Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | R S Krishna | -- | 1637 | 2022-11-28 19:55:55 | | | |
| 2 | Jessie Wu | Meta information modification | 1637 | 2022-11-29 04:15:01 | | | | |
| 3 | Jessie Wu | Meta information modification | 1637 | 2022-11-29 04:17:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R.S. The Chemical Compositions of Industrial Wastes. Encyclopedia. Available online: https://encyclopedia.pub/entry/36919 (accessed on 07 February 2026).
Mishra J, Nanda B, Patro SK, Krishna RS. The Chemical Compositions of Industrial Wastes. Encyclopedia. Available at: https://encyclopedia.pub/entry/36919. Accessed February 07, 2026.
Mishra, Jyotirmoy, Bharadwaj Nanda, Sanjaya Kumar Patro, R. S. Krishna. "The Chemical Compositions of Industrial Wastes" Encyclopedia, https://encyclopedia.pub/entry/36919 (accessed February 07, 2026).
Mishra, J., Nanda, B., Patro, S.K., & Krishna, R.S. (2022, November 28). The Chemical Compositions of Industrial Wastes. In Encyclopedia. https://encyclopedia.pub/entry/36919
Mishra, Jyotirmoy, et al. "The Chemical Compositions of Industrial Wastes." Encyclopedia. Web. 28 November, 2022.
Copy Citation
As a result of global warming, the pursuance of low-carbon, sustainable building materials has been prioritized. The development of geopolymer/cement-less binders can be considered an innovative and green way forward to minimize carbon footprint and tackle industrial waste material utilization.
Industrial Waste
Waste Encapsulation
Sustainability
Geopolymer
Carbon Footprint
Building Material
1. Introduction
Cement production contributes nearly 5–8% of global CO2 emissions [1]. For this reason, issues such as climate change, due to global warming, are considered severe areas of concern. In order to significantly scale down carbon emissions, efforts have been made to develop and implement greener alternatives to cement. The greener (low-carbon) alternatives could not only curb carbon emissions, but also provide an effective waste management strategy to achieve the sustainable development goals of the 21st century. Geopolymer (cement-less) binders are the most promising green building material. Figure 1 demonstrates the use of industrial wastes for the production of geopolymer composites which, from an environmental perspective, leads to several benefits.
Figure 1. Utilization of industrial wastes for the production of geopolymer composites-leading to several merits.
Since the inception of the term ‘geopolymers’ by J. Davidovits in 1978, research and development in the field of geopolymers has steadily increased and is now considered a highly dynamic area of scientific investigation from both the commercial and environmental viewpoint. The formation of geopolymers includes a reaction between aluminosilicate raw materials (industrial wastes) and an aqueous alkaline composition (usually a mixture of alkali hydroxides and silicates). The result is the inorganic polymer matrix, consisting of a three-dimensional framework of covalently bonded Si-O-Al-O (polysialate), with outstanding strength and microstructural and durable properties compared to cement-based materials [2][3][4][5][6]. However, as proposed in various studies [7][8][9], the reaction mechanism is a multiphase and complex process and still requires extensive investigation.
On the other hand, the rise in manufacturing industries calls for effective industrial waste management strategies. Therefore, developing industrial waste-derived geopolymer binders could potentially be the solution, promoting the concept of a circular economy—waste to wealth [7][8]. The various industrial wastes contain a significant amount of aluminium (Al) and silicon (Si), which are essential for the formation of a strong and durable geopolymer matrix [9][10] and serve as source material in the preparation of geopolymer binders. In some studies [11][12], the presence of magnesium oxide (Mg) and iron (Fe), alongside Al and Si, in some source materials have also been reported to be beneficial for the strength development of resulting geopolymer binders. For this reason, the utilization of such wastes for the production of eco-friendly mortars and concrete could be a step forward in reducing our dependence on cement [13].
Over the last decade, numerous researchers have determined FA as an exceptional geopolymer binder due to its physical and chemical characteristics, aiding in geopolymerization reactions. A high amount of alumino silicate content in fly ash facilitates increased reactivity, with a higher degree of geopolymerization in an alkaline environment, while supporting the reduction of hydration heat and thermal cracking. The development of aluminosilicate gel and the early strength gain of FA-based geopolymers has been demonstrated upon premature high-temperature curing while increasing the rate of geopolymerization due to the requirement of higher activation energy of fly ash. Due to their amorphous nature, FA-based geopolymers possess excellent durability against acid and alkali attacks compared with other geopolymer binders.
