Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Niraj Tripathi | -- | 3570 | 2022-11-28 04:29:54 | | | |
| 2 | Conner Chen | Meta information modification | 3570 | 2022-11-29 08:59:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Asati, R.; Tripathi, M.K.; Tiwari, S.; Yadav, R.K.; Tripathi, N. Drought in Chickpea. Encyclopedia. Available online: https://encyclopedia.pub/entry/36702 (accessed on 07 February 2026).
Asati R, Tripathi MK, Tiwari S, Yadav RK, Tripathi N. Drought in Chickpea. Encyclopedia. Available at: https://encyclopedia.pub/entry/36702. Accessed February 07, 2026.
Asati, Ruchi, Manoj Kumar Tripathi, Sushma Tiwari, Rakesh Kumar Yadav, Niraj Tripathi. "Drought in Chickpea" Encyclopedia, https://encyclopedia.pub/entry/36702 (accessed February 07, 2026).
Asati, R., Tripathi, M.K., Tiwari, S., Yadav, R.K., & Tripathi, N. (2022, November 28). Drought in Chickpea. In Encyclopedia. https://encyclopedia.pub/entry/36702
Asati, Ruchi, et al. "Drought in Chickpea." Encyclopedia. Web. 28 November, 2022.
Copy Citation
Chickpea productivity has been found to be around 995 kg ha−1 on a global scale, which is quite low. Drought, terminal heat, excessive salt, and cold are abiotic variables. Several factors are responsible for complexity of drought stress, including severity of drought, stage of crop, and duration of drought stress.
abiotic stress
candidate genes
drought tolerance
1. Introduction
Chickpea is a diploid annual crop that is extremely self-pollinated [1]. After the faba bean and field pea, it is the world’s third most significant pules crop [2]. It is a popular cool-season legume crop with a 738-megabyte genome size [3]. With an annual production of 10.13 million tonnes from a land area of 9.44 million hectares and a productivity of 1073 kg ha−1, India is the greatest producer of chickpeas in the world [4]. Chickpeas are grown in 52 countries, together with Africa, Asia, Australia, and South Europe [5]. Mexico, Turkey, Canada, Iran, Australia, Tanzania, Ethiopia, Spain, and Burma are also notable producers of chickpea. Its seeds come in two varieties. The ‘desi’ chickpea is hardy in character, while the Kabuli chickpea has a delicate seed coat and appears to have evolved from the desi varieties [6][7]. In semi-arid zones, chickpea is cultivated in the form of a dry weather crop [8]; however, in cold climatic zones, it is grown as a rainfed crop [9][10]. In actuality, about 90% of the chickpea crop is cultivated in a rainfed environment [11][12][13]. Without irrigation, the crop is affected [14] at vegetative as well as reproductive phases. After illnesses, drought is the second most significant constraint to the yield of chickpea crop [15]. Drought has been reported as a factor of 40–50 percent yield reduction in chickpea [11][12][16][17][18].
The chickpea is also termed as the “poor man’s meat” [19], since it is important for supplying protein sources [20]. Nutritionists have also highlighted its importance due to high nutritional contents in it [21]. Chickpea is high in lysine and arginine [22], but low in methionine and cystine [23]. In general, the Kabuli type contains more protein than the desi kinds. It contains more calcium and phosphorus than most other pulse crops [24][25]. Chickpea seeds comprise 23% protein, 64% total carbohydrates (47% starch, 6% soluble sugar), 5% fat, 6% crude fibre, and 2% ash on average, as well as micronutrients, for example phosphorus, calcium, magnesium, iron, and zinc [26]. Recently, Singh et al. [21] also reported chickpea as good source of Fe and Zn. Consequently, Samineni et al. [27] examined the effects of drought stress on nutritional parameters of chickpea and observed significant differences in the nutritional contents due to stress.
Chickpea is mostly cultivated in the post-rainy season [28], using soil moisture that has been retained from the previous rainy season [29]. As a result, the crop is frequently subjected to severe heat and drought pressure [12][13][22]. Drought, among other abiotic factors, has a significant impact on chickpea output [30]. Drought and heat stress have been reported to have reduced chickpea yields by about 50% due to the damaging effects of the membrane and reduced photosynthesis [31].
The four climatic elements that are changing will have an impact on how much water plants consume [32]. These elements include rising CO2 concentrations and temperatures, more erratic precipitation, and changes in humidity. Due to the increased variability in precipitation during the growing season and more so in soils with low water holding capacity, these climate changes may result in an increase in the atmospheric water demand by crops and an increase in the potential for limitations in the availability of water in the soil. In the long run, breeding cultivars with high water use efficiency (WUE) is a more realistic and cost-effective strategy for raising yields in drought-prone locations. WUE promotes modest water absorption while maintaining elevated WUE, which is a crucial component of breeding programmes because of its yields in drought-prone areas. Any WUE is impacted by changes above the soil surface because they have an impact on the soil water balance by the evaporation and penetration of soil water. The majority of the chickpea crop is grown on residual moisture; however, additional irrigation can increase yields. At some sites in India, irrigation during the pre-flowering stage and at the beginning of the pod fill led to an increase in yield. Chickpeas’ reproductive cycle was prolonged by irrigation, which also increased plant biomass and increased the number of pods per plant.
The greatest sustained surface winds of tropical storms range from 39 to 73 mph, and they are fast rotating storm systems with an organized centre over warm tropical oceans. These storms have a wide range in size and can cause a variety of dangers for the impacted areas, including tornadoes, catastrophic winds, coastal floods, and inland flooding. The effects of tropical cyclones on drought have been extensively studied, but less research has been conducted on how smaller tropical storms affect the severity of drought. According to research, rainfall is not necessarily inversely correlated with the strength of a tropical cyclone; therefore, tropical storms can sometimes provide more rain than expected. The question of whether tropical storms can help to lessen and mitigate drought conditions is now being researched. Water deficit and surplus are related to drought and tropical storms (TS), respectively. When it comes to monitoring dryness, soil moisture is a crucial element of the hydrological cycle, since it reflects the water that TS rainfall has penetrated or stored. Soil moisture data can be used to determine whether TS can alleviate extremely severe drought situations [33]. The authors calculated the frequency of TS afflicted places in the US, including the ratio of droughts that TS exacerbated and alleviated, and the regions where TS have a significant impact on the offset of drought. Based on a high-resolution data set, the findings demonstrate extensive spatial information about the offset of drought conditions and offer potential guidance for future drought and TS mitigation.
Drought has a substantial influence on crop growth and photosynthesis, both of which are directly related to production [34][35]. Drought researchers must assess growth as well as physiological responses such as chlorophyll index [36], relative water content [37], membrane stability index, and biomass when determining the influence of drought on various crop metrics. The quantitative character of attributes and the prevalence of linkage between desired and undesired genes make developing drought-tolerant agricultural variants difficult [38]. Many experiments on the effects of drought on numerous chickpea features, such as root attributes, shoot biomass, and early maturity, have been conducted [39]. In this crop, various experiments have been performed successfully and published with specific conclusions on different aspects, such as morphological, physiological, biochemical, and molecular characteristics [40][41][42].
Advances genomics has made it possible to tag genes [43] associated to agronomic qualities, as well as the tolerance/resistance to abiotic and biotic challenges [44]. It is playing a significant role in the transfer of labelled genes through molecular breeding [45][46], quickly and accurately. In chickpeas, microsatellite and sequence-tagged microsatellite site markers have been found to be more beneficial [47][48]. As stress resistance/tolerance is governed by numerous genes [49], quantitative trait loci (QTL) mapping has proven to be effective in identifying and tagging the genes [50] involved for disease resistance/tolerance in plants. Foreground, recombinant and background selection are all examples of marker-assisted backcrossing. Linkage disequilibrium (LD) and association mapping are also determined using markers [51].
