Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yulin Ren | -- | 2264 | 2022-11-30 01:14:35 | | | |
| 2 | Conner Chen | Meta information modification | 2264 | 2022-11-30 10:32:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Antioxidant and Potential Antitumor Activity of Aronia Berries. Encyclopedia. Available online: https://encyclopedia.pub/entry/37178 (accessed on 28 February 2026).
Ren Y, Frank T, Meyer G, Lei J, Grebenc JR, Slaughter R, et al. Antioxidant and Potential Antitumor Activity of Aronia Berries. Encyclopedia. Available at: https://encyclopedia.pub/entry/37178. Accessed February 28, 2026.
Ren, Yulin, Tyler Frank, Gunnar Meyer, Jizhou Lei, Jessica R. Grebenc, Ryan Slaughter, Yu G. Gao, A. Douglas Kinghorn. "Antioxidant and Potential Antitumor Activity of Aronia Berries" Encyclopedia, https://encyclopedia.pub/entry/37178 (accessed February 28, 2026).
Ren, Y., Frank, T., Meyer, G., Lei, J., Grebenc, J.R., Slaughter, R., Gao, Y.G., & Kinghorn, A.D. (2022, November 30). Antioxidant and Potential Antitumor Activity of Aronia Berries. In Encyclopedia. https://encyclopedia.pub/entry/37178
Ren, Yulin, et al. "Antioxidant and Potential Antitumor Activity of Aronia Berries." Encyclopedia. Web. 30 November, 2022.
Copy Citation
Aronia berry (black chokeberry) is a shrub native to North America, of which the fresh fruits are used in the food industry to produce different types of dietary products. The fruits of Aronia melanocarpa (Aronia berries) have been found to show multiple bioactivities potentially beneficial to human health, including antidiabetic, anti-infective, antineoplastic, antiobesity, and antioxidant activities, as well as heart-, liver-, and neuroprotective effects.
antioxidants
anti-infectives
antitumor effects
human health
1. Introduction
Aronia berry or black chokeberry, Aronia melanocarpa (Michx.) Elliott (Rosaceae), is a shrub native to North America, of which the fresh fruits are not typically consumed directly, owing to their bitter taste. However, these berries are used in the food and beverage industry for producing juices, syrups, jams, fruit teas and wine, and are also utilized in dietary supplements [1][2]. Phytochemical investigations have shown that the fruits of Aronia melanocarpa (Aronia berries) are an abundant source of phenolic compounds, including procyanidins, anthocyanins, phenolic acids, and their analogues [2][3]. These berries also produce several novel compounds containing a fused flavanol-coumarin-phenol unit and showing hydroxyl radical scavenging and quinone reductase-inducing activities [4]. The content of the major bioactive components of Aronia berries is relatively high, from 10 mg to 5500 mg per 100 g of the dried fruits, including procyanidins, cyanidin-3-O-galactoside, chlorogenic acid, and quercetin (Figure 1). Of these, the content of procyanidins may be greater than 5% of the dried fruits. The high concentrations of these bioactive compounds not only contribute to the biological effects observed for Aronia fruits but also present the potential for the discovery of useful therapeutic agents from this plant part [1][2][3].
