Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | takahiro kitsuka | -- | 2584 | 2022-11-30 00:42:57 | | | |
| 2 | Dean Liu | -3 word(s) | 2581 | 2022-11-30 03:51:09 | | | | |
| 3 | Dean Liu | Meta information modification | 2581 | 2022-12-01 02:26:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kitsuka, T.; Takahashi, F.; Reinhardt, J.; Shinoka, T. Human Cardiac Muscle Patches in Cardiac Tissue Engineering. Encyclopedia. Available online: https://encyclopedia.pub/entry/37187 (accessed on 07 February 2026).
Kitsuka T, Takahashi F, Reinhardt J, Shinoka T. Human Cardiac Muscle Patches in Cardiac Tissue Engineering. Encyclopedia. Available at: https://encyclopedia.pub/entry/37187. Accessed February 07, 2026.
Kitsuka, Takahiro, Fuga Takahashi, James Reinhardt, Toshiharu Shinoka. "Human Cardiac Muscle Patches in Cardiac Tissue Engineering" Encyclopedia, https://encyclopedia.pub/entry/37187 (accessed February 07, 2026).
Kitsuka, T., Takahashi, F., Reinhardt, J., & Shinoka, T. (2022, November 30). Human Cardiac Muscle Patches in Cardiac Tissue Engineering. In Encyclopedia. https://encyclopedia.pub/entry/37187
Kitsuka, Takahiro, et al. "Human Cardiac Muscle Patches in Cardiac Tissue Engineering." Encyclopedia. Web. 30 November, 2022.
Copy Citation
Tissue engineering has paved the way for the development of artificial human cardiac muscle patches (hCMPs) and cardiac tissue analogs, especially for treating Myocardial infarction (MI), often by increasing its regenerative abilities.
tissue engineering
iPS-derived cardiac myocyte
biodegradable scaffolds
1. Cell Types and Sources for Artificial hCMP Fabrication
Originally, many investigations in myocardial regenerative medicine have focused on CMs, cells difficult to proliferate and recover once damaged. CMs are responsible for generating a contractile force in the myocardium [1]. In the heart, the newborn ratio for the number and volume ratio of CMs to non-CMs is 7:3. As the heart grows to adult size. However, the volume ratio does not change, the ratio for the number of CMs to non-CMs becomes 3:7 with the non-CMs increasing in proportion [2]. The cause of this ratio change has been hypothesized to be the proliferation of non-CMs; from this, it is thought that the function of the heart is due to not only the CMs but also the interaction of non-CMs and CMs.
CMs, endothelial cells (ECs), smooth muscle cells (SMCs), and cardiac fibroblasts (CFs) have been previously used for hCMP production, in order to comprehensively reproduce the physical structures and signaling pathways present in native heart tissue. ESCs and iPSCs are the most readily available sources of CM for human strains because they can multiply indefinitely and differentiate into cells of different lineages. Both ESC-derived and iPSC-derived CM are also more structurally similar to neonatal cells than adult CM, hindering applications of hCMPs composed of immature iPSC-derived CM. However, clusters of other cell types (e.g., progenitor cells and spheroids) have also been incorporated into the heart patch and evaluated in preclinical models of myocardial injury [3]. It is also important to note how Zhao et al. is working on differentiating muscle cells into specific ventricular and atrial cells [3].