2. Chemical Properties of Industrial Wastes/Source Materials
The rise in the manufacturing industries to attend to the needs of 21st-century civilization has led to a significant generation of industrial waste. With the ultimate aim of achieving a circular economy and sustainable development, these wastes could be utilized efficiently for making geopolymer binders. It is imperative to study the chemical properties of each of these wastes before their implementation as source materials. The chemical compositions play a significant role in the formation of the geopolymer network (Si-O-Al-O), geopolymer gels (C-A-S-H/N-A-S-H) [14][15][16], that in turn determine the strength and other characteristics of the final product. Hence, understanding geopolymer binders requires the utmost attention to the chemistry involved. The SiO2 and Al2O3 content in the source materials are responsible for the formation of the principal geopolymer network (-Si-O-Al-), while the CaO, Fe2O3, MgO contents provide additional mineral compounds, such as calcium aluminosilicate hydrate (C-A-S-H), magnesium aluminosilicate hydrate (M-A-S-H) and ferrosiliate (-Fe-O-Si-O-) type gels, respectively. These additional mineral phases improve the binder matrix, leading to a denser binder structure. Therefore, in this section, the authors’ primary focus is on the chemical properties, and in particular, SiO2, Al2O3, CaO, Fe2O3, MgO of FA, GGBFS, RM, IOT, FCA, MK and SF, before moving toward the strength and microstructure properties.
According to Davidovits, geopolymerization involves the formation of the Si-O-Al-O network [17]. Therefore, the presence of a high amount of Si and/or Al makes industrial waste a potential candidate for geopolymerization. However, apart from oxides of Si and Al, the oxides of Ca, Fe, and Mg are also responsible for geopolymer compound formation. These minerals co-exist alongside the primary polymer chain of Si-O-Al-O-, which further influences the strength of the overall binder matrix.
The chemical compositions (esp. SiO2, Al2O3, CaO, Fe2O3, MgO %) of the FA, GGBFS, IOT, RM, FCA, MK, and SF are graphically presented in Figure 2. Table 1 shows that the chemical compositions of each of these wastes vary across the source of their origin (region) and method of processing.
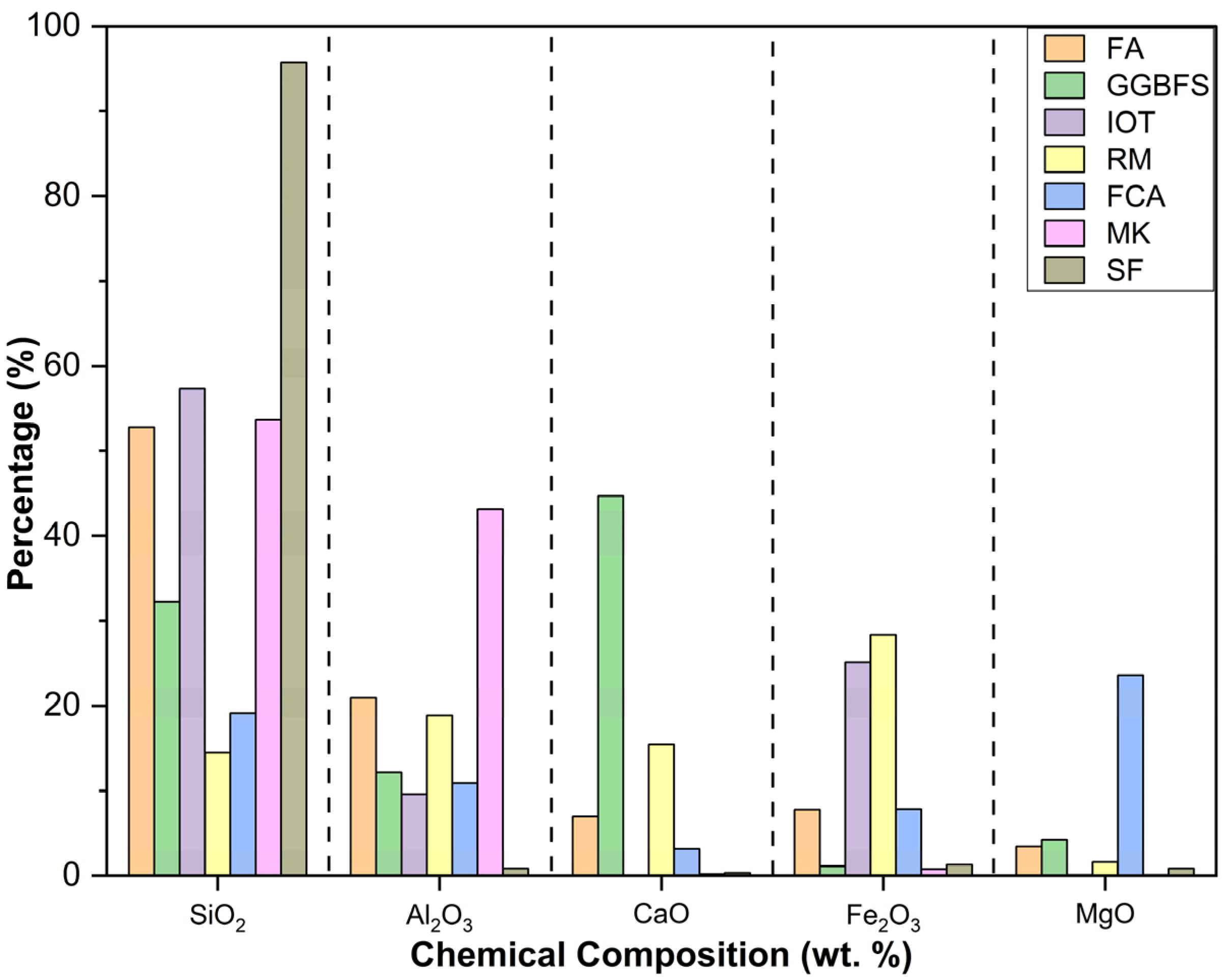
Table 1. Chemical compositions of FA, GGBFS, IOT, RM, and FCA; detected from XRF analysis.
| Industrial Source & Region | Authors | Chemical Compositions (Wt.%) | |||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | MgO | |||
| FLY ASH | NTPC Ramagundam Thermal Power Plant, India | [25] | 60.11 | 26.53 | 4.00 | 4.25 | 1.25 |