Next-generation sequencing (NGS) is a segment of revolutionary biology being a frontier area in crop science and produces correct data, with the results of significant throughput [52] and reduction in the need for fragment-cloning processes, which were the initial requirement for Sanger sequencing. NGS is used for the identification and mapping of mutations in targeted genotype [53][54]. Aside from whole genome sequencing (WGS), NGS also provides a platform for whole transcriptome shotgun sequencing, which is also termed as RNA sequencing (RNA-seq) [55][56] and whole-exome sequencing [57], which exhibits for functional variations [58], targeted or candidate gene sequencing [59].In the examination of large numbers of samples, RNA-seq enables a more precise and sensitive measurement of gene expression levels than microarrays [60].
Transcriptomics is the technology used to study the transcriptome of an organism [61]. Transcriptome is the complete set of genes [62] expressed under specific conditions by the genome of the targeted organism. MicroRNA (miRNA), transfer RNA (tRNA), messenger RNA (mRNA), ribosomal RNA (rRNA) and other non-coding RNA are all found in the transcriptome (ncRNA). Transcriptomics of chickpea [63] has provided insight into mechanisms of drought tolerance/avoidance, as well as pathogenesis-related and developmental processes [64]. Transcriptomics may undoubtedly have a greater impact on chickpea breeding in the future, including the use of microarrays.
Proteomics is the study of whole protein complement in a cell, tissue or organism in detail [65]. Mass spectrometry and protein microarrays can be used to analyse the proteome [66].
Role of various genes of plants under drought stress conditions have been recognized clearly [67]. Drought responsive mechanisms are activated in response to drought stress, which is a regular occurrence in plants. Morphological and structural changes [68], drought-resistant gene expression, hormonal and other biochemical changes are among these pathways [69]. Environmental stresses have the ability to change the developmental behaviour of plants. These alterations in plant growth and development [70][71] mostly resulted in lower yields [72].
2. Drought in Chickpea
Chickpea productivity has been found to be around 995 kg ha−1 on a global scale, which is quite low [73]. Drought, terminal heat [74], excessive salt, and cold are abiotic variables [75], whereas Ascochyta blight, Fusarium wilt, and Helicoverpa are biotic factors that have been recognised as key drivers of yield reduction in chickpea [76]. Drought stress was identified as a major cause in around 50% of chickpea output losses worldwide.
Several factors are responsible for complexity of drought stress (Table 1), including severity of drought, stage of crop, and duration of drought stress [77]. Two types of drought stresses, i.e., terminal and intermittent, have been reported with their impacts on crop plants [78]. During terminal drought, soil water availability diminishes over time, potentially leading to severe drought stress later in crop development. Intermittent drought is defined as a series of short episodes of insufficient rain or irrigation that occur at different times during the growing season [79]. Due to its limited cultivation on marginal terrain, chickpea is suffering from terminal drought stress. Intermittent and terminal drought stress is caused by breaks in rainfall combined with less moisture in terminal growth stages [80]. Apart from morpho-physiological factors various genes and proteins are also responsible for drought tolerance in chickpea crop (Table 2).
Table 1. Relevance of various physiological traits contributing to drought adaptation in chickpea.
| Physiological Traits | Related with | References |
|---|---|---|
| Early phenology (early flowering, early podding) |
Drought escape/conservative water-use strategy |
[81][82][83] |
| Crop growth rate | High water harvest | [47] |
| Shoot biomass | High shoot biomass at maturity contribute to a higher grain yield under drought |
[84] |
| Pod abortion and seed filling | High seed/grain yield could help in drought and heat stress tolerance | [85] |
| Biomass partitioning | Greater biomass partitioning to grain helps in drought and heat stress tolerance | [46][47][86] |
| Pod number; high pod number | Grain yield and contributes to heat, drought tolerance | [87] |
| Pod production | Number of pods/plants is more affected at early stage than late stage under drought stress |
[88] |
| Specific leaf area | SLA has a positive effect on grain yield at reproductive stage |
[89] |
| Cell membrane stability | Related to drought, heat, and cold tolerance |
[30][90][91][92] |
| Canopy temperature depression |
Cooler canopy contributes to drought avoidance and has a positive association with seed yield under drought stress, and it also contributes to heat stress tolerance |
[93][94][95] |
| Canopy conductance | Associated to both heat and drought stress tolerance | [96] |
| Carbon isotope Discrimination |
Transpiration efficiency | [97] |
| Recycling of CO2 inside the pod | Maintain seed filling | [98] |
| Antioxidants enzymes, proline, anthocyanin content, trehalose, sucrose, and nonreducing sugars |
Increase in antioxidant enzymes, proline, trehalose and anthocyanin content during vegetative stage causes drought and cold stress tolerance |
[99] |
| Relative water content | Increase in relative water content causes drought stress tolerance |
[100][101] |
| Chlorophyll content; carotenoid content |
Higher chlorophyll content and carotenoid content helps in heat stress tolerance |
[55][96] |
| (Na+ and K+) ion uptake | (Na+ and K+) ion uptake cause drought tolerance |
[102] |
| Chlorophyll a fluorescence FO, FM, PSII, ETR, FV/FM |
Enable preventing PSII photochemistry from damage and helps in both drought and heat stress tolerance |
[102][103] |
| Plant transpiration rate | Low plant transpiration rate helps in conserving soil water | [104][105] |
| Transpiration efficiency | It decides ultimate yield | [106][107] |
| Early vigour | Associated to both heat and drought stress tolerance | [108] |
| Pollen traits (pollen viability, fertility, and pollentube germination) | High pollen viability and fertility under heat stress are associated to heat stress tolerance |
[109] |
| Abscisic acid (ABA) | Under drought increase in ABA causes closure of stomata, thus reducing assimilate production that leads to the inhibition of seed set |
[108] |
| Root architectural trait prolific root system, root branch, root density root depth, root area, and root volume | Prolific root system is associated to grain yield | [47] |
| Deep rooting helps in using conserved soil moisture from subsoil and helps in avoiding terminal drought stress |
[108] |
Table 2. List of some genes conferring adaptation to drought and other abiotic stresses in chickpea.
| Treatment | Traits | Gene | References |
|---|---|---|---|
| Drought | Abiotic stress response | CarERF116 | [110] |
| Drought | Biotic and abiotic stresses | Aquaporins gene family (CaAQPs) | [111] |
| Drought | Drought stress response | DEGs | [112] |
| Drought, heat and cold stress | Process of plant development | CarLEA4 | [113] |
| Drought and heat stress | Root traits, plat morphology, transpiration, and yield traits | Marker–trait association | [47] |
Due to the overabundance of wheat in irrigated areas in India, chickpea growth is primarily limited to rainfed areas. Crops in rainfed areas are experiencing water shortages, particularly during the sowing and terminal growth periods.
Soil and plant management are important for minimizing water stress. For this purpose, various experiments have been conducted with the applications of different agents. Gypsum can enhance overall plant growth, since it is a moderately soluble source of the crucial plant nutrients, calcium and sulphur. Gypsum supplements can also enhance the physical and chemical characteristics of soils, hence lowering nutrient concentrations in surface water runoff and reducing soil erosion losses. The most often used addition for reclaiming sodic soil is gypsum, which can also be found in synthetic soils used in nursery, greenhouse, and landscaping applications. Gypsum can be used for a variety of purposes in agriculture and horticulture, which could be advantageous to users. There are currently no recognized standards that outline the broad best management practices for using gypsum in agricultural applications.
