
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beatrix Zheng | -- | 1821 | 2022-11-29 01:40:52 |
Video Upload Options
The artificial pancreas is a technology in development to help people with diabetes, primarily type 1, automatically and continuously control their blood glucose level by providing the substitute endocrine functionality of a healthy pancreas. The endocrine functionality of the pancreas is provided by islet cells which produce the hormones insulin and glucagon. Artificial pancreatic technology mimics the secretion of these hormones into the bloodstream in response to the body's changing blood glucose levels. Maintaining balanced blood sugar levels is crucial to the function of the brain, liver, and kidneys. Therefore, for type 1 patients, it is necessary that the levels be kept balanced when the body cannot produce insulin itself. The artificial pancreas is a broad term for different bio-engineering strategies currently in development to achieve these requirements. Different bio-engineering approaches under consideration include:
1. Approaches
1.1. Medical Equipment
The medical equipment approach involves combining a continuous glucose monitor and an implanted insulin pump that can function together with a computer-controlled algorithm to replace the normal function of the pancreas.[1][2][3] The development of continuous glucose monitors has led to the progress in artificial pancreas technology using this integrated system.[4]
Continuous glucose monitors
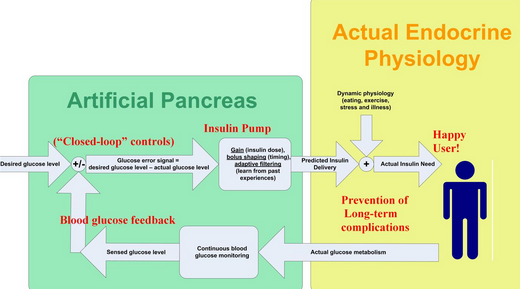
The original devices for use in type 1 diabetes were blood glucose meters. Continuous blood glucose monitors are one of the set of devices that make up an artificial pancreas device system, the other being an insulin pump, and a glucose meter to calibrate the device.[5] Continuous glucose monitors are a more recent breakthrough and have begun to hit the markets for patient use after approval from the FDA. Both the traditional and the continuous monitor require manual insulin delivery or carbohydrate intake depending on the readings from the devices. While the traditional blood glucose meters require the user to prick their finger every few hours to obtain data, continuous monitors use sensors placed just under the skin on the arm or abdomen to deliver blood sugar level data to receivers or smartphone apps as often as every few minutes.[6] The sensors can be used for up to fourteen days. A number of different continuous monitors are currently approved by the FDA.[7]
The first continuous glucose monitor (CGM) was approved in December 2016. Developed by Dexcom, the G5 Mobile Continuous Monitoring System requires users to prick their fingers twice a day (as opposed to the typical average 8 times daily with the traditional meters) in order to calibrate the sensors. The sensors last up to seven days. The device uses Bluetooth technology to warn the user either through a handheld receiver or app on a smartphone if blood glucose levels reach below a certain point. The cost for this device excluding any co-insurance is an estimated $4,800 a year.[7]
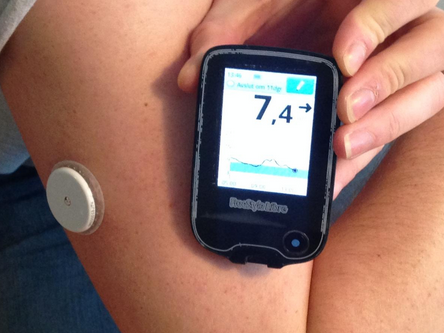
Abbott Laboratories' FreeStyle Libre CGM was approved in September 2017. Recently, the technology was modified to support smartphone use through the LibreLink app. This device does not require finger pricks at all and the sensor, placed on the upper arm, lasts 14 days. The estimated cost for this monitor is $1,300 a year.[7]
Dexcom's next G6 model CGM was approved in March 2018, which can last up to ten days and does not need finger prick calibration. Like Medtronic's monitor, it can predict glucose level trends. It is compatible for integration into insulin pumps.[7]
Closed-loop systems
Unlike the continuous sensor alone, the closed-loop system requires no user input in response to reading from the monitor; the monitor and insulin pump system automatically delivers the correct amount of hormone calculated from the readings transmitted. The system is what makes up the artificial pancreas device.[5][6]
Inreda Diabetic
In collaboration with the Academic Medical Centre (AMC) in Amsterdam, Inreda is developing a closed loop system with insulin and glucagon. The initiator, Robin Koops, started to develop the device in 2004 and ran the first tests on himself. After several highly successful trials it received the European EC license in 2016. The product is expected to market in the second half of 2020. A smaller improved version is scheduled for 2023.