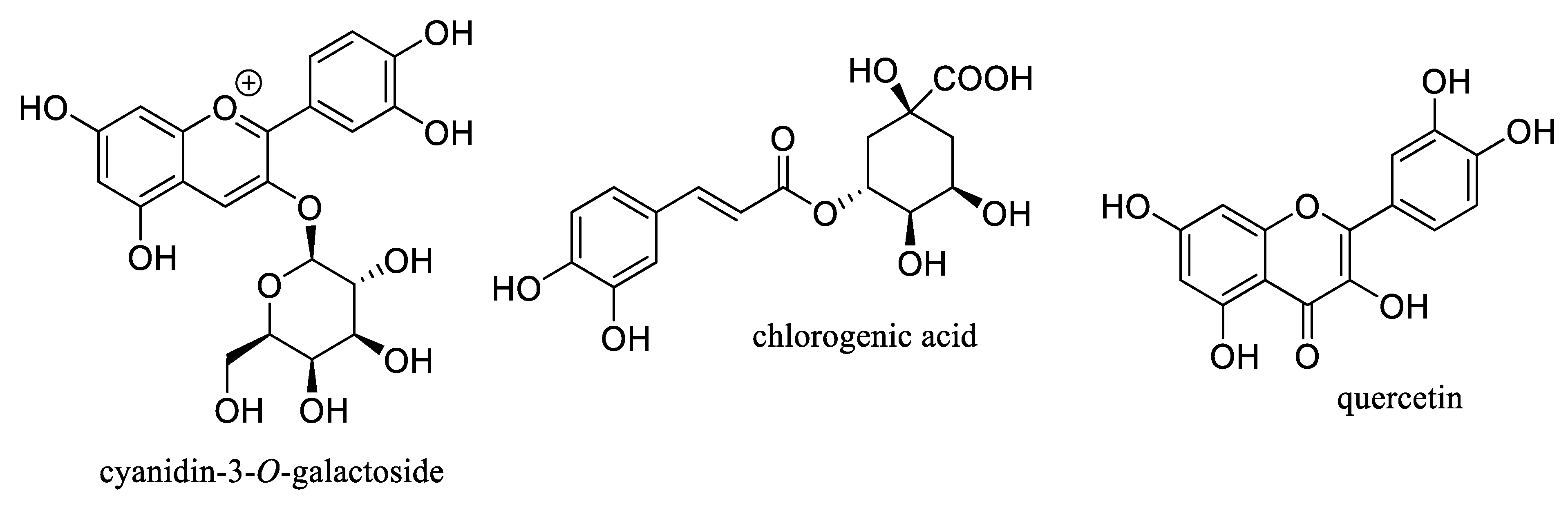
Figure 1. Structures of selected major phenolic compounds isolated from Aronia berries.
The phenolic compounds present potent antioxidative activities that contribute to the health-promoting activities of Aronia berries. These include antidiabetic, anti-infective, antimutagenic, and cytotoxic activities and cardio-, gastro-, hepato-, and radio-protective and immunomodulatory effects [5]. Thus, consumption of Aronia berries could be supportive of the prevention of some chronic diseases, including metabolic disorder [6], and may reduce the toxic effects of some xenobiotics on human health [7].
2. Antioxidant Activity of Aronia Berries
The antioxidant activity of Aronia berries and their major phenolic constituents has been well documented recently. These natural products have been found to inhibit the activity of several types of radicals through different mechanisms of action to contribute to other bioactivities [8][9]. The extracts and several phenolic compounds of Aronia berries showed radical scavenging activity when tested by a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, and they also inhibited 15-lipoxygenase and xanthine oxidase, which are peroxidative and prooxidative enzymes, respectively, the sources of reactive oxygen species (ROS) in vascular cells [8]. Previously, several small phenolic compounds isolated from Aronia berries in one research group were found to show potent antioxidant activity when tested in a hydroxyl radical-scavenging assay, and the catechol group in these compounds seems to play an important role in mediating such a property [10][11]. The antioxidant activity of Aronia berries has also been investigated in a clinical trial on 11 healthy human volunteer subjects who drank daily 250 mL of the juice of Aronia berries (Aronia juice) for three weeks. The serum antioxidant capacity of the participants was increased significantly when tested by a spectrophotometric method, using DPPH stable radical cations [12].
However, a subsequent clinical trial investigation indicated that consumption of Aronia berries did not change the biomarkers of oxidative stress and the total antioxidant activity measured in both plasma and urine of the participants when a 12-week, randomized, placebo-controlled trial was conducted on 49 healthy adult former smokers who consumed daily 500 mg of the ethanolic extract of Aronia berries [13]. Similarly, Aronia juice supplementation was not found to affect the parameters measured in the participants when the diet of 12 young male athletes was supplemented daily with 200 mL of Aronia juice (equivalent to 330.6 mg of anthocyanins) for seven weeks [14]. These results indicate that the antioxidant capacity of Aronia berries may not have been sufficient in these studies, and the dose of their phenolic constituents could be important in mediating the resultant activity. Thus, future clinical trials could focus on both optimizing the active dose and studying the toxic effects of Aronia berries [15].