1.1. Skeletal Myoblasts (SMs)
SMs is the first discovered cell source for cell sheets and is now attracting many researchers for the treatment of acute myocardial infarction (AMI). In basic research, SM has been applied following MI in various animal models such as rats, hamsters, dogs, pigs, etc. [4]. SMs have an advantage as they can be autologously sourced, thereby reducing concerns of eliciting an immune response and they possess ischemic resistance, the ability to differentiate into non-myocyte lines, and high proliferative potential. However, myoblasts do not express myocardium-specific contractile proteins or connexin 43 when transplanted into the heart, causing them to be electrically isolated and beat asynchronously with the recipient’s heart. SMs cannot form a gap junction with CMs, which can cause arrhythmias. Myoblasts are generally known to compensate for damaged skeletal muscle by proliferating and differentiating. When myoblasts are implanted as part of cardiac tissue engineering strategies, part of their therapeutic benefit may be due to paracrine signaling due to their production and release of growth factors and cytokines. Regarding the release of growth factors and cytokines, a study utilizing myoblast sheets implanted in the heart tissue in a chronic MI rat model hypothesized that various cytokines such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and stromal-derived factor-1 (SDF-1) were released from transplanted cells [5]. This hypothesis was largely due to the high expression of insulin growth factor (IGF), improved cardiac function, and the increased expression of HGF and VEGF in the recipient myocardium [6]. Furthermore, a high concentration of SDF-1, a chemokine for bone marrow-derived cells, as well as integration of some stem cell-derived factors such as c-Kit and Sca-1 positive cells were reported. Uchiha et al. reported that laminin α 2 secretory fibroblasts enhance the therapeutic effect of skeletal myoblast sheets by inhibiting the detachment of transplanted myoblasts from transplanted myocardium [7]. For this, it was suggested that myoblast sheet transplantation may improve cardiac function self-repair by inducing stem cells with growth factors and chemokines. On another note, previous non-clinical studies have shown that transplanted myoblasts cannot be detected histologically six months after transplantation. When transplanted myoblast sheets fall off in the late stages after transplantation, their function is maintained and the myoblasts express the hypoxia-inducible factor-1 (HIF-1) gene at a high rate [8]. HIF-1 is responsible for angiogenesis at the transplant site as well as the induction and recruitment of bone marrow mesenchymal stem cells (MSCs) [9][10]. Although clinical trials utilizing cell sheets of skeletal myoblast have demonstrated safety and feasibility before clinical use one should consider improving the current SM-derived cell sheet by combining it with synchronous contraction devices to avoid the unknown effect of myoblast sheets on advanced heart failure and the potential for fibrosis.
1.2. MSCs