Drought tolerance is a complex phenomenon that involves defence mechanisms as well as stress-induced signal responses [114][115]. Drought stress triggers a number of physiological, biochemical, and molecular responses (Figure 1) that can be classified into six categories: drought escape [116], avoidance [117], tolerance [118], resistance [119], abandonment [120], and drought adaptation [12][121]. Some chickpea genotypes have been identified as drought sensitive [122] and others as drought tolerant [123][124]. Plant breeders apply different ways of selection and development of drought tolerant crop genotypes. Different strategies are important to protect plants from harmful effects of drought.
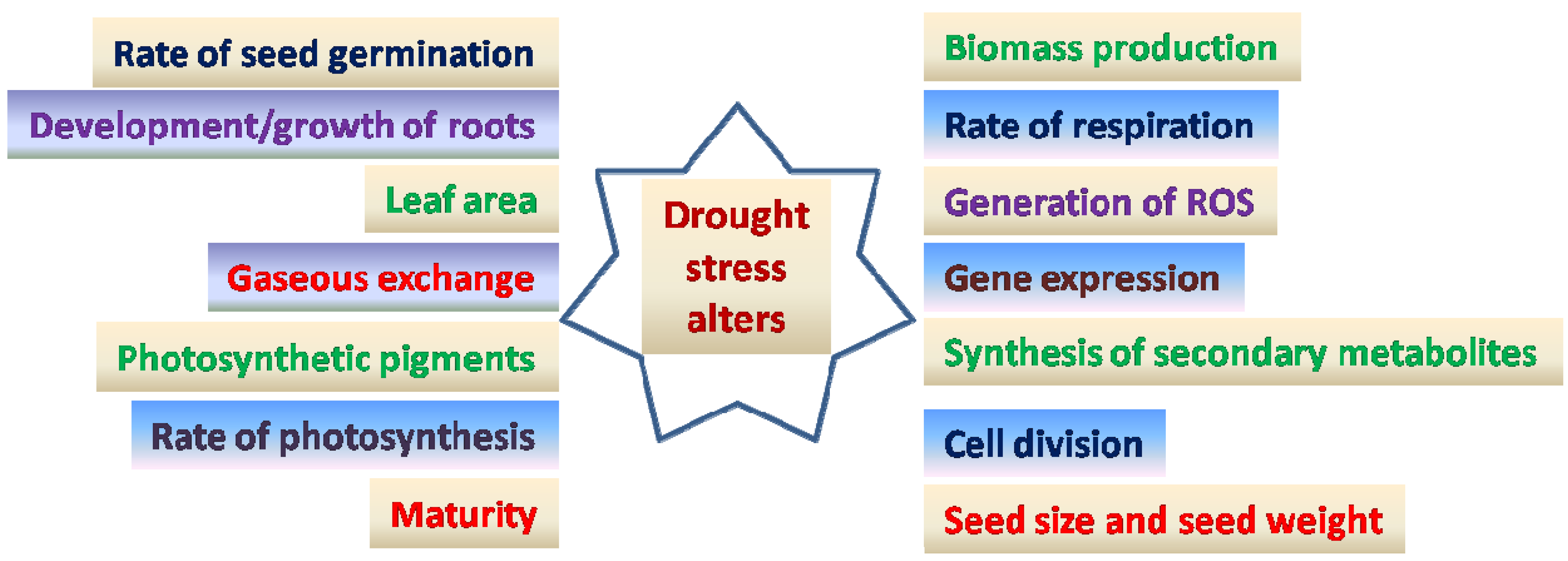
Figure 1. Diagrammatic representation of effects of drought stress on chickpea.
Drought escape is the capacity of the plant to complete its life cycle before experiencing a major water deficit. Drought escape causes early flowering and maturity, as well as better yield potential, allowing plants to finish reproduction before drought strikes [125]. Crop longevity is governed in part by genotype and in part by the environment, and it impacts the crop’s ability to withstand climatic conditions such as drought. To achieve large seed yields, it is necessary to match the plant growth time to soil moisture availability. The genotypes with early maturity have the capacity to escape the terminal drought stress, whereas the genotypes with late maturity generally needs well-watered environments. The length of the growing phase and yield potential are positively associated with each other. In this consequence, the development of shorter duration crop is important for the reliable management of drought stress in the chickpea. The timing of flowering is a key feature of a plant’s response to extreme drought and high temperatures [126]. Early maturity lets the crop circumvent the passé of stress, hence short duration cultivars may be produced to minimise production loss from terminal dryness. However, under ideal growth conditions, the yield is often associated with crop duration, and any decrease in crop interval underneath the optimal will tax yield [29][52].
Drought escape is a critical strategy for preventing chickpea crop from drought [12]. Water supply is harmonised with phenological development in drought escape. Early maturity aids in escaping terminal dryness and is a key feature in germplasm screening. However, growers are frequently incapable to reorganize for early planting owing to climatic factors [52].
Drought avoidance is explained as a plant’s aptitude to retain a high tissue water potential contempt in a lack of soil moisture [125]. Processes involved in the enhancement of water intake, its storage in plant cells, and limiting water loss are associated with drought avoidance. Other mechanisms, including deep rooting, increased level of hydraulic conductance, reduced level of epidermal conductance, radiation absorption and reduced leaf area have also been reported to be linked with drought avoidance in plants. Deep rooting promotes water intake, which is helpful in reducing water losses. In the chickpea, the stomata remains closed during the day to minimise water loss during drought, and as a result, the carbon assimilation is impeded, lowering production [126][127].
Root biomass plays major role in absorbing water [128], as it is advantageous even in the condition of less moisture in the soil. It means there is a linkage between the root system and water stress tolerance [129][130], thus, in the current scenario, breeders are focused in the development of cultivars with larger root systems [131]. From integrating large root features, cultivars have been developed by chickpea breeders with increased drought tolerance [43][132]. Because root size is governed by intrinsic genetic variables [133][134] and modified by multiple environmental signals, such as nutrition and moisture accessibility in the soil, it is a complicated feature [135]. During the vegetative growth stage, susceptible genotypes absorb more water than tolerant genotypes, whereas tolerant genotypes absorb more water during the reproductive stage [136]. The intake of water during the vegetative as well as reproductive stages of plants has a direct relation with seed yield [137]. The importance of roots, rather than just root growth, is determined by their temporal water intake [138]. The best method for screening the germplasm for water usage competence (WUE) is carbon isotope discernment (13C), and this method has also been adopted in the chickpea [139][140].
One of the important impacts of drought stress is stomatal closure. Drought stress reduces the stomatal conductance and transpiration rate. This declines the CO2 fixation and photosynthesis due to the reduction in the internal CO2 concentration of the leaf (Ci). All of these factors have their role on the reduction in yield due to the reduced rate of photosynthesis [141].
The reduced rate of photosynthesis is directly related to extreme drought stress, and it is a result of the decreased chlorophyll content. Because of the lowered chlorophyll content, continuous poor moisture availability reduces light collecting capacity, triggering the generation of reactive oxygen species due to excessive energy absorption [142]. This is also a cause of damaged photosynthetic machinery. The principal cause of chlorophyll depletion is reactive oxygen species [143]. Reduction in photosynthetic activities under drought stress, have been experimented in chickpea genotypes [55] and this reduction was found to be linked to reduced ATP synthesis [144][145]. The yield reduction in chickpea genotypes due to the flower and pod drop under heat and drought stress circumstances was also noticed [146][147].
The leaf surface is also an imperative characteristic of plants in relation to drought stress. As tiny leaf surfaces lose less water [148], waxy leaves have high water preservation potential. Waxy leaves have the ability of a reflectance of irradiation and the reduction of water loss. This helps in the reduction of leaf temperature and provides tolerance against drought condition. The preservation of water in leaves with a reduced leaf temperature are directly related to the drought tolerant behaviour of plants. Drought stress raises leaf temperature in a variable manner, as tolerance genotypes have lower leaf temperatures than sensitive genotypes [149]. One drought tolerant chickpea variety ‘Gokce’ has been developed by ICARDA through the gene pyramiding method, which can be survived under severe drought conditions. This variety possess some other important features, such as early maturity, resistance to Ascochyta blight, increased seed size, and suitability for mechanised harvesting [150].