Antioxidants are important in supporting human health, owing to their ability to inhibit free radicals that damage normal cells. Thus, antioxidant effects could be of value in alleviating other conditions that result from oxidative stress, including cancer, infection, heart disease, and diabetes. In this regard, the abundant phenolic compounds and other natural products in Aronia berries that exhibit potent antioxidant activity could be supportive in improving human health [8].
3. Potential Antitumor Activity of Aronia Berries
Oxidative stress is also found in various cancer cells, and antioxidants have been regarded as having potential value in cancer chemotherapy. Antioxidant extracts, constituents, or their semi-synthetic derivatives of Aronia berries have been well documented for their potential therapeutic effects on different cancer cells, including human breast, cervical, colon, glioblastoma, liver, and lung cancer and leukemia cells [1][2][3]. For example, the antioxidant activity of Aronia berries was found to be correlated with the total procyanidin and anthocyanin content, and the cyanidin glycosides present inhibited HeLa human cervical cancer cell proliferation and increased the generation of reactive oxygen species (ROS) in these cancer cells [16]. When tested in a further in vitro assay, the phenolic components present in Aronia berries were found to exhibit potent antioxidant activity and cytotoxicity toward HepG2 human liver cancer cells [17].
Aronia berries show promising activity toward human colon cancer cells. Commercially available extracts of the fruits of red [Aronia arbutifolia (L.) Pers.], purple [Aronia prunifolia (Marshall) Rehder], and black [Aronia melanocarpa (Michx.) Elliott] chokeberry species were tested for their total phenols and antioxidant activity and growth inhibitory activity against HT-29 human colon cancer cells. The results showed that only the extract of black chokeberry (Aronia berries) was active toward HT-29 cells, and this activity correlated with its total phenolic content, antioxidant activity, and levels of caffeic and chlorogenic acids [18]. This was supported by another study, which showed that the anthocyanin-enriched blackberry extract possessed antioxidant and anti-inflammatory activities and antiproliferative property against HT-29 cells [19]. Interestingly, the anthocyanin-rich extract of Aronia berries was selectively cytotoxic toward HT-29 cells but not human NCM460 normal colon cells [20]. Additionally, Caco-2 human colon cancer cell proliferation was inhibited when exposed to the Aronia juice [21].
Mechanistically, Aronia berries suppress HT-29 cell growth by dual blockage at the G1/G0 and G2/M phases of the cell cycle through upregulation of cyclin-dependent kinase inhibitors (CDKIs) and downregulation of cyclin A and cyclin B1 [20]. They inhibit Caco-2 cell growth by causing G2/M cell cycle arrest through upregulation of the tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) [21]. Anthocyanins present in a methanol extract of Aronia berries were found to inhibit inflammatory cytokines, the expression of solute carrier family 1 member 5 (SLC1A5), and the phosphorylation of mammalian target of rapamycin (mTOR) and its downstream targets in Caco-2 cells. Of these, SLC1A5 is an important amino acid carrier highly expressed in cancer cells to regulate cell proliferation and invasion. Hence, inhibition of this protein by an extract of Aronia berries implies some promise for the discovery of novel anticancer agents from these berries by targeting SLC1A5 [22]. In addition, anthocyanins of Aronia berries were found to inhibit Caco-2 cell growth through the Wnt/β-catenin signaling pathway [23].
Interestingly, the berry anthocyanidins exhibited selective cell growth inhibitory activity against A549 and H1299 human non-small-cell lung cancer (NSCLC) cells, and an equimolar combination of these compounds showed synergistic activity in cell proliferation, invasion, and migration. These types of activities have been proposed to result from their effects on the oncogenic Notch and Wnt signaling pathways and their downstream targets [24]. Cell migration is involved critically in angiogenesis and wound healing, for which GTPases, as hydrolase enzymes, bind to the nucleotide guanosine triphosphate (GTP) that controls cytoskeleton conformation and alters cell motility, in relation to the PI3K/Akt pathway [25]. Thus, berry-derived anthocyanidins could show some therapeutic potential for the targeted treatment of NSCLC and other cancers and for the prevention of cancer recurrence and metastasis [24][25][26].