| Tarong Power Plant, Australia | [26] | 75.66 | 19.00 | 0.30 | 1.38 | 0.00 | |
| Gladstone Power Station, Australia | [27] | 47.83 | 28.49 | 5.51 | 11.38 | 1.43 | |
| Cates Electrical Prod. Plant, Turkey | [28] | 54.08 | 26.08 | 35.58 | 6.68 | 2.67 | |
| Lethabo Power Plant, South Africa | [29] | 56.45 | 30.27 | 4.59 | 3.58 | 1.06 | |
| GGBFS | Nippon Steel & Sumitomo Metal Corporation, Japan |
[30] | 30.53 | 13.67 | 46.00 | 0.33 | 5.09 |
| Vizag steel plant, India | [31] | 30.61 | 16.24 | 34.48 | 0.58 | 6.79 | |
| JSW Iron and Steel Plant, Bellary, India | [32] | 32.52 | 17.14 | 34.22 | 1.22 | 9.65 | |
| Rourkela Steel Plant, India | [33] | 30.82 | 21.06 | 32.02 | 1.37 | 9.52 | |
| Esfahan Steel Company, Iran | [34] | 35.85 | 13.39 | 37.71 | 1.06 | 9.10 | |
| IOT | Lake Superior Iron Ore District facility, USA | [35] | 68.77 | 0.8675 | 0.275 | 28.17 | 0.74 |
| Copper-iron mine, Zibo city, China | [36] | 70.32 | 5.1 | 4.71 | 10.93 | 4.51 | |
| Iron ore tailing dam, Brazil. | [37] | 30.00 | 21.20 | 0.10 | 47.80 | - | |
| Iron Ore mine site, Kuancheng Chengde, China | [38] | 67.58 | 8.70 | 5.78 | 7.42 | 4.37 | |
| Iron ore tailings dam, Ouro Preto, Brazil. | [39] | 40.00 | 8.70 | 0.00 | 48.90 | - | |
| RM | Rio Tinto Aluminium company, Canada | [40] | 10.52 | 22.12 | 1.36 | 38.92 | 0.10 |
| Hindalco Industries, Belgaum, India | [41] | 9.93 | 18.1 | 2.3 | 42.9 | - | |
| Tan Rai Bauxite Plant, Vietnam | [42] | 4.52 | 18.98 | 0.87 | 49.90 | - | |
| Aluminium Smelter, Alcoa, Spain | [43] | 5.67 | 14.63 | 1.88 | 52.25 | 3.35 | |
| Zhaofeng Aluminium Company, China | [44] | 21.43 | 22.72 | 16.49 | 9.98 | 0.00 | |
| FCA | Balasore Alloys Limited, India | [45] | 19.60 | 11.2 | 4.2 | 6.1 | 15.6 |
| Balasore Alloys Limited, India | [46] | 19.10 | 10.91 | 3.14 | 7.84 | 23.60 | |
| Balasore Alloys Limited, India | [22] | 19.10 | 10.91 | 3.14 | 7.84 | 23.60 | |
| MK | Cˇ LUZ (Nové Strašecí, Czech Republic) | [47] | 55.01 | 40.94 | 0.55 | 0.55 | 0.14 |
| Astrra Chemicals, Chennai, India | [48] | 47.64 | 50.22 | 0.05 | 0.24 | 0.05 | |
| Hongle, Inc. (Henan, China). | [23] | 53.65 | 43.12 | 0.17 | 0.76 | 0.06 | |
| SF | Counto Microfine Products Pvt.Ltd., Goa, India | [49] | 93.67 | 0.83 | 0.31 | 1.30 | 0.84 |
| Shenhua Junggar Energy Corporation, Junggar, China |
[50] | 95.72 | 0.09 | 0.23 | 0.63 | 0.37 | |
| SMS ASIA Pvt. Ltd., Rourkela, India |
[24] | 93.67 | 0.83 | 0.31 | |||
Role of SiO2, Al2O3, CaO, Fe2O3, and MgO in the Formation of Geopolymer Binder Structure
The reaction process leading to the formation of a geopolymer binder structure is complicated and is controlled by various parameters [51]. The most significant factor remains the reactivity of SiO2, Al2O3, CaO, MgO, and Fe2O3 present in the source materials used, which are discussed herein.
