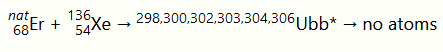Video Upload Options
Unbibium /uːnˈbɪbiəm/, also known as eka-thorium or simply element 122, is the currently hypothetical chemical element in the periodic table with the placeholder symbol of Ubb and atomic number 122. Unbibium and Ubb are the temporary systematic IUPAC name and symbol respectively, until a permanent name is decided upon. In the periodic table of the elements, it is expected to follow unbiunium as the second element of the superactinides, or the g-block and the fourth element of the 8th period. It has attracted recent attention, for similarly to unbiunium, it is expected to fall within the range of the island of stability. Despite many attempts, unbibium has not yet been synthesized, and therefore no natural isotopes have been found to exist. It is currently predicted that it has a g-orbital, the second element of which to have such besides unbiunium, which also has yet to be synthesized. It will most likely require nuclear fission to be produced artificially. In 2008, it was claimed to have been discovered in natural thorium samples but that claim has now been dismissed by recent repetitions of the experiment using more accurate techniques.
1. History
1.1. Neutron Evaporation
The first attempt to synthesize unbibium was performed in 1972 by Flerov et al. at the Joint Institute for Nuclear Research (JINR), using the hot fusion reaction:[1]
No atoms were detected and a yield limit of 5 mb (5,000,000,000 pb) was measured. Current results (see flerovium) have shown that the sensitivity of this experiment was too low by at least 6 orders of magnitude.
In 2000, the Gesellschaft für Schwerionenforschung (GSI) Hemholtz Center for Heavy Ion Research performed a very similar experiment with much higher sensitivity:[1]
These results indicate that the synthesis of such heavier elements remains a significant challenge and further improvements of beam intensity and experimental efficiency is required. The sensitivity should be increased to 1 fb in the future for more quality results.
Another unsuccessful attempt to synthesize unbibium was carried out in 1978 at the GSI Helmholtz Center, where a natural erbium target was bombarded with xenon-136 ions:[1]
These two attempts in the 1970s to synthesize unbibium were both propelled by the research investigating whether superheavy elements could potentially be naturally occurring.[1]
1.2. Compound Nucleus Fission
Several experiments have been performed between 2000–2004 at the Flerov laboratory of Nuclear Reactions studying the fission characteristics of the compound nucleus 306Ubb. Two nuclear reactions have been used, namely 248Cm + 58Fe and 242Pu + 64Ni.[1] The results have revealed how nuclei such as this fission predominantly by expelling closed shell nuclei such as 132Sn (Z=50, N=82). It was also found that the yield for the fusion-fission pathway was similar between 48Ca and 58Fe projectiles, indicating a possible future use of 58Fe projectiles in superheavy element formation.[2]
1.3. Claimed Discovery as a Naturally Occurring Element
On April 24, 2008, a group led by Amnon Marinov at the Hebrew University of Jerusalem claimed to have found single atoms of unbibium-292 in naturally occurring thorium deposits at an abundance of between 10−11 and 10−12, relative to thorium.[3] The claim of Marinov et al. was criticized by a part of the scientific community, and Marinov says he has submitted the article to the journals Nature and Nature Physics but both turned it down without sending it for peer review.[4] The unbibium-292 atoms were claimed to be superdeformed or hyperdeformed isomers, with a half-life of at least 100 million years.[1]
A criticism of the technique, previously used in purportedly identifying lighter thorium isotopes by mass spectrometry,[5] was published in Physical Review C in 2008.[6] A rebuttal by the Marinov group was published in Physical Review C after the published comment.[7]
A repeat of the thorium-experiment using the superior method of Accelerator Mass Spectrometry (AMS) failed to confirm the results, despite a 100-fold better sensitivity.[8] This result throws considerable doubt on the results of the Marinov collaboration with regards to their claims of long-lived isotopes of thorium,[5] roentgenium[9] and unbibium.[3] It is still possible that traces of unbibium might only exist in some thorium samples, although this is very unlikely.[1]
2. Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, unbihexium should instead be known as eka-thorium or dvi-cerium. After the recommendations of the IUPAC in 1979, the element has since been largely referred to as unbibium with the atomic symbol of (Ubb),[10] as its temporary name until the element is officially discovered and synthesized, and a permanent name is decided on. Scientists largely ignore this naming convention, and instead simply refer to unbibium as "Element 122" with the symbol of (122), or sometimes even E122 or 122.[11]
3. Predicted Properties
3.1. Nuclear Stability and Isotopes
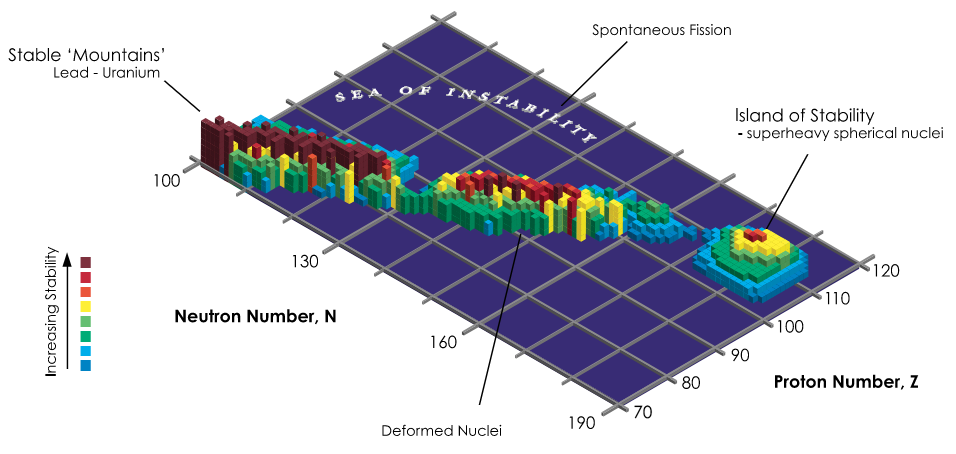