It is well known that cancer stem cells (CSCs) are responsible for tumor initiation, development, metastasis, and resistance to radiotherapy and chemotherapy. Aronia juice was found to inhibit selectively the proliferation of P19 mouse embryonal carcinoma stem cells through upregulation of tumor suppressors p53 and p73 and downregulation of the antiapoptotic protein UHRF1 and the stemness factor Oct-4 [27]. This indicates that phenolic compounds of Aronia berries may inhibit the resistance of cancer cells to cancer therapies to potentiate the effectiveness of other anticancer agents. Thus, the cytotoxicity of gemcitabine against AsPC-1 human pancreatic cancer cells was found to be enhanced by the phenolic compounds present in Aronia berries [28].
It has been well demonstrated that the major phenolic compounds of Aronia berries show potential anticancer activity. Of these, the anticancer potential of cyanidin-3-O-galactoside has been well described, even though there are no clinical trial studies reported thus far. For example, the berry extracts containing cyanidin-3-O-galactoside as a predominant component were found to inhibit BGC-803 human gastric cancer cell growth through induction of cell apoptosis by various gene changes, including increases in Bax and Bak expression and decreases in Bcl-2 and Bcl-xl expression [29]. The small-molecule phenolic acid, chlorogenic acid, acts on p53 and related proteins, p38 mitogen-activated protein kinase (p38 MAPK), c-Jun amino-terminal kinase (JNK), c-Myc, ROS, and on other targets to inhibit the proliferation, migration, and invasion of cancer cells [30].
Liver and lung tumor growth was inhibited significantly when male NOD/SCID mice (18–22 g) were inoculated by human Huh7 hepatoma or H446 lung cancer cells and treated intraperitoneally (i.p.) with chlorogenic acid (25 mg/kg, daily) for 30 days after the tumors reached 100 mm3. No obvious toxicity was observed in mice even at a high dose (>200 mg/kg, i.p.) [31]. Mechanistically, chlorogenic acid inhibited hepatocellular carcinoma growth by down-regulating DNA methyltransferase 1 (DNMT1) that assists with DNA methylation, the most prevalent epigenetic modification [32]. Interestingly, chlorogenic acid was also found to show a synergistic effect with the anticancer drug, doxorubicin, against human U2OS and MG-63 osteosarcoma cells [33].
A phase I trial of chlorogenic acid in patients with advanced cancer with no effective treatment has been posted (NCT02136342, sponsor: Chinese Academy of Medical Sciences). This trial started on 13 May 2014 but terminated on 21 October 2014 (https://www.clinicaltrials.gov/ct2/show/NCT02136342?cond=chlorogenic+acid&draw=4&rank=6, accessed on 5 September 2022). Another phase I study concerning the tolerance and pharmacokinetics of an injection of chlorogenic acid for the treatment of advanced cancer started in September 2014 and was completed in October 2016 (NCT02245204, sponsor: Sichuan J.Z. Bio-chemical Science and Technology Development Co., Ltd., Chengdu, China) (https://www.clinicaltrials.gov/ct2/show/NCT02245204?cond=chlorogenic+acid&draw=2&rank=7, accessed on 5 September 2022). Following these, a trial on single arm, open-label, multicenter, phase Ib/IIa studies of chlorogenic acid for injection for safety and efficacy of advanced lung cancer patients was posted on 23 November 2018 (NCT03751592, sponsor: Sichuan J.Z. Bio-chemical Science and Technology Development Co., Ltd., Chengdu, China) (https://www.clinicaltrials.gov/ct2/show/NCT03751592?cond=chlorogenic+acid&draw=2&rank=9, accessed on 5 September 2022). These clinical trials indicate the anticancer potential of chlorogenic acid.
Quercetin has been documented in terms of its potential antitumor activity. It suppresses cancer cell growth through induction of apoptosis and autophagy by targeting the PI3K/Akt/mTOR, Wnt/β-catenin, and MAPK/ERK1/2 pathways, which are involved in tumor metabolism and mitochondrial function [34]. It also inhibits GLUT1-, 3-, and 4-mediated 2-deoxy-glucose transport to show potential anticancer activity. Thus, quercetin could be a promising compound lead for the development of anticancer agents by targeting tumor metabolites [35].