MSCs’ immunomodulatory abilities and growth factor secretion capabilities show promise in the field of tissue engineering, leading to many clinical trials for their use in the extensive treatment of ischemic heart disease [11]. A few studies have already suspended bone marrow MSCs on 3D hydrogels to test MI in rat models. After 8 weeks, the autologous MSCs significantly improved LV function, promoted angiogenesis at the periphery of the infarction, and reduced infarction volume as well as suppressed apoptosis of host cardiomyocytes 4 weeks after transplantation [12]. Transplanted MSC sheets using cells obtained from bone tissue, adipose tissue, and menstrual blood also showed some myocardial formation and dramatic paracrine effects that contribute to angiogenesis, cardioprotection, improved LV function, and myocardial repair (Figure 1). MSCs can be applied not only in heart sheets but also in regenerative blood vessels, for the cells contribute to the relaxation of the immune response of tissue-engineered vascular grafts (TEVGs) [13]. These applications confirm the safety of MSC and its partial mechanism of action.
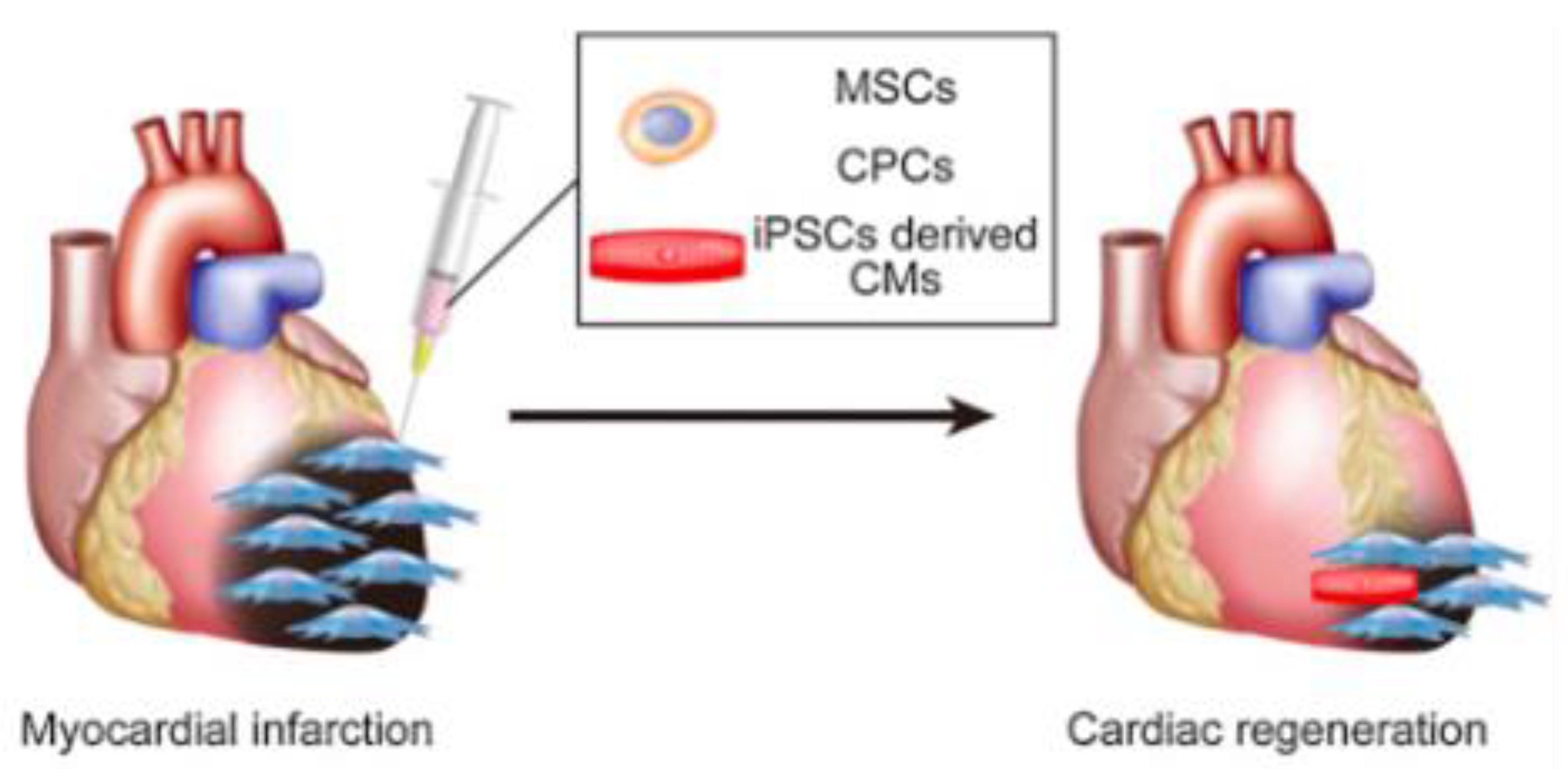
Figure 1. MSCs and CMs can be used to regenerate the myocardium [8].
1.3. Cardiomyocytes (CMs) (Fetal Myocardium and iPSCs)
The myocardium is similar to a skeletal muscle but is instead made up of many CMs, where each CM is electrically connected to adjacent CMs by gap junctions allowing for synchronization of contraction and relaxation. CMs, like skeletal muscle, are difficult to differentiate and mass-culture because CMs in healthy adults are rarely available. Several research teams have reported that cell sheets derived from fetal or neonatal CMs can be used to treat MI in animals. Pioneering studies, using fetal CMs from 15-day-old mouse embryos have demonstrated that donor cells survive after transplantation and administration to the heart [1]. While this approach holds promise, the use of fetal or neonatal CMs in humans would present a complex ethical dilemma.
In 1981, the discovery of pluripotent stem cells (PSCs) using mouse ESCs led to the creation of myocardium using PSCs with the characteristics of infinite proliferation and highly efficient CM differentiation [14]. ESCs and PSCs are both created by extracting the internal cell mass of an early embryo, and the products have the versatility to differentiate into all cells in the living body; a quality very advantageous as cell sources for the cell sheets [15]. A cell sheet using ESC-derived CMs was developed, where a new cardiovascular cell differentiation system can be established from mouse ESCs, to collect CMs and vascular cells in vitro [16]. However, the engraftment efficiency of the ESC-derived (CTS) was considerably lower 4 weeks after transplantation, requiring further studies to investigate another cell source for better cell survival and myocardial regeneration. Compared to the ESCs, iPSCs that Yamanaka et al. developed can be generated from a patient’s somatic cells without ethical issues, and therefore have been studied for the treatment of MI [17].
It is also worth noting how electrical stimulation or excitation-contraction coupling can affect the amount and duration of action potentials in CMs, thus increasing the number of synchronous contractions. Ruan-Circ et al. conducted a study with collagen-based, bioengineered tissue created with CMs derived from hiPSCs; electrical pacing combined with static stress conditioning led to a ~1.34 mN/mm2 increase in force production, suggesting maturation [18]. Electrical stimulation can trigger several transcription factors such as NRF-1, GATA4, NFAT3, and cytochrome c, leading to increased growth and maturation in CMs [19]. However, electrical stimulation could lead to arrhythmias due to the changes in electrical coupling [20]. Moreover, most biomaterials used in 3D constructs are still not synchronized, requiring new materials such as graphene to be added [21][22][23].