The initial step, in the case of geopolymerization, is the dissolution of SiO2 and Al2O3 from the source materials under an alkaline environment, which leads to the development of Si-OH and Al-OH bonds [52]. Subsequently, due to subsequent condensation and polymerization reactions, a 3D network structure is produced that involves the tetrahedral networks of [SiO4]− and [AlO4], linked via shared oxygen atoms [53]. However, these tetrahedral connection types of [SiO4] and [AlO4] are often very distinct and intricate; therefore, it can be implied that the properties of the final geopolymer product vary with different connection types [17]. For this reason, it is crucial to select source materials with an optimum composition of SiO2 and Al2O3 in order to achieve enhanced geopolymer properties [18].
The CaO in the source materials does not participate in the core geopolymer network formation, i.e., Si-O-Al-. However, it aids in the formation of hydration products such as C-S-H (a space-filler) and a geopolymer gel, i.e., C-A-S-H, inside the geopolymer binder structure [54]; however, this geopolymer gel also comprises of Al and Si in a tetrahedral connection type, as mentioned earlier. It is also acknowledged that the final orientation and properties of geopolymer structures vary depending on the CaO content in the source materials [55]. Several researchers have favored the presence of CaO in the source materials to achieve improved properties [56][57][58][59]. Furthermore, the hardening time of geopolymers is also significantly affected by the presence of low/high CaO content in the source material [60]; hence, the role of CaO is considered crucial during the selection of source material for geopolymerization.
The presence of iron oxide (from the iron-rich source materials) is indicated by the replacement of Al3+ by Fe3+ (ferric ions) in the geopolymer binder structure (Si-O-Al), yielding a Ferro-sialate network (-Fe-O-Si-O-Al-O-) [61][62]. In contrast, some studies in the past have also found that iron, in its Fe2+ (ferrous) state, remains inert and does not participate in the geopolymerization process [63]. However, Lemougna et al. [64] noticed that some of the ferrous ions are present in the tetrahedral connections of the Si-O-Al network. This observation is corroborated by Kaze et al. [65], who reports that crystalline phases of ferrous ions exist, such as fayalite and siderite, in the final geopolymer binder system. Hence, it is confirmed that the presence of iron in its ferric or ferrous state affects the performance of the geopolymer binder structure and its formation.