In addition, the pentacyclic triterpene ursolic acid that has been isolated from Aronia berries [36][37] (Figure 2) has shown potential anticancer activity through inhibiting NF-κB activation and other mechanisms involving angiogenesis and metastasis. A phase I study to assess the multiple-dose tolerability, efficacy, and pharmacokinetics of a liposomal form of ursolic acid indicated that this agent is safe and well-tolerated, and it showed some potential for improving patient remission rates [38][39]. Additionally, ursolic acid exhibited anti-inflammatory activity by targeting histamine, lipoxygenase, cyclooxygenase, phospholipase, nitric oxide, and ROS, with all of these found to play an important role in mediating potential antitumor activity of this triterpenoid [40][41].
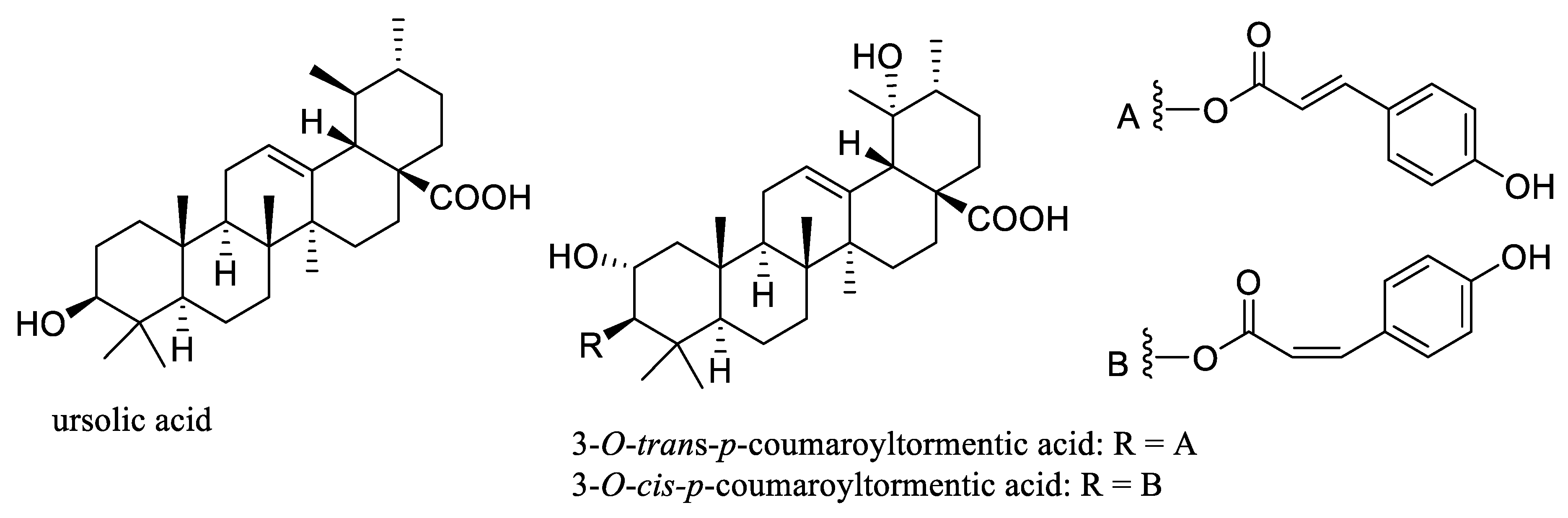
Figure 2. Structures of the triterpene ursolic acid and its derivatives, 3-O-trans- and 3-O-cis-p-coumaroyltormentic acids isolated from Aronia berries.
Recently, two ester derivatives of ursolic acid, namely, 3-O-trans- and -cis-p-coumaroyltormentic acids (Figure 2), were identified as the active compounds of an ethyl acetate-soluble extract of Aronia berries by an activity-guided isolation procedure [42]. These esters were found to inhibit MCF-7 and MDA-MB-231 human breast cancer cell proliferation and mammosphere formation through deregulation of the expression of c-Myc, a cancer stem cell survival factor. The results obtained indicate that these triterpene esters could exert inhibitory activity against breast cancer stem cells, and thus they may be promising leads for the development of new breast cancer chemotherapeutic agents via disruption of c-Myc protein [42].