The drought tolerance refers to a plant’s ability to maintain its metabolism in a water shortage [35] condition with low tissue water potential [58]. Two types of traits are responsible for the drought tolerance in plants, i.e., constitutive characters and acquired behaviours. The constitutive traits affect the yield at mild to moderate levels of drought stress, whereas the acquired traits affect the yield at severe levels of drought stress. Drought tolerance features are largely concerned with cellular structural protection against the effects of cellular dehydration. Due to a reduction in the plant tissue water content, dehydrins and late embryogenesis of abundant (LEA) proteins accumulate [151]. These proteins act as chaperones [152].
In recent years, the role of reactive oxygen species (ROS) in stress signalling has been widely researched and evaluated [153][154]. The extreme creation of ROS causes oxidative damage and, lastly, cell death [155]. The role of ROS as a signalling molecule or in the oxidative damage depends upon the equilibrium between production and the scavenging of them [156]. The scavenging of ROS under drought stress depends upon the action of antioxidants in the cell [157][158].
Pushpavalli et al. [159] emphasised the need of selecting chickpea genotypes that can withstand various shocks rather than simply one. High temperature stress, in addition to drought stress, is a new threat to chickpea production [33][160]. According to Kalra et al. [161], a temperature increase of 18 °C above a particular threshold causes a significant loss in chickpea output. Furthermore, it is predicted that a global temperature increase of 2–38 °C, along with erratic rainfall patterns, would pose a threat to chickpea yield. In agriculture, the yield is the most important parameter for crops, and a reduction in yield cannot be compromised at any level. There is a strong association between drought tolerance with yield in a crop [122][162]. This is because yield-related traits of crops have been found to be sensitive under drought stress [163].
References
- Madurapperumage, A.; Tang, L.; Thavarajah, P.; Bridges, W.; Shipe, E.; Vandemark, G.; Thavarajah, D. Chickpea (Cicer arietinum L.) as a Source of Essential Fatty Acids–A Biofortification Approach. Front. Plant Sci. 2021, 12, 734980.
- Singh, A.; Nath, O.; Singh, S.; Kumar, S.; Singh, I.K. Genome-wide identification of the MAPK gene family in chickpea and expression analysis during development and stress response. Plant Gene 2018, 13, 25–35.
- Varshney, R.K.; Gaur, P.M.; Chamarthi, S.K.; Krishnamurthy, L.; Tripathi, S.; Kashiwagi, J.; Samineni, S.; Singh, V.K.; Thudi, M.; Jaganathan, D. Fast-track introgression of ‘QTL-hotspot’ for root traits and other drought tolerance traits in JG 11, an elite and leading variety of chickpea. Plant Genome 2013, 6, 3.
- Directorate of Economics and Statistics. 2019. Available online: https://eands.dacnet.nic.in/ (accessed on 20 October 2022).
- Ganjeali, A.; Porsa, H.; Bagheri, A. Assessment of Iranian chickpea (Cicer arietinum L.) germplasms for drought tolerance. Agric. Water Manag. 2011, 98, 1477–1484.
- Gaur, P.M.; Samineni, S.; Thudi, M.; Tripathi, S.; Sajja, S.B.; Jayalakshmi, V.; Mannur, D.M.; Vijayakumar, A.G.; Ganga Rao, N.V.; Ojiewo, C.; et al. Integrated breeding approaches for improving drought and heat adaptation in chickpea (Cicer arietinum L.). Plant Breed. 2019, 138, 389–400.
- Gaur, R.; Verma, S.; Pradhan, S.; Ambreen, H.; Bhatia, S. A high-density SNP-based linkage map using genotyping-by-sequencing and its utilization for improved genome assembly of chickpea (Cicer arietinum L.). Funct. Integr. Genom. 2020, 20, 763–773.
- Sahu, V.K.; Tiwari, S.; Gupta, N.; Tripathi, M.K.; Yasin, M. Evaluation of physiological and biochemical contents in desi and Kabuli chickpea. Legume Res. 2020, 45, 1197–1208.
- Kumar, T.; Hamwieh, A.; Swain, N.; Sarker, A. Identification and morphological characterization of promising kabuli chickpea genotypes for short-season environment in central India. J. Genet. 2021, 100, 33.
- Tahir, N.A.R.; Karim, H.F.H. Impact of magnetic application on the parameters related to growth of chickpea (Cicer arietinum L.). Jordan J. Biol. Sci. 2010, 3, 175–184.
- Mohammed, A.; Tana, T.; Singh, P.; Korecha, D.; Mollad, A. Management options for rainfed chickpea (Cicer arietinum L.) in northeast Ethiopia under climate change condition. Clim. Risk Manag. 2017, 16, 222–233.
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.M.; Nayyar, H. Developing Climate-Resilient Chickpea Involving Physiological and Molecular Approaches with a Focus on Temperature and Drought Stresses. Front. Plant Sci. 2020, 10, 1759.
- Arif, A.; Parveen, N.; Waheed, M.Q.; Atif, R.M.; Waqar, I.; Shah, T.M. A Comparative Study for Assessing the Drought-Tolerance of Chickpea Under Varying Natural Growth Environments. Front. Plant Sci. 2021, 11, 607869.
- Kumar, J.; Abbo, S. Genetics of flowering time in chickpea and its bearing on productivity in semiarid environments. Adv. Agron. 2001, 72, 107–138.
- Singh, K.B.; Malhotra, R.S.; Halila, M.H.; Knights, E.J.; Verma, M.M. Current status and future strategy in breeding chickpea for resistance to biotic and abiotic stresses. Euphytica 1994, 73, 137–149.
- Millan, T.; Clarke, H.J.; Siddique, K.H.; Buhariwalla, H.K.; Gaur, P.M.; Kumar, J.; Gil, J.; Kahl, G.; Winter, P. Chickpea molecular breeding: New tools and concepts. Euphytica 2006, 147, 81–103.
- Jameel, S.; Hameed, A.; Shah, T.M. Investigation of Distinctive Morpho-Physio and Biochemical Alterations in Desi Chickpea at Seedling Stage Under Irrigation, Heat, and Combined Stress. Front. Plant Sci. 2021, 12, 692745.
- Sachdeva, S.; Bharadwaj, C.; Patil, B.S.; Pal, M.; Roorkiwal, M.; Varshney, R.K. Agronomic Performance of Chickpea Affected by Drought Stress at Different Growth Stages. Agronomy 2022, 12, 995.
- Grewal, S.K.; Sharma, K.P.; Bharadwaj, R.D.; Hegde, V.; Tripathi, S.; Singh, S.; Jain, P.K.; Agrawal, P.K.; Mondal, B. Understanding genotypic variation and identification of promising genotypes for iron and zinc content in chickpea (Cicer arietinum L.). J. Food Compos. Anal. 2020, 88, 103458.
- Sahu, V.K.; Tiwari, S.; Tripathi, M.K.; Gupta, N.; Tomar, R.S.; Yasin, M. Morpho-physiological and biochemical traits analysis for Fusarium wilt disease using gene-basedmarkers in desi and Kabuli genotypes of chickpea (Cicerarietinum L.). Indian J. Genet. 2020, 80, 163–172.
- Singh, S.; Babu, K.S.; Arora, A.; Panwar, R.K.; Verma, S.K. Genetic studies for biofortification traits in chickpea. J. Food Legumes 2021, 34, 17–20.
- Wallace, T.C.; Murray, R.; Zelman, K.M. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766.