1.4. Supporting Cells: Vascular Endothelial Cells, Fibroblasts, and SMCs
Blood vessels have a major role in the heart, transporting nutrients and oxygen using the flow of blood. Blood flow is essential in regenerative medicine due to the large size of myocardial tissue, which requires a functional vascular network within. Stevens et al. and others have shown that a cohesive heart patch composed of CMs, ECs, and fibroblasts derived from human ESCs develops vascular structures that are essential for successful transplantation in vivo. Arai et al. also used a combination of iCell, ECs, and fibroblasts to form a cardiac spheroid of interest. Based on this idea, Arai et al. hope that blood flow can be ensured by mixing human umbilical vascular ECs when creating structures based on iPSC-derived CMs [24]. Recently, cardiac fibroblasts have been successfully distinguished from human pluripotent stem cells(hPSCs); cells similar to natural cardiac fibroblasts in morphology, gene expression, and proliferation. hPSC-derived cardiac fibroblast cocultured with hPSC-CM would increase the rate of the action potential propagation compared to coculture with skin fibroblasts [23][25]. Optimal combinations/ratios of cell types for reproducing complex 3D environments of native heart tissue continue to be an active area of research. There is no doubt that both fibroblasts play an important role in scaffolds to create a large structure such as that of myocardial tissue. SMCs secrete several angiogenic factors, including basic fibroblast growth factors (bFGF), VEGFs, and HGFs [26], and a paracrine angiogenic effect is expected. SMC cells can also induce differentiation from iPSC, and various methods have been reported [27].
2. How to Create Sheets
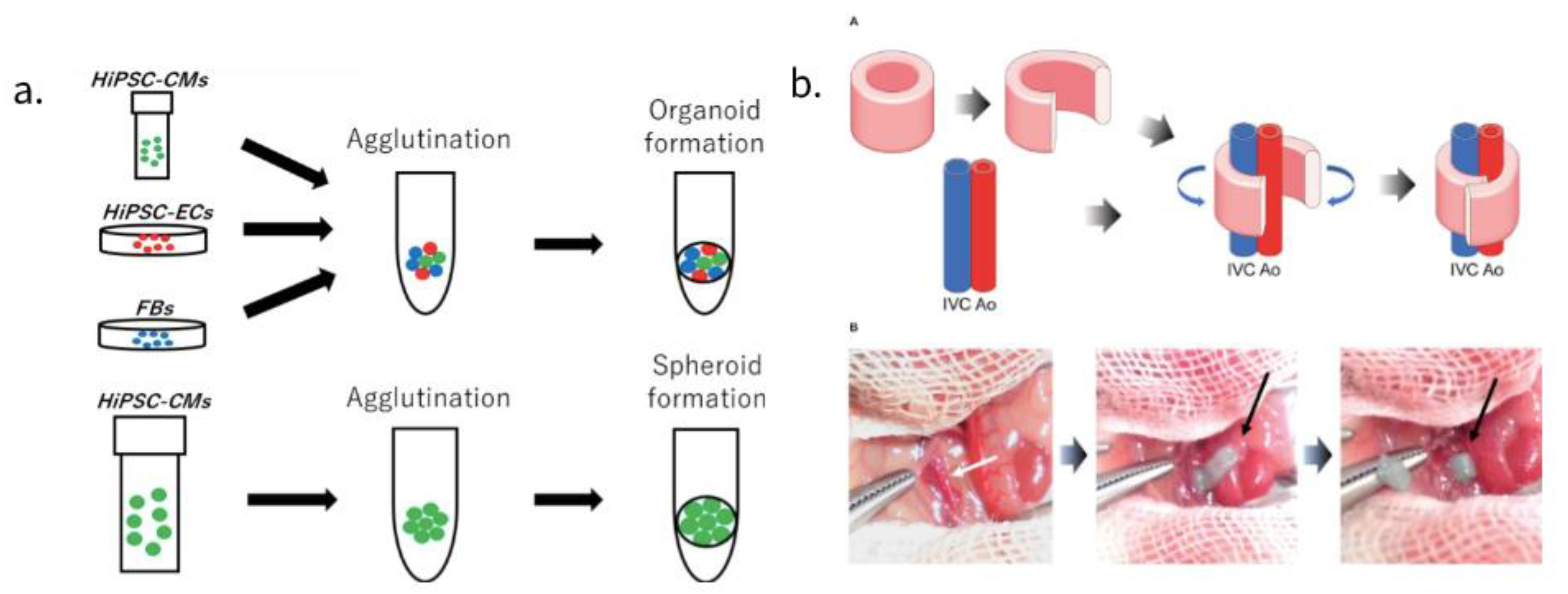
Advances in stem cell biology have made it possible to use various cells such as adult stem cells and iPSCs, and advances in bio-3D printing have made it possible to create large tissue structures for transplantation. An approach utilizing scaffolds, a method of seeding a vesicle into a three-dimensional and porous scaffold using a biodegradable polymer material involves mixing and pouring cells and gel-like scaffolds (e.g., collagen) into a mold, then arranging the cells using a 3D printer and a small amount of gel. Another method, bioprinting, has been pursued as well where fiber-like cell-containing gels are bundled together. Spheroids and organoids-clumps of cells have also attracted attention. By seeding these cell masses in metal needles or metal devices, organizing then extracting them, one can create a three-dimensional structure that does not contain scaffolds (Figure 2a).