Hence, the abundance of potent antioxidant phenolic compounds and triterpenoid constituents support the anticancer potential of Aronia berries, which could be mediated by some unusual mechanisms of action. As an example, the levels of oxidative/nitrative stress and hemostatic activity in plasma collected from breast cancer patients were reduced significantly when the blood samples were treated with a commercial extract of Aronia berries (50 μg/mL) in vitro. This indicates that Aronia berries may represent a promising antioxidant therapy or co-therapy for breast cancer patients [43]. Thus, Aronia berries and their constituents could be promising leads for the development of new anticancer agents, as indicated by the cancer clinical trial investigations for chlorogenic acid in particular, a major phenolic acid of Aronia berries, as discussed above.
References
- Sidor, A.; Drożdżyńska, A.; Gramza-Michalowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors–an overview. Trends Food Sci. Technol. 2019, 89, 45–60.
- Yang, S.-Q.; Wang, D.; Gao, Y.-X. Advances in studies on the function and application of Aronia melanocarpa. Food Res. Dev. 2021, 42, 206–213.
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antioxidants 2021, 10, 1052.
- Naman, C.B.; Li, J.; Moser, A.; Hendrycks, J.M.; Benatrehina, P.A.; Chai, H.; Yuan, C.; Keller, W.J.; Kinghorn, A.D. Computer-assisted structure elucidation of black chokeberry (Aronia melanocarpa) fruit juice isolates with a new fused pentacyclic flavonoid skeleton. Org. Lett. 2015, 17, 2988–2991.
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269.
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944.
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Safety 2016, 15, 982–1017.
- Sun, Z.-M.; Zhou, X.; Zhang, J.-L.; Li, T. Research progress of anthocyanin antioxidant function in Aronia melanocarpa. Food Res. Dev. 2017, 38, 220–224.
- Sidor, A.; Gramza-Michalowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710.
- Li, J.; Deng, Y.; Yuan, C.; Pan, L.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Antioxidant and quinone reductase-inducing constituents of black chokeberry (Aronia melanocarpa) fruits. J. Agric. Food Chem. 2012, 60, 11551–11559.
- Li, J.; Benatrehina, P.A.; Rague, A.L.; Pan, L.; Kinghorn, A.D.; Naman, C.B. Isolation and analysis of antioxidant phytochemicals from black chokeberry, maqui, and goji berry dietary supplements. In Advances in Plant Phenolics: From Chemistry to Human Health; ACS Symposium Series; American Chemical Society: Washington, WA, USA, 2018; Volume 1286, pp. 3–19.
- Nowak, D.; Grabczewska, Z.; Gośliński, M.; Obońska, K.; Dabrowska, A.; Kubica, J. Effect of chokeberry juice consumption on antioxidant capacity, lipids profile and endothelial function in healthy people: A pilot study. Czech J. Food Sci. 2016, 34, 39–46.
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.-Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77.
- Stankiewicz, B.; Cieślicka, M.; Kujawski, S.; Piskorska, E.; Kowalik, T.; Korycka, J.; Skarpańska-Stejnborn, A. Effects of antioxidant supplementation on oxidative stress balance in young footballer—A randomized double-blind trial. J. Int. Soc. Sports Nutr. 2021, 18, 44.
- Olas, B. Berry phenolic antioxidants–implications for human health? Front. Pharmacol. 2018, 9, 78.
- Ruginǎ, D.; Sconta, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J. Med. Food 2012, 15, 700–706.
- Gao, N.; Wang, Y.; Jiao, X.; Chou, S.; Li, E.; Li, B. Preparative purification of polyphenols from Aronia melanocarpa (chokeberry) with cellular antioxidant and antiproliferative activity. Molecules 2018, 23, 139.
- Gill, N.K.; Rios, D.; Osorio-Camacena, E.; Mojica, B.E.; Kaur, B.; Soderstrom, M.A.; Gonzalez, M.; Plaat, B.; Poblete, C.; Kaur, N.; et al. Anticancer effects of extracts from three different chokeberry species. Nutr. Cancer 2021, 73, 1168–1174.
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J Med. Food 2007, 10, 258–265.
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196.
- Bermứdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271.
- Yu, W.; Gao, J.; Hao, R.; Zhang, C.; Liu, H.; Fan, J.; Wei, J. Aronia melanocarpa Elliot anthocyanins inhibit colon cancer by regulating glutamine metabolism. Food Biosci. 2021, 40, 100910.