- Iqbal, A.; Ateeq, N.; Khalil, I.A.; Perveen, S.; Saleemullah, S. Physicochemical characteristics and amino acid profile of chickpea cultivars grown in Pakistan. J. FoodSer. 2006, 17, 94–101.
- Singh, S.; Singh, D.; Rao, V.U.M. Seedling establishment of chickpea cultivars in varying sowing environments under field conditions. J. Agrometeorol. 2009, 11, 148–151.
- Hirdyani, H. Nutritional composition of Chickpea (Cicer arietinum-L) and value added products. Indian J. Community Health Haryana J. Agron. 2014, 4, 116–118.
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Chickpea: Nutritional properties and its benefits. Br. J. Nutr. 2012, 108, S11–S26.
- Samineni, S.; Mahendrakar, M.D.; Shankar, N.; Hotti, A.; Chand, U.; Rathore, A.; Gaur, P.M. Impact of heat and drought stresses on grain nutrient content in chickpea: Genome-wide marker-trait associations for protein, Fe and Zn. Environ. Exp. Bot. 2022, 194, 104688.
- Korbu, L.; Fikre, A.; Tesfaye, K.; Funga, A.; Bekele, D.; Ojiewo, C.O. Response of chickpea to varying moisture stress conditions in Ethiopia. Agrosyst. Geosci. Environ. 2022, 5, e20234.
- Marjani, A.; Farsi, M.; Hervan, E.M.; Ganjeali, A. Comparative analysis of LEA and Dehydrin genes in response to drought stress in chickpea phonological different. Int. J. Biosci. 2014, 4, 49–57.
- Krishnamurthy, L.; Kashiwagi, J.; Gaur, P.M.; Upadhyaya, H.D.; Vadez, V. Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crops Res. 2010, 119, 322–330.
- Upadhyaya, H.D.; Kashiwagi, J.; Varshney, R.K.; Gaur, P.M.; Saxena, K.B.; Krishnamurthy, L.; Gowda, C.L.L.; Pundir, R.P.S.; Chaturvedi, S.K.; Basu, P.S.; et al. Phenotyping chickpeas and pigeonpeas for adaptation to drought. Front. Physiol. 2012, 3, 179.
- Awasthi, R.; Gaur, P.; Turner, N.C.; Vadez, V.; Siddique, K.H.M.; Nayyar, H. Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer arietinum) genotypes differing in heat and drought tolerance. Crop Pasture Sci. 2017, 68, 823–841.
- Devasirvatham, V.; Tan, D.K.Y. Impact of High Temperature and Drought Stresses on Chickpea Production. Agronomy 2018, 8, 145.
- Choudhary, M.L.; Tripathi, M.K.; Gupta, N.; Tiwari, S.; Tripathi, N.; Parihar, P.; Pandya, R.K. Screening of pearl millet R Br] germplasm lines against drought tolerance based on biochemical traits. Curr. J. Appl. Sci. Technol. 2021, 40, 1–12.
- Choudhary, M.L.; Tripathi, M.K.; Tiwari, S.; Pandya, R.K.; Gupta, N.; Tripathi, N.; Parihar, P. Screening of pearl millet germplasm lines for drought tolerance based on morpho-physiological traits and SSR markers. Curr. J. Appl. Sci. Technol. 2021, 40, 46–63.
- Raddi, S.; Giannetti, F.; Martini, S.; Farinella, F.; Chirici, G.; Tani, A.; Maltoni, A.; Mariotti, B. Monitoring drought response and chlorophyll content in Quercus by consumer-grade, near-infrared (NIR) camera: A comparison with reflectance spectroscopy. New For. 2022, 53, 241–265.
- Sapes, A.; Sala, G. Relative water content consistently predicts drought mortality risk in seedling populations with different morphology, physiology and times to death. Plant Cell Environ. 2021, 44, 3322–3335.
- Maqbool, M.A.; Aslam, M.; Ali, H.; Shah, T.M.; Farid, B.; Zaman, Q.U. Drought tolerance indices-based evaluation of chickpea advanced lines under different water treatments. Res. Crops 2015, 16, 336–344.
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Krishna, H.; Chandra, S.; Vadez, V.; Serraj, R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpeas (Cicer arietinum L). Euphytica 2005, 146, 213–222.
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Gaur, P.M. Rapid screening technique for canopy temperature status and its relevance to drought tolerance improvement in chickpea. J. SAT Agric. Res. 2008, 6, 1–4.
- Krishnamurthy, L.; Kashiwagi, J.; Upadhyaya, H.D.; Serraj, R. Genetic diversity of drought-avoidance root traits in the mini-core germplasm collection of chickpeas. Int. Chick. Pigeonpea Newslett. 2003, 10, 21–24.
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.). Field Crops Res. 2017, 201, 146–161.
- Kumar, N.; Soren, K.R.; Bharadwaj, C.; Sneha Priya, P.R.; Shrivastava, A.K.; Pal, M.; Roorkiwal, M.; Kumar, K.; Patil, B.S.; Soni, A.; et al. Genome-wide transcriptome analysis and physiological variation modulates gene regulatory networks acclimating salinity tolerance in chickpea. Environ. Exp. Bot. 2021, 187, 104478.
- Hosseinzadeh, S.R.; Amiri, H.; Ismaili, A. Evaluation of photosynthesis, physiological, and biochemical responses of chickpea (Cicer arietinum L. cv. Pirouz) under water deficit stress and use of vermicompost fertilizer. J. Integrat. Agric. 2018.
- Hussain, T.; Akram, Z.; Shabbir, G.; Manaf, A.; Ahmed, M. Identification of drought tolerant Chickpea genotypes through multi trait stability index. Saudi J. Biol. Sci. 2021, 28, 6818–6828.
- Salahvarzi, M.; Nasr Esfahani, M.; Shirzadi, N.; Burritt, D.J.; Tran, L.P. Genotype- and tissue-specific physiological and biochemical changes of two chickpea (Cicer arietinum) varieties following a rapid dehydration. Physiol. Plant 2021, 172, 1822–1834.
- Thudi, M.; Upadhyaya, H.D.; Rathore, A.; Gaur, P.M.; Krishnamurthy, L.; Roorkiwal, M.; Nayak, S.N.; Chaturvedi, S.K.; Basu, P.S.; Gangarao, N.V.P.R.; et al. Genetic dissection of drought and heat tolerance in chickpea through genomewide and candidate gene-based association mapping approaches. PLoS ONE 2014, 9, e96758.
- Palit, P.; Ghosh, R.; Tolani, P.; Tarafdar, A.; Chitikineni, A.; Bajaj, P.; Sharma, M.; Kudapa, H.; Varshney, R.K. Molecular and Physiological Alterations in Chickpea under Elevated CO2 Concentrations. Plant Cell Physiol. 2020, 61, 1449–1463.
- Kanca, O.; Bellen, H.J.; Schnorrer, F. Gene Tagging Strategies to Assess Protein Expression, Localization, and Function in Drosophila. Genetics 2017, 207, 389–412, Erratum in Genetics 2017, 207, 1711.
- Santiago, C.R.d.N.; Assis, R.d.A.B.; Moreira, L.M.; Digiampietri, L.A. Gene Tags Assessment by Comparative Genomics (GTACG): A User-Friendly Framework for Bacterial Comparative Genomics. Front. Genet. 2019, 10, 725.
- Wanga, M.A.; Shimelis, H.; Mashilo, J.; Laing, M.D. Opportunities and challenges of speed breeding: A review. Plant Breed. 2021, 140, 185–194.
- Ali, Z.; Merrium, S.; Habib-ur-Rahman, M.; Hakeem, S.; Saddique, M.A.B.; Sher, M.A. Wetting mechanism and morphological adaptation; leaf rolling enhancing atmospheric water acquisition in wheat crop—A review. Environ. Sci. Pollut. Res. 2022, 29, 30967–30985.