The stability of nuclei decreases greatly with the increase in atomic number after plutonium, the heaviest primordial element, so that all isotopes with an atomic number above 101 decay radioactively with a half-life under a day, with an exception of dubnium-268. No elements with atomic numbers above 82 (after lead) have stable isotopes.[12] Nevertheless, because of reasons not very well understood yet, there is a slight increased nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg, explains why superheavy elements last longer than predicted.[13] In this region of the periodic table, N=184 and N=196 have been suggested as closed neutron shells. Therefore the isotopes of most interest are 306Ubb and 318Ubb, for these might be considerably longer-lived than other isotopes. Element 122 is predicted to lie within the island of stability, with element 126 predicted to lie near its peak.[1]
3.2. Atomic and Physical
Unbibium is predicted to belong to a new block of valence g-electron atoms, although the g-block's position left of the f-block is speculative.[14] It has been predicted by Ephraim Eliav et al. that unbibium will have the electron configuration [Og] 8s27d18p1,[15] although there may be a sort of smearing of the energies within 5g, 6f, 7d and 8p orbitals.[14]
3.3. Chemical
If group reactivity is followed, unbibium should be a reactive metal, more reactive than cerium or thorium. Unbibium would most likely form the dioxide, UbbO2, and trihalides, such as UbbF3 and UbbCl3. The predicted oxidation states are III and IV (and perhaps II). Being a congener of thorium, it should have similar chemistry to thorium, with multiple oxidation states.[1]
References
- Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 588. ISBN 978-0-19-960563-7.
- see Flerov lab annual reports 2000–2004 inclusive http://www1.jinr.ru/Reports/Reports_eng_arh.html
- Marinov, A.; Rodushkin, I.; Kolb, D.; Pape, A.; Kashiv, Y.; Brandt, R.; Gentry, R. V.; Miller, H. W. (2008). "Evidence for a long-lived superheavy nucleus with atomic mass number A=292 and atomic number Z=~122 in natural Th". International Journal of Modern Physics E 19: 131. doi:10.1142/S0218301310014662. Bibcode: 2010IJMPE..19..131M. https://dx.doi.org/10.1142%2FS0218301310014662
- Royal Society of Chemistry, "Heaviest element claim criticised", Chemical World. http://rsc.org/chemistryworld/News/2008/May/02050802.asp
- Marinov, A.; Rodushkin, I.; Kashiv, Y.; Halicz, L.; Segal, I.; Pape, A.; Gentry, R. V.; Miller, H. W. et al. (2007). "Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes". Phys. Rev. C 76 (2): 021303(R). doi:10.1103/PhysRevC.76.021303. Bibcode: 2007PhRvC..76b1303M. https://dx.doi.org/10.1103%2FPhysRevC.76.021303
- R. C. Barber; J. R. De Laeter (2009). "Comment on "Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes"". Phys. Rev. C 79 (4): 049801. doi:10.1103/PhysRevC.79.049801. Bibcode: 2009PhRvC..79d9801B. https://dx.doi.org/10.1103%2FPhysRevC.79.049801
- A. Marinov; I. Rodushkin; Y. Kashiv; L. Halicz; I. Segal; A. Pape; R. V. Gentry; H. W. Miller; D. Kolb; R. Brandt (2009). "Reply to "Comment on 'Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes'"". Phys. Rev. C 79 (4): 049802. doi:10.1103/PhysRevC.79.049802. Bibcode: 2009PhRvC..79d9802M. https://dx.doi.org/10.1103%2FPhysRevC.79.049802
- J. Lachner; I. Dillmann; T. Faestermann; G. Korschinek; M. Poutivtsev; G. Rugel (2008). "Search for long-lived isomeric states in neutron-deficient thorium isotopes". Phys. Rev. C 78 (6): 064313. doi:10.1103/PhysRevC.78.064313. Bibcode: 2008PhRvC..78f4313L. https://dx.doi.org/10.1103%2FPhysRevC.78.064313
- Marinov, A.; Rodushkin, I.; Pape, A.; Kashiv, Y.; Kolb, D.; Brandt, R.; Gentry, R. V.; Miller, H. W. et al. (2009). "Existence of Long-Lived Isotopes of a Superheavy Element in Natural Au". International Journal of Modern Physics E (World Scientific Publishing Company) 18 (3): 621–629. doi:10.1142/S021830130901280X. Bibcode: 2009IJMPE..18..621M. http://www.phys.huji.ac.il/~marinov/publications/Au_paper_IJMPE_73.pdf. Retrieved February 12, 2012.
- Chatt, J. (1979). "Recommendations for the Naming of Elements of Atomic Numbers Greater than 100". Pure Appl. Chem. 51 (2): 381–384. doi:10.1351/pac197951020381. https://dx.doi.org/10.1351%2Fpac197951020381
- Haire, Richard G. (2006). "Transactinides and the future elements". in Morss; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. p. 1724. ISBN 1-4020-3555-1.
- Marcillac, Pierre de; Noël Coron; Gérard Dambier; Jacques Leblanc; Jean-Pierre Moalic (April 2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature 422 (6934): 876–878. doi:10.1038/nature01541. PMID 12712201. Bibcode: 2003Natur.422..876D. https://dx.doi.org/10.1038%2Fnature01541
- Considine, Glenn D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9 ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096. http://www.worldcat.org/oclc/223349096
- Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. http://www.britannica.com/EBchecked/topic/603220/transuranium-element. Retrieved 2010-03-16.
- Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". in Morss; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. p. 1659. ISBN 1-4020-3555-1.