MiniMed 670G
In September 2016, the FDA approved the Medtronic MiniMed 670G, which was the first approved hybrid closed loop system. The device senses a diabetic person's basal insulin requirement and automatically adjusts its delivery to the body.[8] It is made up of a continuous glucose monitor, an insulin pump, and a glucose meter for calibration. It automatically functions to modify the level of insulin delivery based off the detection of blood glucose levels by continuous monitor. It does this by sending the blood glucose data through an algorithm that analyzes and makes the subsequent adjustments.[8] The system has two modes. Manual mode lets the user choose the rate at which basal insulin is delivered. Auto mode regulates basal insulin levels from the continuous monitor's readings every five minutes.[9]
The device was originally available only to those aged 14 or older, and in June 2018 was approved by the FDA for use in children aged 7–14. Families have reported better sleep quality from use of the new system, as they do not have to worry about manually checking blood glucose levels during the night.[10] The full cost of the system is $3700, but patients have the opportunity to get it for less.[11]
Ilet Bionic Pancreas
A team at Boston University working in collaboration with Massachusetts General Hospital on a dual hormone artificial pancreas system [12] began clinical trials on their device called the Bionic Pancreas in 2008.[13] In 2016, the Public Benefit Corporation Beta Bionics was formed. In conjunction with the formation of the company, Beta Bionics changed the preliminary name for their device from the Bionic Pancreas to the iLet.[13] The device uses a closed-loop system to deliver both insulin and glucagon in response to sensed blood glucose levels. While not yet approved for public use, the 4th generation iLet prototype, presented in 2017, is around the size of an iPhone, with a touchscreen interface. It contains two chambers for both insulin and glucagon, and the device is configurable for use with only one hormone, or both.[14] While trials continue to be run, the iLet has a projected final approval for the insulin-only system in 2020.[13]
Current studies
Four studies on different artificial pancreas systems are being conducted starting in 2017 and going into the near future. The projects are funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and are the final part of testing the devices before applying for approval for use. Participants in the studies are able to live their lives at home while using the devices and being monitored remotely for safety, efficacy, and a number of other factors.[15]
The International Diabetes Closed-Loop trial,[16] led by researchers from the University of Virginia, is testing a closed-loop system called inControl, which has a smartphone user interface. 240 people of ages 14 and up are participating for 6 months.[15]
A full-year trial led by researchers from the University of Cambridge started in May 2017 and has enrolled an estimated 150 participants of ages 6 to 18 years.[15] The artificial pancreas system being studied uses a smartphone and has a low glucose feature to improve glucose level control.[17]
The International Diabetes Center in Minneapolis, Minnesota, in collaboration with Schneider Children's Medical Center in Petah Tikva, Israel, are planning a 6-month study that will begin in early 2019 and will involve 112 adolescents and young adults, ages 14 to 30.[15][18] The main object of the study is to compare the current Medtronic 670G system to a new Medtronic-developed system. The new system has programming that aims to improve glucose control around mealtime, which is still a big challenge in the field.[18]
The current 6-month study lead by the Bionic Pancreas team started in mid-2018 and enrolled 312 participants of ages 18 and above.[15]
1.2. Physiological
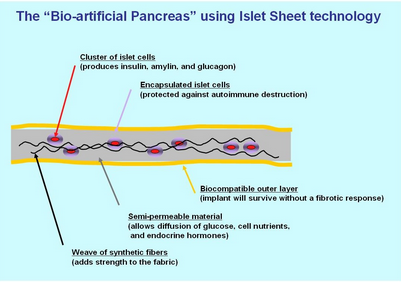
The biotechnical company Defymed, based in France, is developing an implantable bio-artificial device called MailPan which features a bio-compatible membrane with selective permeability to encapsulate different cell types, including pancreatic beta cells.[19] The implantation of the device does not require conjunctive immuno-suppressive therapy because the membrane prevents antibodies of the patient from entering the device and damaging the encapsulated cells. After being surgically implanted, the membrane sheet will be viable for years. The cells that the device holds can be produced from stem cells rather than human donors, and may also be replaced over time using input and output connections without surgery.[19][20] Defymed is partially funded by JDRF, formerly known as the Juvenile Diabetes Research Foundation, but is now defined as an organization for all ages and all stages of type 1 diabetes.[21][22]