The role of MgO in the source materials has been studied in [11][66][67]. It is demonstrated that the existence of MgO did not aid in developing or reorganizing the geopolymer chain (Si-O-Al). However, its presence led to the formation of hydrotalcite. This magnesium and aluminium hydroxycarbonate mineral form act as a filler agent while aiding in accelerating the generation of hydration products such as C-S-H. This reveals that, although MgO does not participate in the core development of geopolymer tetrahedral network formation, it necessitates forming other chemical compounds that directly affect the final geopolymer binder structure.
References
- Akbar, A.; Farooq, F.; Shafique, M.; Aslam, F.; Alyousef, R.; Alabduljabbar, H. Sugarcane Bagasse Ash-Based Engineered Geopolymer Mortar Incorporating Propylene Fibers. J. Build. Eng. 2021, 33, 101492.
- Kumar, M.; Kumar, A. Geopolymer Cement: An Initiative towards the Replacement of Grey Cement by Green Cement in Future. J. Build. Mater. Struct. 2021, 8, 1–8.
- Zhang, Z.; Yu, H.; Xu, M.; Cui, X. Preparation, Characterization and Application of Geopolymer-Based Tubular Inorganic Membrane. Appl. Clay Sci. 2021, 203, 106001.
- Guo, H.; Yuan, P.; Zhang, B.; Wang, Q.; Deng, L.; Liu, D. Realization of High-Percentage Addition of Fly Ash in the Materials for the Preparation of Geopolymer Derived from Acid-Activated Metakaolin. J. Clean. Prod. 2021, 285, 125430.
- Saranya, P.; Nagarajan, P.; Shashikala, A.P. Experimental Investigation on Bond Strength Properties of Geopolymer Concrete. In Proceedings of the Lecture Notes in Civil Engineering; Springer: Singapore, 2021; Volume 83, pp. 731–740.
- Amri, A.; Najib, A.A.; Olivia, M.; Altarawneh, M.; Syam, A.; Rahman, M.M.; Saputro, S.; Wahyuadi, J.; Jiang, Z.T. Physicochemical Properties of Geopolymer Composites with DFT Calculations of In-Situ Reduction of Graphene Oxide. Ceram. Int. 2021, 47, 13440–13445.
- Ongpeng, J.M.C.; Guades, E.J.; Promentilla, M.A.B. Cross-Organizational Learning Approach in the Sustainable Use of Fly Ash for Geopolymer in the Philippine Construction Industry. Sustainability 2021, 13, 2454.
- Almalkawi, A.T.; Balchandra, A.; Soroushian, P. Potential of Using Industrial Wastes for Production of Geopolymer Binder as Green Construction Materials. Constr. Build. Mater. 2019, 220, 516–524.
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933.
- Hardjito, D.; Wallah, S.; Sumajouw, D.M.J.; Rangan, B.V. Brief Review of Development of Geopolymer. In Proceedings of the 8th CANMET/ACI International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolans in Conerete, Las Vegas, NV, USA, 23–29 May 2004; pp. 1–10.
- Bernal, S.A.; San Nicolas, R.; Myers, R.J.; Mejía De Gutiérrez, R.; Puertas, F.; Van Deventer, J.S.J.; Provis, J.L. MgO Content of Slag Controls Phase Evolution and Structural Changes Induced by Accelerated Carbonation in Alkali-Activated Binders. Cem. Concr. Res. 2014, 57, 33–43.
- Hu, Y.; Liang, S.; Yang, J.; Chen, Y.; Ye, N.; Ke, Y.; Tao, S.; Xiao, K.; Hu, J.; Hou, H.; et al. Role of Fe Species in Geopolymer Synthesized from Alkali-Thermal Pretreated Fe-Rich Bayer Red Mud. Constr. Build. Mater. 2019, 200, 398–407.
- Liu, M.; Wu, H.; Yao, P.; Wang, C.; Ma, Z. Microstructure and Macro Properties of Sustainable Alkali-Activated Fly Ash Mortar with Various Construction Waste Fines as Binder Replacement up to 100%. Cem. Concr. Compos. 2022, 134, 104733.
