
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Audrey Ferrand | + 1136 word(s) | 1136 | 2020-12-16 07:58:25 | | | |
| 2 | Vicky Zhou | Meta information modification | 1136 | 2020-12-22 04:49:14 | | |
Video Upload Options
Intestinal stem cells (ISC) are crucial players in colon epithelium physiology. The accurate control of their auto-renewal, proliferation and differentiation capacities provides a constant flow of regeneration, maintaining the epithelial intestinal barrier integrity. Under stress conditions, colon epithelium homeostasis in disrupted, evolving towards pathologies such as inflammatory bowel diseases or colorectal cancer. A specific environment, namely the ISC niche constituted by the surrounding mesenchymal stem cells, the factors they secrete and the extracellular matrix (ECM), tightly controls ISC homeostasis. Colon ECM controls ISC homeostasis by exerting physical constraint on the enclosed stem cells through peculiar topography, stiffness and deformability.
1. Introduction
The colon is part of the digestive system, one of the 11 major organ systems of the human body. Its well-known digestive function is to compact the alimentary bolus while absorbing electrolytes and water. However, the colon also plays a critical role in protecting the inner body against external aggressions, whether biological (pathogenic bacteria, virus or parasites); physical (bolus) or chemical (pollutants or food contaminants). This ability to control the uptake across the mucosa while protecting from damage caused by harmful substances is defined as the intestinal barrier function (IBF). A main player of the IBF is a healthy and fully functional epithelium. This epithelium is composed of diverse specialized cell types, all originating from the intestinal stem cells (ISC) [1]. Indeed, ISC ensure the complete renewal of the intestinal epithelial linen within only three to five days, putting great demand on the mechanisms regulating ISC homeostasis and capacities but, also, on the entire tissue cellular organization. Therefore, the colon constitutes one of the most adequate tissues to study stem cells capacities.
The identification of the ISC population during the past decade led to their successful ex-vivo culture, allowing recreating 3D intestinal epithelial mini-organs, namely organoids. This technological breakthrough consists in an isolated intestinal crypt, which includes the ISC, embedded in Matrigel, put in the presence of a culture medium favoring either the ISC renewal or the differentiation process. As a result, these 3D intestinal organoids display all the epithelial cells constituting the colon epithelial linen and represent an excellent model to study the ISC capacity and ability to reconstitute a fully polarized and functional epithelium with all its cell populations (stem cells, transit-amplifying progenitors, enterocytes, enteroendocrine and goblet cells) [2]. This model thus represents an excellent tool to investigate the cellular and molecular mechanisms involved in proliferation and differentiation processes under (patho)physiological conditions.
The ISC surrounding environment, namely the ISC niche, critically controls ISC homeostasis. This niche comprises the supportive mesenchymal cells, fibroblasts being the major population, and the differentiated epithelial progeny, all secreting factors tightly regulating ISC self-renewal, as well as the proliferation and differentiation processes along the crypt. Interestingly, recent findings highlight that physical constraints exerted by the extracellular matrix (ECM) on the epithelium participate in the regulation of the cell phenotypes and behaviors [3]. ECM exerts these constraints on the epithelial cells through specific topography, stiffness and deformability. Under pathological contexts, such as chronic inflammation or cancer, ECM mechanical properties are profoundly modified, participating in the development and progression of diseases such as inflammatory bowel disease (IBD) or colorectal cancer (CRC). Understanding how these ECM constraints affect the intestinal epithelium and which roles they play in the intestinal (patho)physiology is thus of high interest; however, investigating in vivo the impact of ECM mechanical properties on the ISC regulation and the intestinal epithelial linen is challenging. Therefore, using the organoid model represents an excellent alternative to address these questions.