- Ray, S.; Satya, P. Next generation sequencing technologies for next generation plant breeding. Front. Plant Sci. 2014, 5, 367.
- Verma, S.; Gupta, S.; Bandhiwal, N.; Kumar, T.; Bharadwaj, C.; Bhatia, S. High-density linkage map construction and mapping of seed trait QTLs in chickpea (Cicer arietinum L.) using Genotyping-by-Sequencing (GBS). Sci. Rep. 2015, 5, 17512.
- Sahu, P.K.; Sao, R.; Mondal, S.; Vishwakarma, G.; Gupta, S.K.; Kumar, V.; Singh, S.; Sharma, D.; Das, B.K. Next Generation Sequencing Based Forward Genetic Approaches for Identification and Mapping of Causal Mutations in Crop Plants: A Comprehensive Review. Plants 2020, 9, 1355.
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63.
- Kaur, P.; Gaikwad, K. From Genomes to GENE-omes: Exome Sequencing Concept and Applications in Crop Improvement. Front. Plant Sci. 2017, 8, 2164.
- Leo, V.C.; Morgan, N.V.; Bern, D.; Jones, M.L.; Lowe, G.C.; Lordkipanidzé, M.; Drake, S.; Simpson, M.A.; Gissen, P.; Mumford, A.; et al. Use of next-generation sequencing and candidate gene analysis to identify underlying defects in patients with inherited platelet function disorders. J. Thromb. Haemost. 2015, 13, 643–650.
- Kulski, J.K.; Suzuki, S.; Ozaki, Y.; Mitsunaga, S.; Inoko, H.; Shiina, T. Phase HLA genotyping by next generation sequencing—A comparison between two massively parallel sequencing bench-top systems, the Roche GS Junior and Ion Torrent PGM. In HLA and Associated Important Diseases; Xi, Y., Ed.; Intech.: Rijeka, Croatia, 2014; pp. 141–181.
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver from Short-Term Rat Toxicity Studies. Front. Genet. 2019, 9, 636.
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457.
- Wang, B.; Kumar, V.; Olson, A.; Ware, D. Reviving the Transcriptome Studies: An Insight into the Emergence of Single-Molecule Transcriptome Sequencing. Front. Genet. 2019, 10, 384.
- Bhaskarla, V.; Zinta, G.; Ford, R.; Jain, M.; Varshney, R.K.; Mantri, N. Comparative Root Transcriptomics Provide Insights into Drought Adaptation Strategies in Chickpea (Cicer arietinum L.). Int. J. Mol. Sci. 2020, 21, 1781.
- Upasani, M.L.; Limaye, B.M.; Gurjar, G.S.; Kasibhatla, S.M.; Joshi, R.R.; Kadoo, N.Y.; Gupta, V.S. Chickpea-Fusarium oxysporum interaction transcriptome reveals differential modulation of plant defense strategies. Sci. Rep. 2017, 7, 7746.
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196.
- Ahrens, C.H.; Brunner, E.; Qeli, E.; Basler, K.; Aebersold, R. Generating and navigating proteome maps using mass spectrometry. Nat. Rev. Mol. Cell Biol. 2010, 11, 789–801.
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50.
- Mishra, N.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Gupta, N.; Sharma, A. Morphological and physiological performance of Indian soybean genotypes in respect to drought. Legume Res. Int. J. 2021.
- Mishra, N.; Tripathi, M.K.; Tripathi, N.; Tiwari, S.; Gupta, N.; Sharma, A.; Shrivastav, M.K. Changes in biochemical and antioxidant enzymes activities play significant role in drought tolerance in soybean. Int. J. Agric. Technol. 2021, 17, 1425–1446.
- Atta, K.; Singh, A.P.; Adhikary, S.; Mondal, S.; Dewanjee, S. Drought Stress: Manifestation and Mechanisms of Alleviation in Plants. In Drought ; Eyvaz, A.P.M., Albahnasawi, A., Tekbaş, M.M., Gürbulak, M.E., Eds.; IntechOpen: London, UK, 2022; Available online: https://www.intechopen.com/online-first/80834 (accessed on 30 April 2022).
- Mishra, N.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Ahuja, A.; Sapre, S.; Tiwari, S. Cell suspension culture and in vitro screening for drought tolerance in soybean using poly-ethylene glycol. Plants 2021, 10, 517.
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117.
- Toker, C.; Canci, H. Selection for drought and heat resistance in chickpea under terminal drought conditions. In Food Legumes for Nutritional Security and Sustainable Agriculture: 4th International Food Legumes Research Conference; Kharkwal, M.C., Ed.; Indian Agricultural Research Institute: New Delhi, India, 2006; pp. 18–22.
- Varshney, R.K.; Thudi, M.; Pandey, M.K.; Tardieu, F.; Ojiewo, C.; Vadez, V.; Whitbread, A.M.; Siddique, K.H.M.; Nguyen, H.T.; Carberry, P.S.; et al. Accelerating genetic gains in legumes for the development of prosperous smallholder agriculture: Integrating genomics, phenotyping, systems modelling and agronomy. J. Exp. Bot. 2018, 69, 3293–3312.
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Res 2016, 5, F1000 Faculty Rev-1554.
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442.
- Martignago, D.; Rico-Medina, A.; Blasco-Escámez, D.; Fontanet-Manzaneque, J.B.; Caño-Delgado, A.I. Drought Resistance by Engineering Plant Tissue-Specific Responses. Front. Plant Sci. 2020, 10, 1676.
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654.
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2012, 132, 21–32.
- Keskin, M.E.; Terzi, Ö.; Taylan, E.D.; Küçükyaman, D. Meteorological drought analysis using artificial neural networks. Acad. J. 2011, 6, 4469–4477.
- Berger, J.; Palta, J.; Vadez, V. An integrated framework for crop adaptation to dry environments: Responses to transient and terminal drought. Plant Sci. 2016, 253, 58–67.
- Maliro, M.F.A.; MacNeil, D.; Redden, B.; Kollmorgen, J.F.; Pittock, C. Sampling strategies and screening of chickpea (Cicerarietinum L.) germplasm for salt tolerance. Genet. Resour. Crop. Evol. 2008, 55, 53–63.
- Kashiwagi, J.; Krishnamurthy, L.; Purushothaman, R.; Upadhyaya, H.D.; Gaur, P.M.; Gowda, C.L.L.; Ito, O.; Varshney, R.K. Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). Field Crops Res. 2015, 170, 47–54.
- Serraj, R.; Sinclair, T.R. Osmolyte Accumulation: Can It Really Help Increase Crop Yield under Drought Conditions? Plant Cell Environ. 2002, 25, 333–341.
- Goulet, B.E.; Roda, F.; Hopkins, R. Hybridization in Plants: Old Ideas, New Techniques. Plant Physiol. 2017, 173, 65–78.
- Purushothaman, R.; Krishnamurthy, L.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Shoot traits and their relevance in terminal drought tolerance of chickpea (Cicer arietinum L.). Field Crop. Res. 2016, 197, 10–27.
- Leport, L.; Turner, N.C.; Davies, S.L.; Siddique, K.H.M. Variation in pod production and abortion among chickpea cultivars under terminal drought. Eur. J. Agron. 2006, 24, 236–246.
- Nayyar, H.; Bains, T.; Kumar, S. Low temperature induced floral abortion in chickpea: Relationship to abscisic acid and cryoprotectants in reproductive organs. Environ. Exp. Bot. 2005, 53, 39–47.
- Rahbarian, R.; Nejad, R.K.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietınum L.) genotypes. Acta Biol. Crac. Ser. Bot. 2011, 53, 47–56.
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930.