- Cristelo, N.; Segadães, L.; Coelho, J.; Chaves, B.; Sousa, N.R.; de Lurdes Lopes, M. Recycling Municipal Solid Waste Incineration Slag and Fly Ash as Precursors in Low-Range Alkaline Cements. Waste Manag. 2020, 104, 60–73.
- Cristelo, N.; Coelho, J.; Miranda, T.; Palomo, Á.; Fernández-Jiménez, A. Alkali Activated Composites–An Innovative Concept Using Iron and Steel Slag as Both Precursor and Aggregate. Cem. Concr. Compos. 2019, 103, 11–21.
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; Van Deventer, J.S.J.; Provis, J.L. Phase Evolution of C-(A)-S-H/N-A-S-H Gel Blends Investigated via Alkali-Activation of Synthetic Calcium Aluminosilicate Precursors. Elsevier 2016, 89, 120–135.
- Davidovits, J. Geopolymers-Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656.
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al Ratio on the Efflorescence and Properties of Fly Ash Based Geopolymer. J. Clean. Prod. 2020, 244, 118852.
- Bellum, R.R.; Muniraj, K.; Indukuri, C.S.R.; Madduru, S.R.C. Investigation on Performance Enhancement of Fly Ash-GGBFS Based Graphene Geopolymer Concrete. J. Build. Eng. 2020, 32, 101659.
- Kuranchie, F.A.; Shukla, S.K.; Habibi, D. Utilisation of Iron Ore Mine Tailings for the Production of Geopolymer Bricks. Int. J. Min. Reclam. Environ. 2016, 30, 92–114.
- Zhou, W.; Shi, X.; Lu, X.; Qi, C.; Luan, B.; Liu, F. The Mechanical and Microstructural Properties of Refuse Mudstone-GGBS-Red Mud Based Geopolymer Composites Made with Sand. Constr. Build. Mater. 2020, 253, 119193.
- Mishra, J.; Kumar Das, S.; Krishna, R.S.; Nanda, B.; Kumar Patro, S.; Mohammed Mustakim, S. Synthesis and Characterization of a New Class of Geopolymer Binder Utilizing Ferrochrome Ash (FCA) for Sustainable Industrial Waste Management. Mater. Today Proc. 2020, 33, 5001–5006.
- Chen, K.; Wu, D.; Yi, M.; Cai, Q.; Zhang, Z. Mechanical and Durability Properties of Metakaolin Blended with Slag Geopolymer Mortars Used for Pavement Repair. Constr. Build. Mater. 2021, 281, 122566.
- Jena, S.; Panigrahi, R.; Sahu, P. Effect of Silica Fume on the Properties of Fly Ash Geopolymer Concrete. In Sustainable Construction and Building Materials. Lecture Notes in Civil Engineering; Springer: Singapore, 2019; pp. 145–153.
- Mallikarjuna Rao, G.; Gunneswara Rao, T.D. Final Setting Time and Compressive Strength of Fly Ash and GGBS-Based Geopolymer Paste and Mortar. Arab. J. Sci. Eng. 2015, 40, 3067–3074.
- Gunasekara, C.; Law, D.W.; Setunge, S. Long Term Permeation Properties of Different Fly Ash Geopolymer Concretes. Constr. Build. Mater. 2016, 124, 352–362.
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of One-Part Geopolymer Binders Made from Fly Ash. Waste Biomass Valorization 2017, 8, 225–233.
- Al-mashhadani, M.M.; Canpolat, O.; Aygörmez, Y.; Uysal, M.; Erdem, S. Mechanical and Microstructural Characterization of Fiber Reinforced Fly Ash Based Geopolymer Composites. Constr. Build. Mater. 2018, 167, 505–513.
- Naghizadeh, A.; Ekolu, S.O. Method for Comprehensive Mix Design of Fly Ash Geopolymer Mortars. Constr. Build. Mater. 2019, 202, 704–717.
- Phoo-Ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of Sodium Hydroxide and Sodium Silicate Solutions on Compressive and Shear Bond Strengths of FA-GBFS Geopolymer. Constr. Build. Mater. 2015, 91, 1–8.
- Jawahar, J.G.; Mounika, G. Strength Properties of Fly Ash and GGBS Based Geopolymer Concrete. Asian J. Civ. Eng 2016, 17, 127–135.
