
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ulrike Leiter | -- | 2262 | 2022-11-30 09:41:42 | | | |
| 2 | Lindsay Dong | -6 word(s) | 2256 | 2022-12-01 03:23:08 | | |
Video Upload Options
The first-line therapy for locally advanced basal cell carcinoma (laBCC) is Hedgehog pathway inhibitors (HHIs), as they achieve good efficacy and duration of response. However, toxicity in the course of long-term treatment may lead to a decrease in the quality of life, and consequently to interruption or even discontinuation of therapy. As HHI therapy is a balancing act between effectiveness, adverse events, quality of life, and adherence, numerous successful treatment strategies have evolved, such as dose reduction and dose interruptions with on-off treatment schedules or interruptions with re-challenge after progression. As a small percentage of patients show primary or acquired resistance to HHIs, the inhibition of programmed cell death protein 1 (PD-1) has been approved as a second-line therapy, which may also be accompanied by immune-related toxicities and non-response. Thus, optimization of current treatment schedules, novel agents, and combination strategies are urgently needed for laBCC.
1. Introduction
2. First-Line Therapy: Hedgehog Inhibitors
3. Targeted Treatment with HHIs—How to Balance Efficacy and Toxicity
3.1. On-Off Treatment Schedules
Several studies aimed at improving the tolerability of HHIs while maintaining their efficacy. This should reduce the number of patients who discontinue HHI therapy due to AEs. Thus, some studies have sought to determine whether an intermittent dosing regimen leads to a reduction of AEs, and thus to a prolonged duration of treatment (DOT).
Villani et al. reported a case series of 10 patients with multiple BCCs treated with different vismodegib treatment regimens [25]. Three patients were treated with a regular dosing regimen (150 mg daily), four patients received a modified treatment regimen based on dose adjustment (on-off scheme with eight or twelve weeks interruption) according to the number and severity of reported AEs, and three patients received a prophylactic dose reduction (150 mg every other day) from baseline in order to avoid severe AEs. Patients who received a holiday regimen (during treatment or at baseline) reported a good AE profile characterized by mild adverse events leading to longer treatment durations.
3.2. Dose Reduction
3.3. Re-Challenge after Relapse without Other Intercurrent Treatments
In a retrospective multicenter observational study, 116 patients with laBCC who had CR on vismodegib and subsequently discontinued treatment were studied [28]. The median relapse-free survival (RFS) was 18.4 months (95% CI 13.5–24.8 months). The RFS rate at 36 months was 35.4% (95% CI 22.5–47.9%) for the total population and 40.0% (95% CI 25.7–53.7%) for patients with Gorlin–Goltz syndrome. The median overall survival (OS) was not reached, and the rate at 36 months was 85% (95% CI 74.6–96%). Risk factors associated with RFS were laBCC for limbs and trunk (hazard ratio 2.77; 95% CI 1.23–6.22). A total of 50% of patients who relapsed during follow-up were re-treated with vismodegib, of whom 85% experienced an objective response, 37% a CR, and 48% a PR.