2.1. Cell Sheet Approach to Producing hCMP
Most clinical studies use sheets for their approach; for example, the cell sheet method developed by Professor Okano of Tokyo Women’s Medical University consists of synthesizing myocardial tissues with a pulsating ability, without utilizing cell supports [28]. The cell sheets are created using a special culture dish coated with poly N-isopropyl acrylamide. To create sheets with sheet-like cells, one must generate and transplant tissues by stacking each monolayer. Furthermore, a culture dish, with the ability to control cell desorption by changing the hydrophilicity according to surface temperature, would be used for the recovery of the cell sheet. At a normal culture temperature of 37 °C., it becomes a hydrophobic surface, and in this state, cell adhesion factors such as fibronectin adhere to the surface of the normal culture dish. When the temperature drop treatment (32 °C or lower) is performed, the surface of the instrument becomes hydrophilic, and the cells desorb from the surface of the culture dish together with membrane proteins.
Because the cell sheet lacks exogenous/synthetic scaffolding, there are no concerns about the potential immunogenicity of the scaffold material. A major advantage of creating tissues this way is that they maintain adhesion proteins on the surface of the cell tissue body, so when transplanted into living organs, they have an admirable integration function with the transplanted organ. Furthermore, cells in adjacent layers can form connections between the layers that facilitate communication, including gap connections necessary for electrical bonding.
2.2. 3D Printing; Spheroids, Contractile Forces, and Tubular EHTs
Early research used tissue printing techniques, where the printed constructs worked well with the cardiac system and demonstrated high survival rates after 7 days of culture [29]. However, these scaffolding-based tissue engineering approaches also have some drawbacks; collagen and other biological molecules deteriorate the scaffolds rapidly, showing that further research is needed for appropriate tissue engineering and/or cell delivery techniques. 3D printing stacks up the previously mentioned spheroids, a method that leads to a higher function expression compared to two-dimensional cultures. Spheroids also promote the differentiation of the myocardium, promoting maturation [30]. Some of these spheroids use CMs, due to the advancement in IPS CM purification methods that can create cells with 99% purity [31]. In one study, transplanted spheroids composed of CPCs, expressing ISL1-LIM-homeodomain transcription factors differentiated into CM and EC, contribute to the formation of new blood vessels in the heart of infarcted mice [32]. On a new note, bioprinting techniques are gaining traction because they use CAD modeling to create large organs with controllable, native-like substances such as living cells and other biological materials [33]. Bioprinted hCMP without scaffolding is produced by loading spheroids one by one into an array of needles, fusing them, then removing the hCMP and culturing it until the holes in the needle are filled with the surrounding tissue.
Breckwoldt et al. developed hCMPs by differentiating human ESCs into hiPSC-CMs, a design that allows one to analyze human heart diseases by checking the EHT force strength [34].
A system for evaluating the 3D contractile force of the heart’s structure is important for new drug development. Although some research groups have already reported scaffold-based heart constructs, these scaffolding-based heart structures cannot accurately predict the heart’s drug response in vivo due to the interaction between the drug and scaffolding material [34]. Heart structures without scaffolding, such as patches and spheroids, have been manufactured but have not been reported to accurately assess the contractile force of the 3D heart structure. Although several studies have reported that contractile forces could be measured with the use of myocardial structures such as organoids, the contraction evaluation of said studies cannot be accepted because the shrinkage of the structure is very small and the reproducibility is poor [35]. Therefore, after creating a scaffolded three-dimensional (3D) tubular heart structure with a bio-3D printer, Arai et al. established an analysis system that can measure changes in needle tip movement as an index of the contractile forces.