- Palankar, N.; Ravi Shankar, A.U.; Mithun, B.M. Investigations on Alkali-Activated Slag/Fly Ash Concrete with Steel Slag Coarse Aggregate for Pavement Structures. Int. J. Pavement Eng. 2017, 18, 500–512.
- Samantasinghar, S.; Singh, S.P. Synthesis of Fly Ash-GGBS-Blended Geopolymer Composits; Springer: Singapore, 2019; Volume 16, ISBN 9789811308994.
- Shahmansouri, A.A.; Akbarzadeh Bengar, H.; Ghanbari, S. Compressive Strength Prediction of Eco-Efficient GGBS-Based Geopolymer Concrete Using GEP Method. J. Build. Eng. 2020, 31, 101326.
- McDonald, J.E.D.; Roache, S.C.; Kawatra, S.K. Repurposing Mine Tailings: Cold Bonding of Siliceous Iron Ore Tailings. Miner. Metall. Process. 2016, 33, 47–52.
- Tian, Z.X.; Zhao, Z.H.; Dai, C.Q.; Liu, S.J. Experimental Study on the Properties of Concrete Mixed with Iron Ore Tailings. Adv. Mater. Sci. Eng. 2016, 2016, 8606505.
- Galvão, J.L.B.; Andrade, H.D.; Brigolini, G.J.; Peixoto, R.A.F.; Mendes, J.C. Reuse of Iron Ore Tailings from Tailings Dams as Pigment for Sustainable Paints. J. Clean. Prod. 2018, 200, 412–422.
- Zhang, C.; Li, S. Utilization of Iron Ore Tailing for the Synthesis of Zeolite A by Hydrothermal Method. J. Mater. Cycles Waste Manag. 2018, 20, 1605–1614.
- do Carmo e Silva Defáveri, K.; dos Santos, L.F.; Franco de Carvalho, J.M.; Peixoto, R.A.F.; Brigolini, G.J. Iron Ore Tailing-Based Geopolymer Containing Glass Wool Residue: A Study of Mechanical and Microstructural Properties. Constr. Build. Mater. 2019, 220, 375–385.
- Hairi, S.N.M.; Jameson, G.N.L.; Rogers, J.J.; MacKenzie, K.J.D. Synthesis and Properties of Inorganic Polymers (Geopolymers) Derived from Bayer Process Residue (Red Mud) and Bauxite. J. Mater. Sci. 2015, 50, 7713–7724.
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Durability of Red Mud Based Geopolymer Paste in Acid Solutions. Mater. Sci. Forum 2016, 866, 99–105.
- Hoc Thang, N.; Kien, P.T.; Abdullah, M.M.A.B. Lightweight Heat Resistant Geopolymer-Based Materials Synthesized from Red Mud and Rice Husk Ash Using Sodium Silicate Solution as Alkaline Activator. MATEC Web Conf. 2017, 97, 01119.
- Novais, R.M.; Carvalheiras, J.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Innovative Application for Bauxite Residue: Red Mud-Based Inorganic Polymer Spheres as PH Regulators. J. Hazard. Mater. 2018, 358, 69–81.
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of Red Mud in Geopolymer-Based Pervious Concrete with Function of Adsorption of Heavy Metal Ions. J. Clean. Prod. 2019, 207, 789–800.
- Chethan, K.B.; Yaragal, S.C.; Das, B.B. Ferrochrome Ash–Its Usage Potential in Alkali Activated Slag Mortars. J. Clean. Prod. 2020, 257, 120577.
- Mishra, J.; Das, S.K.; Krishna, R.S.; Nanda, B. Utilization of Ferrochrome Ash as a Source Material for Production of Geopolymer Concrete for a Cleaner Sustainable Environment. Indian Concr. J. 2020, 94, 40–49.
- Rovnaník, P.; Šafránková, K. Thermal Behaviour of Metakaolin/Fly Ash Geopolymers with Chamotte Aggregate. Materials 2016, 9, 535.
- Praveen Kumar, V.V.; Prasad, N.; Dey, S. Influence of Metakaolin on Strength and Durability Characteristics of Ground Granulated Blast Furnace Slag Based Geopolymer Concrete. Struct. Concr. 2020, 21, 1040–1050.