2. The Colon Epithelial Cell Populations
The colon epithelium is a single layer of polarized cells, their basal pole leaning on the basement membrane and their apical pole facing the lumen (Figure 1). Observed from the lumen, the colon epithelium is flat, contrary to the small intestine epithelium that displays villi, finger-like protrusions that project into the lumen of the gut. However, like the small intestine, the colon presents invaginations called crypts of Lieberkühn. The peculiar architecture of the intestinal epithelium allows compartmentalization of ISC at the bottom of the crypts and differentiated cells at their top (Figure 1) [1]. The intestinal epithelium is subjected to a fast renewal, occurring within three to five days, to maintain its integrity and optimal function. Constituted up to 80% by absorptive enterocytes, the colon epithelium also contains goblet cells (18%) secreting the mucus, tuft cells (0.4%) playing an antiparasite function and enteroendocrine cells (1% of the cell population) secreting diverse hormones controlling the intestinal motility and secretions and colon peristalsis but, also, visceral sensation, appetite or pancreas exocrine functions. Contrary to the small intestine, Paneth cells are absent from the colon epithelium, where deep crypt secretory (DCS) cells function as their colon equivalent [4]. Differentiated cells originate from transit-amplifying (TA) cells, progenitor cells that are characterized by a rapid proliferation rate before migrating along the crypt and differentiating while reaching the luminal surface. The TA cells rise from the ISC.
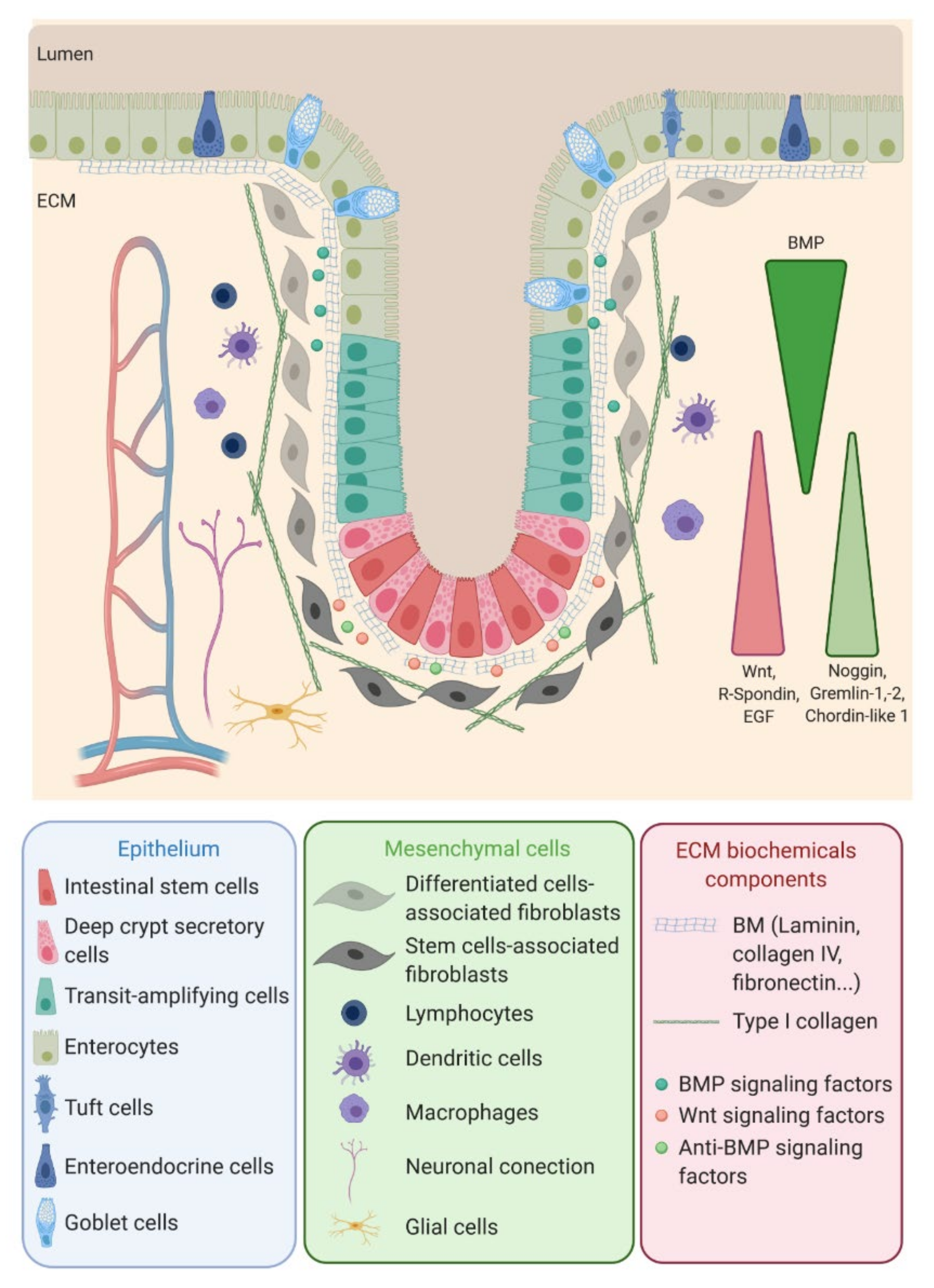
ISC function and behavior are precisely regulated by their niche. Along the crypt axis, the expression profiles of signaling pathways in epithelial cells along the intestinal tract, as well as the crypt-villus axis, have been studied for the past decade in the small intestine and colon of pigs [5], mice [6], rats [7] and humans [8] and recently reviewed by Wang et al. [9]. Using complementary DNA (cDNA) microarrays, Kosinski et al. were able to discriminate key pathways involved along the human colon crypts [8], corroborating results obtained in mice small intestines by Mariadason et al. two years before [6]. Logically, at the bottom of the crypt, the expression of genes regulating the cell cycle or, more specifically, the ISC and progenitors (Wnt, R-spondin, HedgeHog (Hhg) and Epidermal Growth Factor (EGF)) are increased, while those inducing apoptosis are downregulated. On the other hand, pro-differentiative bone marrow protein (BMP) pathways are preferentially expressed at the top of the crypt. Interestingly, BMP antagonists such as Noggin, Gremlin 