- Wei, J.; Yu, W.; Hao, R.; Fan, J.; Gao, J. Anthocyanins from Aronia melanocarpa induce apoptosis in Caco-2 cells through Wnt/β-catenin signaling pathway. Chem. Biodiversity 2020, 17, e2000654.
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62.
- Tsakiroglou, P.; Vandenakker, N.E.; Del Bo’, C.; Riso, P.; Klimis-Zacas, D. Role of berry anthocyanins and phenolic acids on cell migration and angiogenesis: An updated overview. Nutrients 2019, 11, 1075.
- Shi, N.; Chen, X.; Chen, T. Anthocyanins in colorectal cancer prevention review. Antioxidants 2021, 10, 1600.
- Sharif, T.; Stambouli, M.; Burrus, B.; Emhemmed, F.; Dandache, I.; Auger, C.; Etienne-Selloum, N.; Schini-Kerth, V.B.; Fuhrmann, G. The polyphenolic-rich Aronia melanocarpa juice kills teratocarcinomal cancer stem-like cells, but not their differentiated counterparts. J. Funct. Foods 2013, 5, 1244–1252.
- Abdullah Thani, N.A.; Keshavarz, S.; Lwaleed, B.A.; Cooper, A.J.; Rooprai, H.K. Cytotoxicity of gemcitabine enhanced by polyphenolics from Aronia melanocarpa in pancreatic cancer cell line AsPC-1. J. Clin. Pathol. 2014, 67, 949–954.
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-O-galactoside: A natural compound with multiple health benefits. Int. J. Mol. Sci. 2021, 22, 2261.
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911.
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763.
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Front. Pharmacol. 2020, 11, 867.
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic acid enhances doxorubicin-mediated cytotoxic effect in osteosarcoma cells. Int. J. Mol. Sci. 2021, 22, 8586.
- Reyes-Farias, M.; Carrasco-Pozo, C. The anticancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177.
- Shriwas, P.; Chen, X.; Kinghorn, A.D.; Ren, Y. Plant-derived glucose transport inhibitors with potential antitumor activity. Phytother. Res. 2020, 34, 1027–1040.
- Yoshida, T.; Maejima, K. Aronia extract for supporting an active life. Food Style 21 2014, 18, 67–69.
- Makanae, Y.; Ato, S.; Kido, K.; Fujita, S. Dietary Aronia melanocarpa extract enhances mTORC1 signaling, but has no effect on protein synthesis and protein breakdown-related signaling, in response to resistance exercise in rat skeletal muscle. J. Int. Soc. Sports Nutr. 2019, 16, 60.
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019, 85, 802–814.
- Shaikh, N.; Sawant, G.; Dixit, N. A review on ursolic acid: A naturally obtained pentacyclic triterpene. Int. J. Pharm. Pharm. Sci. 2021, 13, 1–5.
- Luan, M.; Wang, H.; Wang, J.; Zhang, X.; Zhao, F.; Liu, Z.; Meng, Q. Advances in anti-inflammatory activity, mechanism and therapeutic application of ursolic acid. Mini Rev. Med. Chem. 2022, 22, 422–436.
- Mioc, M.; Milan, A.; Malita, D.; Mioc, A.; Prodea, A.; Racoviceanu, R.; Ghiulai, R.; Cristea, A.; Căruntu, F.; Soica, C. Recent advances regarding the molecular mechanisms of triterpenic acids: A review (part I). Int. J. Mol. Sci. 2022, 23, 7740.
- Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Deng, H.-Y.; Yun, B.-S.; Lee, D.-S. Triterpene acid (3-O-p-coumaroyltormentic acid) isolated from Aronia extracts inhibits breast cancer stem cell formation through downregulation of c-Myc protein. Int. J. Mol. Sci. 2018, 19, 2528.
- Kedzierska, M.; Malinowska, J.; Kontek, B.; Kolodziejczyk-Czepas, J.; Czernek, U.; Potemski, P.; Piekarski, J.; Jeziorski, A.; Olas, B. Chemotherapy modulates the biological activity of breast cancer patients plasma: The protective properties of black chokeberry extract. Food Chem. Toxicol 2013, 53, 126–132.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
2 times
(View History)
Update Date:
30 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