3.4. Maintenance of Remission
3.5. Resistance Mechanisms of HHIs
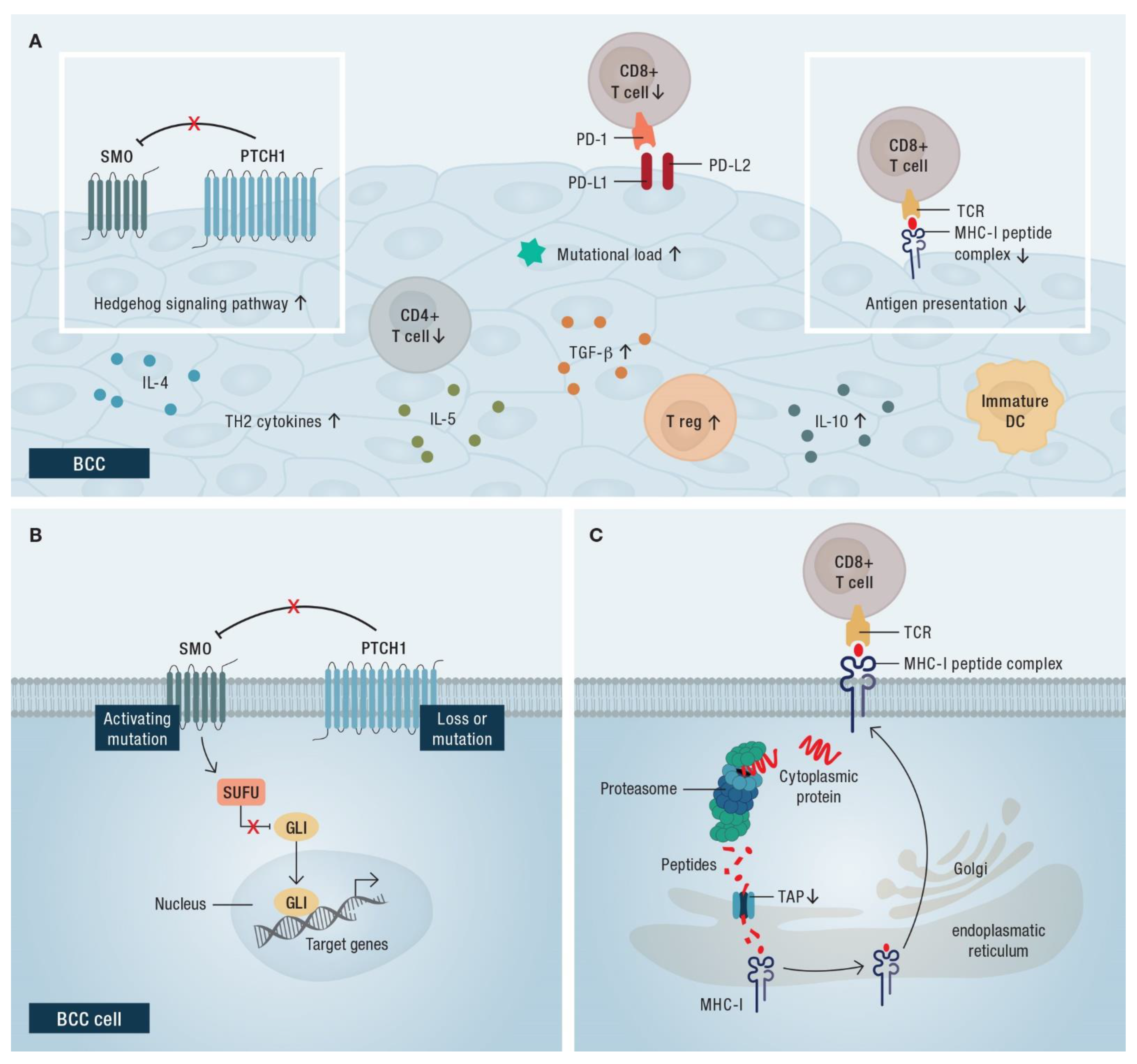
4. Immune Checkpoint Blockade with Anti-PD-1-Blocking Antibodies
4.1. Efficacy of PD-1 Inhibitors in BCC
4.2. Rationale for the Use of Immune Checkpoint Blockade in BCC
5. Conclusions
References
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754.
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317.
- Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olencki, T.; Rodgers, P. Guidelines of care for the management of basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 540–559.
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34.
- Lear, J.T.; Corner, C.; Dziewulski, P.; Fife, K.; Ross, G.L.; Varma, S.; Harwood, C.A. Challenges and new horizons in the management of advanced basal cell carcinoma: A UK perspective. Br. J. Cancer 2014, 111, 1476–1481.
- Hahn, H.; Wicking, C.; Zaphiropoulous, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996, 85, 841–851.
- Johnson, R.L.; Rothman, A.L.; Xie, J.; Goodrich, L.V.; Bare, J.W.; Bonifas, J.M.; Quinn, A.G.; Myers, R.M.; Cox, D.R.; Epstein, E.H., Jr.; et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996, 272, 1668–1671.
- Reifenberger, J.; Wolter, M.; Knobbe, C.B.; Köhler, B.; Schönicke, A.; Scharwächter, C.; Kumar, K.; Blaschke, B.; Ruzicka, T.; Reifenberger, G. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br. J. Dermatol. 2005, 152, 43–51.
- Xie, J.; Murone, M.; Luoh, S.M.; Ryan, A.; Gu, Q.; Zhang, C.; Bonifas, J.M.; Lam, C.W.; Hynes, M.; Goddard, A.; et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 1998, 391, 90–92.
- Low, J.A.; de Sauvage, F.J. Clinical experience with Hedgehog pathway inhibitors. J. Clin. Oncol. 2010, 28, 5321–5326.
- Pan, S.; Wu, X.; Jiang, J.; Gao, W.; Wan, Y.; Cheng, D.; Han, D.; Liu, J.; Englund, N.P.; Wang, Y.; et al. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. ACS Med. Chem. Lett. 2010, 1, 130–134.
- European Medicines Agency. European Public Assessment Report: Erivedge. November 2016. Available online: https://www.ema.europa.eu/en/documents/overview/erivedge-epar-summary-public_en.pdf (accessed on 9 February 2022).
- European Medicines Agency. European Public Assessment Report: Odomzo. September 2015. Available online: https://www.ema.europa.eu/en/documents/overview/odomzo-epar-summary-public_en.pdf (accessed on 9 February 2022).
- Lacouture, M.E.; Dréno, B.; Ascierto, P.A.; Dummer, R.; Basset-Seguin, N.; Fife, K.; Ernst, S.; Licitra, L.; Neves, R.I.; Peris, K.; et al. Characterization and Management of Hedgehog Pathway Inhibitor-Related Adverse Events in Patients with Advanced Basal Cell Carcinoma. Oncologist 2016, 21, 1218–1229.
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: A joint expert opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956.