In November 2018, it was announced that Defymed would partner with the Israel-based Kadimastem, a bio-pharmaceutical company developing stem-cell based regenerative therapies, to receive a two-year grant worth approximately $1.47 million for the development of a bio-artificial pancreas that would treat type 1 diabetes.[19][23] Kadimastem's stem cell technology uses differentiation of human embryonic stem cells to obtain pancreatic endocrine cells. These include insulin-producing beta cells, as well as alpha cells, which produce glucagon. Both cells arrange in islet-like clusters, mimicking the structure of the pancreas.[24] The aim of the partnership is to combine both technologies in a bio-artificial pancreas device, which releases insulin in response to blood glucose levels, to bring to clinical trial stages.[19]
The San Diego, California based biotech company ViaCyte has also developed a product aiming to provide a solution for type 1 diabetes which uses an encapsulation device made of a semi-permeable immune reaction-protective membrane. The device contains pancreatic progenitor cells that have been differentiated from embryonic stem cells.[25][26] After surgical implantation in an outpatient procedure, the cells mature into endocrine cells which arrange in islet-like clusters and mimic the function of the pancreas, producing insulin and glucagon.[27][28] The technology advanced from pre-clinical studies to FDA approval for phase 1 clinical trials in 2014, and presented two-year data from the trial in June 2018.[25][26] They reported that their product, called PEC-Encap, has so far been safe and well tolerated in patients at a dose below therapeutic levels. The encapsulated cells were able to survive and mature after implantation, and immune system rejection was decreased due to the protective membrane. The second phase of the trial will evaluate the efficacy of the product.[25] ViaCyte has also been receiving financial support from JDRF on this project.[28]
2. Initiatives Around the Globe
In the United States in 2006, JDRF (formerly the Juvenile Diabetes Research Foundation) launched a multi-year initiative to help accelerate the development, regulatory approval, and acceptance of continuous glucose monitoring and artificial pancreas technology.[29][30]
Grassroots efforts to create and commercialize a fully automated artificial pancreas system have also arisen directly from patient advocates and the diabetes community.[31] Bigfoot Biomedical, a company founded by parents of children with T1D have created algorithms and are developing a closed loop device that monitor blood sugar and appropriately provide insulin.[32]
References
- "The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes". Diabetes, Obesity & Metabolism 20 (2): 245–256. February 2018. doi:10.1111/dom.13052. PMID 28675686. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5810921
- "Pharmacological aspects of closed loop insulin delivery for type 1 diabetes". Current Opinion in Pharmacology 36: 29–33. October 2017. doi:10.1016/j.coph.2017.07.006. PMID 28802779. https://dx.doi.org/10.1016%2Fj.coph.2017.07.006
- "Moving Toward a Unified Platform for Insulin Delivery and Sensing of Inputs Relevant to an Artificial Pancreas". Journal of Diabetes Science and Technology 11 (2): 308–314. March 2017. doi:10.1177/1932296816682762. PMID 28264192. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5478040
- "[From insulin pump and continuous glucose monitoring to the artificial pancreas]". Revista Médica de Chile 145 (5): 630–640. May 2017. doi:10.4067/S0034-98872017000500011. PMID 28898340. https://dx.doi.org/10.4067%2FS0034-98872017000500011
- Health, Center for Devices and Radiological. "Artificial Pancreas Device System - What is the pancreas? What is an artificial pancreas device system?" (in en). https://www.fda.gov/medicaldevices/productsandmedicalprocedures/homehealthandconsumer/consumerproducts/artificialpancreas/ucm259548.htm#artificial.
- "Closed-loop insulin delivery for treatment of type 1 diabetes" (in En). BMC Medicine 9 (1): 120. November 2011. doi:10.1186/1741-7015-9-120. PMID 22071283. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3229449
- "The First Four Continuous Glucose Monitors" (in en). Managed Care magazine. 2018-07-04. https://www.managedcaremag.com/archives/2018/7/first-four-continuous-glucose-monitors.