- Kashiwagi, J.; Krishnamurty, L.; Gaur, P.M.; Chandra, S.; Upadhyaya, H.D. Estimation of gene effects of the drought avoidance root characteristicsin chickpea (C. arietinum L.). Field Crops Res. 2008, 105, 64–69.
- Purushothaman, R.; Thudi, M.; Krishnamurthy, L.; Upadhyaya, H.D.; Kashiwagi, J.; Gowda, C.L.L.; Varshney, R.K. Association of mid-reproductive stage canopy temperature depression with the molecular markers and grain yields of chickpea (Cicer arietinum L.) germplasm under terminal drought. Field Crops Res. 2015, 174, 1–11.
- Sofi, P.A.; Ara, A.; Gull, M.; Rehman, K. Canopy Temperature Depression as an Effective Physiological Trait for Drought Screening. In Drought-Detection and Solutions; IntechOpen: London, UK, 2019.
- Karimzadeh, S.H.; Nezami, A.; Nabati, J.; Oskoueian, E.; Ahmadi-Lahijani, M.J. The physiological, biochemical, and molecular modifications of chickpea (Cicer arietinum L.) seedlings under freezing stress. J. Plant Growth Regul. 2021, 41, 1109–1124.
- Sivasakthi, K.; Tharanya, M.; Kholová, J.; Wangari Muriuki, R.; Thirunalasundari, T.; Vadez, V. Chickpea Genotypes Contrasting for Vigor and Canopy Conductance Also Differ in Their Dependence on Different Water Transport Pathways. Front. Plant Sci. 2017, 8, 1663.
- Krishnamurthy, L.; Kashiwagi, J.; Tobita, S.; Ito, O.; Upadhyaya, H.D.; Gowda, C.L.L.; Gaur, P.M.; Sheshshayee, M.S.; Singh, S.; Vadez, V.; et al. Variation in carbon isotope discrimination and its relationship with harvest index in the reference collection of chickpea germplasm. Funct. Plant Biol. 2013, 14, 1350–1361.
- Ma, Q.; Behboudian, M.H.; Turner, N.C.; Palta, J.A. Gas exchange by pods and subtending leaves and internal recycling of internal CO2 by pods of chickpea (Cicer arietinum L.) subjected to water stess. J. Exp. Bot. 2001, 52, 123–131.
- Macar, T.K.; Ekmekci, Y. Alterations in Photochemical and Physiological Activities of Chickpea (Cicer arietinum L.) Cultivars under Drought Stress. J. Agron. Crop. Sci. 2009, 195, 335–346.
- Shayla, B.; Inderjit, S.; Satinder, S.; Ashutosh, K.S.G.B.; Sonia, S.; Karan, K.; Kaur, G.S.C.B.; Harsh, N.; Sarvjeet, S. Use of morpho-physiological and biochemical traits to identify sources of drought and heat tolerance in chickpea (Cicer arietinum). Crop. Pasture Sci. 2021, 72, 801–814.
- Talebi, R.; Ensafi, M.H.; Baghebani, N.; Karami, E.; Mohammadi, K. Physiological responses of chickpea (Cicer arietinum) genotypes to drought stress. Environ. Exp. Biol. 2013, 11, 9–15.
- Jangpromma, N.; Songsri, P.; Thammasirirak, S.; Jaisil, P. Rapid assessment of chlorophyll content in sugarcane using a SPAD chlorophyll meter across different water stress conditions. Asian J. Plant Sci. 2010, 9, 368–374.
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26.
- Devasirvatham, V.; Tan, D.K.Y.; Gaur, P.M.; Raju, T.N.; Trethowan, R.M. High temperature tolerance in chickpea and its implications for plant improvement. Crop. Pasture Sci. 2012, 63, 419–428.
- Chen, Y.; Ghanem, M.E.; Siddique, K.H. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2017, 68, 1987–1999.
- Badhan, S.; Kole, P.; Ball, A.; Mantri, N. RNA sequencing of leaf tissues from two contrasting chickpea genotypes reveals mechanisms for drought tolerance. Plant Physiol. Biochem. 2018, 129, 295–304.
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590.
- Stefaniak, T.; McPhee, K. Comparison of Hybridization Techniques in Chickpea. Crop. Sci. 2017, 57, 843–846.
- Deokar, A.A.; Tar’an, B. Genome-wide analysis of the aquaporin gene family in chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 1802.
- Deokar, A.A.; Kondawar, V.; Kohli, D.; Aslam, M.; Jain, P.K.; Karuppayil, S.M.; Varshney, R.K.; Srinivasan, R. The CarERF genes in chickpea (Cicerarietinum L.) and the identification of CarERF116 as abiotic stress responsive transcription factor. Funct. Integr. Genom. 2015, 15, 27–46.
- Hamwieh, A.; Imtiaz, M.; Malhotra, R.S. Multi-environment QTL analyses for drought-related traits in a recombinant inbred population of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2013, 126, 1025–1038.
- Singh, V.K.; Khan, A.W.; Jaganathan, D.; Thudi, M.; Roorkiwal, M.; Takagi, H.; Garg, V.; Kumar, V.; Chitikineni, A.; Gaur, P.M.; et al. QTL-seq for rapid identification of candidate genes for 100-seed weight and root/total plant dry weight ratio under rainfed conditions in chickpea. Plant. Biotechnol. J. 2016, 14, 2110–2119.
- Kudapa, H.; Garg, V.; Chitikineni, A.; Varshney, R.K. The RNA-Seqbased high resolution gene expression atlas of chickpea (Cicer arietinum L.) reveals dynamic spatiotemporal changes associated with growth and development. Plant Cell Environ. 2018, 41, 2209–2225.
- Kumar, M.; Chauhan, A.S.; Yusuf, M.A.; Sanyal, I.; Chauhan, P.S. Transcriptome sequencing of chickpea (Cicer arietinum L.) genotypes for identifcation of drought-responsive genes under drought stress condition. Plant Mol. Biol. Rep. 2019, 37, 186–203.
- Gupta, S.; Singh, A.; Singh, P.; Kewat, R.N. Effect of drought stress or carbohydrate content in drought tolerant and susceptible chickpea genotypes. J. Crop. Sci. Biotechnol. 2015, 4, 35–38.
- Sachdeva, S.; Bharadwaj, C.; Singh, R.K.; Jain, P.K.; Patil, B.S.; Roorkiwal, M.; Varshney, R. Characterization of ASR gene and its role in drought tolerance in chickpea (Cicer arietinum L.). PLoS ONE 2020, 15, e0234550.
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162.
- Siddique, K.H.M.; Loss, S.P.; Thomson, B.D. Cool season grain legumes in dryland Mediterranean environments of Western Australia: Significance of early flowering. In Management of Agricultural Drought; Saxena, N.P., Ed.; Science Publishers: Enfield, NH, USA, 2003; pp. 151–161.
- Saeed, M.; Francis, C.A. Yield stability in relation to maturity in grain sorghum. Crop. Sci. 1983, 23, 683–687.
- Richards, M.F.; Maphosa, L.; Preston, A.L. Impact of Sowing Time on Chickpea (Cicer arietinum L.) Biomass Accumulation and Yield. Agronomy 2022, 12, 160.
- Shimray, P.U.; Bajaj, D.; Shrivastava, R.; Daware, A.; Upadhyaya, H.D.; Kumar, R.; Bhardwaj, C.; Tyagi, A.K.; Parida, S.K. Identifying Transcription Factor Genes Associated with Yield Traits in Chickpea. Plant Mol. Biol. Rep. 2017, 35, 562–574.
- Sabaghpour, S.H.; Kumar, J.; Rao, T.N. Inheritance of growth vigor and its association with other characters in chickpea. Plant Breed. 2003, 122, 542–544.