1, Gremlin 2 and Chordin-like 1 are only expressed by the surrounding stromal cells, myofibroblasts and smooth muscle cells at the bottom of the crypt. The leading role of the epithelial-mesenchymal dialogue in intestinal epithelium differentiation and crypt-villus formation has already been demonstrated using a co-culture of intestinal epithelial cells and mesenchymal cells [10][11]. This suggests a compartmentalization not only of epithelial cells but, also, of mesenchymal cells through specific gene expression profiles along the crypt axis. This compartmentalization is indeed a key determinant in intestinal homeostasis.
References
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33.
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265.
- DiMarco, R.L.; Su, J.; Yan, K.S.; Dewi, R.; Kuo, C.J.; Heilshorn, S.C. Engineering of three-dimensional microenvironments to promote contractile behavior in primary intestinal organoids. Integr. Biol. 2014, 6, 127–142.
- Sasaki, N.; Sachs, N.; Wiebrands, K.; Ellenbroek, S.I.; Fumagalli, A.; Lyubimova, A.; Begthel, H.; van den Born, M.; van Es, J.H.; Karthaus, W.R.; et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl. Acad. Sci. USA 2016, 113, E5399–E5407.
- Gourbeyre, P.; Berri, M.; Lippi, Y.; Meurens, F.; Vincent-Naulleau, S.; Laffitte, J.; Rogel-Gaillard, C.; Pinton, P.; Oswald, I.P. Pattern recognition receptors in the gut: Analysis of their expression along the intestinal tract and the crypt/villus axis. Physiol. Rep. 2015, 3.
- Mariadason, J.M.; Nicholas, C.; L’Italien, K.E.; Zhuang, M.; Smartt, H.J.; Heerdt, B.G.; Yang, W.; Corner, G.A.; Wilson, A.J.; Klampfer, L.; et al. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 2005, 128, 1081–1088.
- Suzuki, T.; Mochizuki, K.; Goda, T. Localized expression of genes related to carbohydrate and lipid absorption along the crypt-villus axis of rat jejunum. Biochim. Biophys. Acta 2009, 1790, 1624–1635.
- Kosinski, C.; Li, V.S.; Chan, A.S.; Zhang, J.; Ho, C.; Tsui, W.Y.; Chan, T.L.; Mifflin, R.C.; Powell, D.W.; Yuen, S.T.; et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl. Acad. Sci. USA 2007, 104, 15418–15423.
- Wang, Y.; Kim, R.; Hinman, S.S.; Zwarycz, B.; Magness, S.T.; Allbritton, N.L. Bioengineered Systems and Designer Matrices That Recapitulate the Intestinal Stem Cell Niche. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 440–453.e441.
- Kedinger, M.; Simon-Assmann, P.; Haffen, K. Growth and differentiation of intestinal endodermal cells in a coculture system. Gut 1987, 28, 237–241.
- Plateroti, M.; Freund, J.N.; Leberquier, C.; Kedinger, M. Mesenchyme-mediated effects of retinoic acid during rat intestinal development. J. Cell Sci. 1997, 110 Pt 10, 1227–1238.