- Health, Center for Devices and Radiological. "Recently-Approved Devices - The 670G System - P160017" (in en). http://wayback.archive-it.org/7993/20170111141252/http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm.
- "MiniMed 670G Insulin Pump System | World's First Hybrid Closed Loop System" (in en). https://www.medtronicdiabetes.com/products/minimed-670g-insulin-pump-system.
- "FDA Approves the MiniMed 670G System for Children 7-13 - The LOOP Blog" (in en-US). 2018-06-22. https://www.medtronicdiabetes.com/loop-blog/fda-approves-minimed-670g-system-children-7-13/.
- "Update on MiniMed 670G Availability" (in en-US). https://www.medtronicdiabetes.com/loop-blog/update-minimed-670g-availability/.
- "bionicpancreas.org". http://www.bionicpancreas.org/.
- "Update on the iLet Bionic Pancreas Closed Loop System" (in en). Healthline. https://www.healthline.com/diabetesmine/beta-bionics-ilet-update#1.
- "Bionic Pancreas Passes Critical Science Hurdle | BU Today | Boston University" (in en). BU Today. http://www.bu.edu/today/2017/bionic-pancreas-clinical-trial/.
- "The Miracle of an Artificial Pancreas" (in en). https://medlineplus.gov/magazine/issues/spring17/articles/spring17pg15-16.html.
- Clinical trial number NCT02844517 for "International Diabetes Closed Loop (iDCL) Trial: Research Site Training Protocol" at ClinicalTrials.gov https://www.clinicaltrials.gov/show/NCT02844517
- Clinical trial number NCT02925299 for "Day and Night Closed-loop in Young People With Type 1 Diabetes" at ClinicalTrials.gov https://www.clinicaltrials.gov/show/NCT02925299
- Clinical trial number NCT03040414 for "Fuzzy Logic Automated Insulin Regulation" at ClinicalTrials.gov https://www.clinicaltrials.gov/show/NCT03040414
- "Israeli and French Biotech Companies Partner to Fight Diabetes With Bio-Artificial Pancreas". CTECH - www.calcalistech.com. 2018-11-12. https://www.calcalistech.com/ctech/articles/0,7340,L-3749642,00.html.
- "MailPan ® BioArtificial Pancreas | Defymed, advanced therapies inspired for you" (in fr-FR). Defymed. https://www.defymed.com/mailpan/.
- "Innovative Medical Devices for the Treatment of Diabetes, Defymed Strengthens Its Position as a Worldwide Leader" (in en). https://www.businesswire.com/news/home/20180212005894/en/Innovative-Medical-Devices-Treatment-Diabetes-Defymed-Strengthens.
- Canada, JDRF. "What Does JDRF Stand For?" (in en). https://www.jdrf.ca/who-we-are/what-does-jdrf-stand-for/.
- "Kadimastem - Stem Cell to Cure Diseases". http://www.kadimastem.com/.
- "Diabetes - Kadimastem". http://www.kadimastem.com/regenerative-medicine/17/diabetes.
- Inc., ViaCyte. "Two-year Data from ViaCyte's STEP ONE Clinical Trial Presented at ADA 2018" (in en). https://www.prnewswire.com/news-releases/two-year-data-from-viacytes-step-one-clinical-trial-presented-at-ada-2018-300671013.html.
- "PEC‐Encap™ (VC-01™)" (in en-US). Viacyte, Inc.. https://viacyte.com/products/pec%E2%80%90encap-vc-01.
- "Home" (in en-US). Viacyte, Inc.. https://viacyte.com/.
- "Concise Review: Manufacturing of Pancreatic Endoderm Cells for Clinical Trials in Type 1 Diabetes". Stem Cells Translational Medicine 4 (8): 927–31. August 2015. doi:10.5966/sctm.2015-0058. PMID 26062982. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4511151
- Artificial Pancreas Project : JDRF http://www.jdrf.org/artificialpancreas
- KMorandi says (2017-08-10). "Insurers can profit while improving the lives of people with type 1 diabetes". https://www.statnews.com/2017/08/10/type-1-diabetes-insurance-companies/.
- Hurley, Dan (24 December 2014) [1] WIRED Magazine, Diabetes Patients Are Hacking Their Way Toward a Bionic Pancreas
- Knutson, Ryan (8 June 2015) [2] The Wall Street Journal, Computer Experts Deliver Insulin to Diabetic Kids