- Lear, J.T.; Migden, M.R.; Lewis, K.D.; Chang, A.L.S.; Guminski, A.; Gutzmer, R.; Dirix, L.; Combemale, P.; Stratigos, A.; Plummer, R.; et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 372–381.
- Sekulic, A.; Migden, M.R.; Lewis, K.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; Marmur, E.; Rudin, C.M.; et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J. Am. Acad. Dermatol. 2015, 72, 1021–1026.e8.
- Gutzmer, R.; Robert, C.; Loquai, C.; Schadendorf, D.; Squittieri, N.; Arntz, R.; Martelli, S.; Dummer, R. Assessment of various efficacy outcomes using ERIVANCE-like criteria in patients with locally advanced basal cell carcinoma receiving sonidegib: Results from a preplanned sensitivity analysis. BMC Cancer 2021, 21, 1244.
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.T.; Lewis, K.D.; Chang, A.L.S.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Dermatol. 2020, 182, 1369–1378.
- Gutzmer, R.; Schulze, H.J.; Hauschild, A.; Leiter, U.; Meier, F.; Haferkamp, S.; Ulrich, C.; Wahl, R.U.; Berking, C.; Herbst, R.; et al. Effectiveness, safety and utilization of vismodegib in locally advanced basal cell carcinoma under real-world conditions in Germany—The non-interventional study NIELS. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1678–1685.
- Herms, F.; Baroudjian, B.; Delyon, J.; Laly, P.; Tetu, P.; Lebbe, C.; Basset-Seguin, N. Sonidegib in the Treatment of Locally Advanced Basal Cell Carcinoma: A retrospective single-center study in France. In Proceedings of the Virtual European Academy of Dermatology and Venereology Congress, Virtual, 29 September–2 October 2021.
- Leow, L.J.; Teh, N. Clinical clearance of complex basal cell carcinoma in patients receiving sonidegib: A case series. Dermatol. Ther. 2022, 35, e15217.
- Villani, A.; Fabbrocini, G.; Scalvenzi, M. Sonidegib treatment in patients with locally advanced basal cell carcinoma. Dermatol. Ther. 2022, 35, e15348.
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179.
- Villani, A.; Costa, C.; Fabbrocini, G.; Scalvenzi, M. Drug holiday regimen for vismodegib treatment in patients with multiple primary basal cell carcinomas. Dermatol. Ther. 2020, 33, e13707.
- EMA. Summary of Product Characteristics, Odomzo 200 mg Hard Capsules. Available online: https://www.ema.europa.eu/en/documents/product-information/odomzo-epar-product-information_en.pdf (accessed on 7 February 2022).
- Villani, A.; Costa, C.; Fabbrocini, G.; Ruggiero, A.; Scalvenzi, M. Dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor sonidegib to manage adverse effects: A retrospective case series. J. Am. Acad. Dermatol. 2021, 84, e211–e212.
- Herms, F.; Lambert, J.; Grob, J.J.; Haudebourg, L.; Bagot, M.; Dalac, S.; Dutriaux, C.; Guillot, B.; Jeudy, G.; Mateus, C.; et al. Follow-Up of Patients with Complete Remission of Locally Advanced Basal Cell Carcinoma After Vismodegib Discontinuation: A Multicenter French Study of 116 Patients. J. Clin. Oncol. 2019, 37, 3275–3282.
- Tang, J.Y.; Ally, M.S.; Chanana, A.M.; Mackay-Wiggan, J.M.; Aszterbaum, M.; Lindgren, J.A.; Ulerio, G.; Rezaee, M.R.; Gildengorin, G.; Marji, J.; et al. Inhibition of the hedgehog pathway in patients with basal-cell nevus syndrome: Final results from the multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1720–1731.
- Doan, H.Q.; Chen, L.; Nawas, Z.; Lee, H.H.; Silapunt, S.; Migden, M. Switching Hedgehog inhibitors and other strategies to address resistance when treating advanced basal cell carcinoma. Oncotarget 2021, 12, 2089–2100.
- Yurchenko, A.A.; Pop, O.T.; Ighilahriz, M.; Padioleau, I.; Rajabi, F.; Sharpe, H.J.; Poulalhon, N.; Dreno, B.; Khammari, A.; Delord, M.; et al. Frequency and Genomic Aspects of Intrinsic Resistance to Vismodegib in Locally Advanced Basal Cell Carcinoma. Clin. Cancer Res. 2022, 28, 1422–1432.
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.S.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857.
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.S.; Dalle, S.; et al. Phase 2 study of cemiplimab in patients with locally advanced basal cell carcinoma after hedgehog inhibitor therapy: Long-term follow-up. In Proceedings of the virtual European Association of Dermato Oncology congress, Seville, Spain, 21–23 April 2022.
- Walter, A.; Barysch, M.J.; Behnke, S.; Dziunycz, P.; Schmid, B.; Ritter, E.; Gnjatic, S.; Kristiansen, G.; Moch, H.; Knuth, A.; et al. Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin. Cancer Res. 2010, 16, 3562–3570.