Another study without the use of a scaffold consists of a tubular EHT (T-EHT), created using bio-3D printers with hiPSC-CO and needle arrays, which produced beating conduits for patients suffering from monoventricular disease. Researchers created a hiPSC-derived cardiac organoid composed of hiPSC-derived CMs, human cord vein endothelial cells, and human fibroblasts [36]. A bio-3D-printed T-EHT has been implanted around the abdominal aorta as well as the inferior vena cava (IVC) of NOG mice. (Figure 2b) Muscle stripes were observed with MLC2a, MLC2v, and α-actinin staining of tissue, all of which are indicators of maturation of myocardial tissue. After the T-EHT, which had been in vivo for 4 weeks, was removed and cultured, researchers observed it to have a spontaneous beat. Although the cultured T-EHT demonstrated an increased beating rate when researchers applied a bipolar electrical pulse, abundant evidence had not been provided to prove whether the T-EHT supported blood flow. Although a few of such non-scaffold myocardial structures have been reported, further advances in research are awaited [37].
References
- Wang, L.; Serpooshan, V.; Zhang, J. Engineering Human Cardiac Muscle Patch Constructs for Prevention of Post-infarction LV Remodeling. Front. Cardiovasc. Med. 2021, 8, 621781.
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409.
- Tadevosyan, K.; Iglesias-García, O.; Mazo, M.M.; Prósper, F.; Raya, A. Engineering and Assessing Cardiac Tissue Complexity. Int. J. Mol. Sci. 2021, 22, 1479.
- Guo, R.; Morimatsu, M.; Feng, T.; Lan, F.; Chang, D.; Wan, F.; Ling, Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 2020, 11, 19.
- Shudo, Y.; Miyagawa, S.; Ohkura, H.; Fukushima, S.; Saito, A.; Shiozaki, M.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T.; et al. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng. Part A 2014, 20, 728–739.
- Augustin, M.; Mahar, M.A.A.; Lakkisto, P.; Tikkanen, I.; Vento, A.; Patila, T.; Harjula, A. Heat shock attenuates VEGF expression in three-dimensional myoblast sheets deteriorating therapeutic efficacy in heart failure. Med. Sci. Monit. 2011, 17, 345–353.
- Uchinaka, A.; Tasaka, K.; Mizuno, Y.; Maeno, Y.; Ban, T.; Mori, S.; Hamada, Y.; Miyagawa, S.; Saito, A.; Sawa, A.; et al. Laminin α2-secreting fibroblasts enhance the therapeutic effect of skeletal myoblast sheets. Eur. J. Cardio-Thorac. Surg. 2017, 51, 457–464.
- Uchinaka, A.; Kawaguchi, N.; Hamada, Y.; Mori, S.; Miyagawa, S.; Saito, A.; Sawa, Y.; Matsuura, N. Transplantation of myoblast sheets that secrete the novel peptide SVVYGLR improves cardiac function in failing hearts. Cardiovasc. Res. 2013, 99, 102–110.
- Chen, L.; Endler, A.; Shibasaki, F. Hypoxia and angiogenesis: Regulation of hypoxia-inducible factors via novel binding factors. Exp. Mol. Med. 2009, 41, 849–857.
- Lv, B.; Hua, T.; Li, F.; Han, J.; Fang, J.; Xu, L.; Sun, C.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia-inducible factor 1 α protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am. J. Transl. Res. 2017, 9, 2492–2499.
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065.
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203.
- Kitsuka, T.; Hama, R.; Ulziibayar, A.; Matsuzaki, Y.; Kelly, J.; Shinoka, T. Clinical Application for Tissue Engineering Focused on Materials. Biomedicines 2022, 10, 1439.
- Romito, A.; Cobellis, G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2016, 2016, 9451492.
- Ciuffi, S.; Zonefrati, R.; Brandi, M.L. Adipose stem cells for bone tissue repair. Clin. Cases Miner. Bone Metab. 2017, 14, 217–226.