- Okoye, F.N.; Durgaprasad, J.; Singh, N.B. Effect of Silica Fume on the Mechanical Properties of Fly Ash Based-Geopolymer Concrete. Ceram. Int. 2016, 42, 3000–3006.
- Duan, P.; Yan, C.; Zhou, W. Compressive Strength and Microstructure of Fly Ash Based Geopolymer Blended with Silica Fume under Thermal Cycle. Cem. Concr. Compos. 2017, 78, 108–119.
- Nath, S.K.; Kumar, S. Role of Particle Fineness on Engineering Properties and Microstructure of Fly Ash Derived Geopolymer. Constr. Build. Mater. 2020, 233, 117294.
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of Water in the Synthesis of Calcined Kaolin-Based Geopolymer. Appl. Clay Sci. 2009, 43, 218–223.
- Zhang, L.W.; Kai, M.F.; Chen, X.H. Si-Doped Graphene in Geopolymer: Its Interfacial Chemical Bonding, Structure Evolution and Ultrastrong Reinforcing Ability. Cem. Concr. Compos. 2020, 109, 103522.
- Walkley, B.; Ke, X.; Hussein, O.H.; Bernal, S.A.; Provis, J.L. Incorporation of Strontium and Calcium in Geopolymer Gels. J. Hazard. Mater. 2020, 382, 121015.
- Ye, H.; Huang, L. Degradation Mechanisms of Alkali-Activated Binders in Sulfuric Acid: The Role of Calcium and Aluminum Availability. Constr. Build. Mater. 2020, 246, 118477.
- Saini, G.; Vattipalli, U. Assessing Properties of Alkali Activated GGBS Based Self-Compacting Geopolymer Concrete Using Nano-Silica. Case Stud. Constr. Mater. 2020, 12, e00352.
- Puligilla, S.; Mondal, P. Role of Slag in Microstructural Development and Hardening of Fly Ash-Slag Geopolymer. Cem. Concr. Res. 2013, 43, 70–80.
- Deb, P.S.; Nath, P.; Sarker, P.K. The Effects of Ground Granulated Blast-Furnace Slag Blending with Fly Ash and Activator Content on the Workability and Strength Properties of Geopolymer Concrete Cured at Ambient Temperature. Mater. Des. (1980–2015) 2014, 62, 32–39.
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The Development of Compressive Strength of Ground Granulated Blast Furnace Slag-Palm Oil Fuel Ash-Fly Ash Based Geopolymer Mortar. Mater. Des. 2014, 56, 833–841.
- Antoni; Wijaya, S.W.; Hardjito, D. Factors Affecting the Setting Time of Fly Ash-Based Geopolymer. Mater. Sci. Forum 2016, 841, 90–97.
- Gomes, K.C.; Lima, G.S.T.; Torres, S.M.; De Barros, S.; Vasconcelos, I.F.; Barbosa, N.P. Iron Distribution in Geopolymer with Ferromagnetic Rich Precursor. Mater. Sci. Forum 2010, 643, 131–138.
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-). Tech. Pap. # 27 2020.
- Perera, D.S.; Cashion, J.D.; Blackford, M.G.; Zhang, Z.; Vance, E.R. Fe Speciation in Geopolymers with Si/Al Molar Ratio of ∼2. J. Eur. Ceram. Soc. 2007, 27, 2697–2703.
- Lemougna, P.N.; MacKenzie, K.J.D.; Jameson, G.N.L.; Rahier, H.; Chinje Melo, U.F. The Role of Iron in the Formation of Inorganic Polymers (Geopolymers) from Volcanic Ash: A 57Fe Mössbauer Spectroscopy Study. J. Mater. Sci. 2013, 48, 5280–5286.
- Kaze, C.R.; Djobo, J.N.Y.; Nana, A.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahier, H. Effect of Silicate Modulus on the Setting, Mechanical Strength and Microstructure of Iron-Rich Aluminosilicate (Laterite) Based-Geopolymer Cured at Room Temperature. Ceram. Int. 2018, 44, 21442–21450.
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and Drying Shrinkage of Reactive MgO Modified Alkali-Activated Slag Paste. Constr. Build. Mater. 2014, 51, 395–404.
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and Hydration Properties of Reactive MgO-Activated Ground Granulated Blastfurnace Slag Paste. Cem. Concr. Compos. 2015, 57, 8–16.
More
Information
Subjects:
Construction & Building Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
29 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




