.; Blaise, C. Ecotoxicology, revisiting its pioneers. Environ. Sci. Pollut. Res. Int. 2021, 28, 3852–3857.
- Fitzgerald, L.; Wikoff, D.S. Persistent organic pollutants. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014; Volume 3, pp. 820–825. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/persistent-organic-pollutant (accessed on 27 September 2022).
- Davidson, T.; Costa, M. Selected Molecular Mechanisms of Metal Toxicity and Carcinogenicity. In Handbook on the Toxicology of Metals; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 79–100.
- Bjerregaard, P.; Andersen, O. Ecotoxicology of Metals—Sources, Transport, and Effects in the Ecosystem. In Handbook on the Toxicology of Metals; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014.
- Bryan, G.W. Heavy Metal Contamination in the Sea, Marine Pollution; Johnston, R., Ed.; Academic Press: Cambridge, MA, USA; Elesvier: London, UK, 1976; pp. 185–302.
- Moore, J.W.; Ramamurthy, S. Heavy Metals in Natural Waters: Applied Monitoring and Impact Assessment; Springer: Berlin/Heidelberg, Germany, 1984; p. 288.
- Venitsianov, E.V.; Lepikhin, A.P. Physico–Chemical Bases of Modeling Migration and Transformation of Heavy Metals in Natural Waters; Publishing House of RosNIIVH: Yekaterinburg, Russia, 2002; p. 236.
- Heath, A.G. Water Pollution and Fish Physiology; Lewis Publishers: Amsterdam, The Netherlands, 2002; p. 506.
- Moiseenko, T.I.; Gashkina, N.A.; Dinu, M.I.; Kremleva, T.A.; Khoroshavin, V.Y. Water Chemistry of Arctic Lakes under Airborne Contamination of Watersheds. Water 2020, 12, 1659.
- Moiseenko, T.I. A Fate of Metals in Arctic Surface Waters. Method for Defining Critical Levels. Sci. Total Environ. 1999, 236, 19–39.
- Moiseenko, T.I.; Gashkina, N.A. Distribution and bioaccumulation of heavy metals (Hg, Cd, and Pb) In fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013.
- Moiseenko, T.; Sharov, A. Large Russian lakes Ladoga, Onega, and Imandra under strong pollution and in the period of revitalization: A review. Geosciences 2019, 9, 492.
- Magalhaes, D.; Marques, M.; Baptista, D.; Forsin, D.; Buss, D. Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 2015, 13, 69–87.
- Merrington, G.; Peters, A.; Schlekat, C.E. Accounting for metal bioavailability in assessing water quality: A step change? Environ. Toxicol. Chem. 2016, 35, 257–265.
- Väänänena, K.; Leppänen, M.T.; Chen, X.; Akkanenaa, J. Metal bioavailability in ecological risk assessment of freshwater ecosystems: From science to environmental management. Ecotoxicol. Environ. Saf. 2018, 147, 430–446.
- European Commission. Directive 2013/39/EC Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. 2013/39/EC; European Commission: Brussels, Belgium, 2013; Available online: https://www.eea.europa.eu/policy-documents/2013-39-ec (accessed on 27 September 2022).
- USA EPA. National Recommended Water Quality Criteria. Report 4304T. Office of Water, Office of Science and Technology. (EPA/600/4-91/002); USA EPA: Springfield, IL, USA, 2007.
- Stockholm Convention on Persistent Organic Pollutants. UN Convention; 2004. Available online: https://www.state.gov/key-topics-office-of-environmental-quality-and-transboundary-issues/stockholm-convention-on-persistent-organic-pollutants (accessed on 2 April 2022).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064.
- Barrick, A.; Champeau, O.; Butler, J.; Wiles, T.; Boundy, M.; Tremblaya, L.A. Hazard/Risk Assessment Investigating the Ecotoxicity of Select Emerging Organic Contaminants Toward the Marine Copepod Gladioferens pectinatus. Environ. Toxicol. Chem. 2022, 41, 792–799.
- Khudoley, V.V. Stockholm Convention, and National Plan of Action Against Persistent Organic Pollutants (POPs); The International Ecological Forum: St.Petersburg, Russia, 2002; pp. 89–91.
- Moiseenko, T.I. Aquatic ecotoxicology: Theoretical principles and practical application. Water Resour. 2008, 35, 530–541.
- Zhulidov, A.V.; Robarts, R.D.; Headley, J.V.; Korotova, L.G.; Pavlov, D.F.; Zhulidov, D.A.; Zhulidova, O.V. A review riverine fluxes of hexachlorcyclohexane and DDT to Azov and Blake seas from the formed USSR and Russian Federation. J. Environ. Sci. Health 2003, 5, 38.
- Blus, L.I. Organochlorine Pesticedes. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Cairnce, J., Jr., Eds.; Lewis Publishers: New York, NY, USA, 2005; pp. 314–329.
- Rice, C.P.; O’Keefe, P.; Kubiak, T.J. Sources, Partweys and effects of PSBs, Dioxins and Dibenzofurans. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Cairnce, J., Jr., Eds.; Lewis Publishers: New York, NY, USA, 2005; pp. 501–556.
More
Information
Subjects:
Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
675
Revisions:
2 times
(View History)
Update Date:
01 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