- Yamashita, J.K.; Takano, M.; Hiraoka-Kanie, M.; Shimazu, C.; Peishi, Y.; Yanagi, K.; Nakano, A.; Inoue, E.; Kita, F.; Nishikawa, S. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005, 19, 1534–1536.
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130.
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567.
- Bikkina, M.; Larson, M.G.; Levy, D. Asymptomatic ventricular arrhythmias and mortality risk in subjects with left ventricular hypertrophy. J. Am. Coll. Cardiol. 1993, 22, 1111–1116.
- Curran, M.E.; Splawski, I.; Timothy, K.W.; Vincent, G.M.; Green, E.D.; Keating, M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 1995, 80, 795–803.
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725.
- Zhou, J.; Chen, J.; Sun, H.; Qiu, X.; Mou, Y.; Liu, Z.; Zhao, Y.; Li, X.; Han, Y.; Duan, C.; et al. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep. 2014, 4, 3733.
- You, J.O.; Rafat, M.; Ye, G.J.; Auguste, D.T. Nanoengineering the heart: Conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011, 11, 3643–3648.
- Sharma, P.; Gentile, C. Cardiac Spheroids as in vitro Bioengineered Heart Tissues to Study Human Heart Pathophysiology. J. Vis. Exp. 2021, e61962.
- Zhang, J.; Tao, R.; Campbell, K.F.; Carvalho, J.L.; Ruiz, E.C.; Kim, G.C.; Schmuck, E.G.; Raval, A.N.; da Rocha, A.M.; Herron, T.J.; et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 2019, 10, 2238.
- Ali, S.; Becker, M.W.; Davis, M.G.; Dorn, G.W., 2nd. Dissociation of vasoconstrictor-stimulated basic fibroblast growth factor expression from hypertrophic growth in cultured vascular smooth muscle cells. Relevant roles Protein of protein kinase C. Circ. Res. 1994, 75, 836–843.
- Ji, H.; Kim, H.S.; Kim, H.W.; Leong, K.W. Application of induced pluripotent stem cells to model smooth muscle cell function in vascular diseases. Curr. Opin. Biomed. Eng. 2017, 1, 38–44.
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng. 2001, 7, 141–151.
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 11, 7948–7969.
- Kitsuka, T.; Itoh, M.; Amamoto, S.; Arai, K.I.; Oyama, J.; Node, K.; Toda, S.; Morita, S.; Nishida, T.; Nakayama, K. 2-Cl-C.OXT-A stimulates contractio n through the suppression of phosphodiesterase activity in human induced pluripotent stem cell-derived cardiac organoids. PLoS ONE 2019, 14, e0213114.
- Ban, K.; Bae, S.; Yoon, Y.S. Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Theranostics 2017, 7, 2067–2077.
- Steinhauser, M.L.; Lee, R.T. Regeneration of the heart. EMBO Mol. Med. 2011, 3, 701–712.
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536.
- Breckwoldt, K.; Letuffe-Brenière, D.; Mannhardt, I.; Schulze, T.; Ulmer, B.; Werner, T.; Benzin, A.; Klampe, B.; Reinsch, M.C.; Laufer, S.; et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017, 12, 1177–1197.
- Sakaguchi, K.; Takahashi, H.; Tobe, Y.; Sasaki, D.; Matsuura, K.; Iwasaki, K.; Shimizu, T.; Umezu, M. Measuring the Contractile Force of Multilayered Human Cardiac Cell Sheets. Tissue Eng. Part C Methods 2020, 26, 485–492.
- Arai, K.; Murata, D.; Takao, S.; Nakamura, A.; Itoh, M.; Kitsuka, T.; Nakayama, K. Drug response analysis for scaffold-free cardiac constructs fabricated using bio-3D printer. Sci. Rep. 2020, 10, 8972.
- Kawai, Y.; Tohyama, S.; Arai, K.; Tamura, T.; Soma, Y.; Fukuda, K.; Shimizu, H.; Nakayama, K.; Kobayashi, E. Scaffold-Free Tubular Engineered Heart Tissue from Human Induced Pluripotent Stem Cells Using Bio-3D Printing Technology in vivo. Front. Cardiovasc. Med. 2021, 8, 806215.
- Kitsuka, T.; Shiraki, A.; Oyama, J.I.; Nakagami, H.; Tanaka, A.; Node, K. A novel soluble epoxide hydrolase vaccine protects murine cardiac muscle against myocardial infarction. Sci. Rep. 2022, 12, 6923.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
01 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No