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Gao, W.; Qu, Y. Screening of Key Drought Tolerance Indices for Cotton at the Flowering and Boll Setting Stage Using the Dimension Reduction Method. Front. Plant Sci. 2021, 12, 619926.
- Sabaghpour, S.H.; Mahmodi, A.A.; Saeed, A.; Kamel, M.; Malhotra, R.S. Study on chickpea drought tolerance lines under dryland condition of Iran. Indian J. Crop. Sci. 2006, 1, 70–73.
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969.
- Levitt, J. Responses of Plants to Environmental Stresses. Volume II. Water, Radiation, Salt, and Other Stresses; Academic Press: Cambridge, MA, USA, 1980.
- Kamanga, R.M.; Mbega, E.; Ndakidemi, P. Drought Tolerance Mechanisms in Plants: Physiological Responses Associated with Water Deficit Stress in Solanum lycopersicum. Adv. Crop. Sci. Technol. 2018, 6, 362.
- Pastori, G.; Foyer, C.H.; Mullineaux, P. Low temperature-induced changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. J. Exp. Bot. 2000, 51, 107–113.
- Li, H.; Ma, X.; Lu, Y.; Ren, R.; Cui, B.; Si, B. Growing deep roots has opposing impacts on the transpiration of apple trees planted in subhumid loess region. Agric. Water Manag. 2021, 258, 107207.
- Maqbool, M.A.; Aslam, M.; Ali, H.; Shah, T.M.; Atta, B.M. GGE biplot analysis-based selection of superior chickpea (Cicerarietinum L.) inbred lines under variable water environments. Pak. J. Bot. 2015, 47, 1901–1908.
- Sohrabi, Y.; Heidari, G.; Weisany, W.; Ghasemi Golezani, K.; Mohammadi, K. Some physiological responses of chickpea cultivars to arbuscular mycorrhiza under drought stress. Russ. J. Plant Physiol. 2012, 59, 708–716.
- Kashiwagi, J.; Krishnamurthy, L.; Crouch, J.H.; Serraj, R. Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L) under terminal drought stress. Field Crops Res. 2006, 95, 171–181.
- Gaur, P.M.; Krishnamurthy, L.; Kashiwagi, J. Improving drought avoidance root traits in chickpea (Cicer arietinum L.) current status of research at ICRISAT. Plant Prod. Sci. 2008, 11, 3–11.
- Davies, W.; Zhang, J. Root signals and the regulation of growth and development of plants in dry soil. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76.
- Pierik, R.; Sasidharan, R.; Voesenek, L.A.C.J. Growth control by ethylene: Adjusting phenotypes to the environment. J. Plant Growth Regul. 2007, 26, 188–200.
- Zaman-Allah, M.; Jenkinson, D.M.; Vandez, V. Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Funct. Plant Biol. 2011, 38, 270–281.
- Pang, J.; Turner, N.C.; Du, Y.-L.; Colmer, T.D.; Siddique, K.H.M. Pattern of Water Use and Seed Yield under Terminal Drought in Chickpea Genotypes. Front. Plant Sci. 2017, 8, 1375.
- Zaman-Allah, M.; Jenkinson, D.M.; Vandez, V. A conservative pattern of water use, rather than deep or profuse rooting, is critical for terminal drought tolerance of chickpea. J. Exp. Bot. 2011, 38, 270–281.
- Kashiwagi, J.; Krishnamurthy, L.; Singh Sube Gaur, P.M.; Upadhyaya, H.D.; Panwar, J.D.S.; Basu, P.S.; Ito, O.; Tobita, S. Relationship between transpiration efficiency and carbon isotope discrimination in chickpea (Cicer arietinum L.). J. SAT Agric. Res. 2006, 2, 1–3.
- Nadal-Sala, D.; Grote, R.; Birami, B.; Knüver, T.; Rehschuh, R.; Schwarz, S.; Ruehr, N.K. Leaf Shedding and Non-Stomatal Limitations of Photosynthesis Mitigate Hydraulic Conductance Losses in Scots Pine Saplings During Severe Drought Stress. Front. Plant Sci. 2021, 12, 715127.
- Li, Y.; Li, H.; Li Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop. J. 2017, 5, 231–239.
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189.
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188.
- Flexas, J.; Galmes, J.; Ribas-Carbo, M.; Medrano, H. The effects of water stress on plant respiration. In Plant Respiration: From Cell to Ecosystem; Lambers, H., Ribas-Carbo, M., Eds.; Advances in Photosynthesis and Respiration Series; Springer: Dordrecht, The Netherlands, 2005; Chapter 6; Volume 18, pp. 85–94.
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop. Sci. 2017, 203, 81–102.
- Xiao, Q.; Bai, X.; Zhang, C.; He, Y. Advanced high-throughput plant phenotyping techniques for genome-wide association studies: A review. J. Adv. Res. 2022, 35, 215–230.
- Nayyar, H.; Singh, S.; Kaur, S.; Kumar, S.; Upadhyaya, H.D. Differential sensitivity of macrocarpa and microcarpa types of chickpea (Cicer arietinum L.) to water stress: Association of contrasting stress response with oxidative injury. J. Integr. Plant Biol. 2006, 48, 1318–1329.
- Masle, J.; Gilmore, S.R.; Farquhar, G.D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 2005, 436, 866–870.
- Saxena, N.P. Management of drought in chickpea-holistic approach. In Management of Agricultural Drought-Agronomic and Genetic Options; Saxena, N.P., Ed.; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 2003; pp. 103–122.
- Hemati, A.; Mofidi-Chelan, M.; Amirifar, A.; Moghiseh, E.; Asgari Lajayer, B. Drought Tolerance Mechanisms in Crop Plants. In Plant Stress Mitigators; Vaishnav, A., Arya, S., Choudhary, D.K., Eds.; Springer: Singapore, 2022.
- Imtiaz, M.; Malhotra, R.S. Reduce stress: Breed for drought tolerance. ICARDA Caravan Sci. Food Sec. 2009, 26, 34–36.
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant 1996, 97, 795–803.
- Hara, M.; Terashima, S.; Kuboi, T. Characterization and cryoprotective activity of cold-responsive dehydrin from Citrus unshiu. J. Plant Physiol. 2001, 158, 1333–1339.
- Hung, S.H.; Yu, C.W. Hydrogen peroxide functions as a stress signal in plants. Bot. Bull. Acad. Sinica 2005, 41, 1–10.
- Chen, Z.; Gallie, D.R. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell. 2004, 16, 1143–1162.
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037.
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53.
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800.
- Gupta, P.K.; Varshney, R.K.; Prasad, M. Molecular markers: Principles and methodology. In Molecular Techniques in Crop Improvement; Jain, S.M., Ahloowalia, B.S., Brar, D.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 9–54.
- Pushpavalli, R.; Krishnamurthy, L.; Thudi, M.; Gaur, P.M.; Rao, M.V.; Siddique, K.H.M.; Colmer, T.D.; Turner, N.C.; Varshney, R.K.; Vadez, V. Two key genomic regions harbour QTLs for salinity tolerance in ICCV 2 JG 11 derived chickpea (Cicerarietinum L.) recombinant inbred lines. BMC Plant Biol. 2015, 15, 124.
- Li, Y.; Ruperao, P.; Batley, J.; Edwards, D.; Khan, T.; Colmer, T.D.; Pang, J.; Siddique, K.H.M.; Sutton, T. Investigating Drought Tolerance in Chickpea Using Genome-Wide Association Mapping and Genomic Selection Based on Whole-Genome Resequencing Data. Front. Plant Sci. 2018, 9, 190.
- Kalra, N.; Chakraborty, D.; Sharma, A.; Rai, H.K.; Jolly, M.; Chander, S.; Kumar, P.R.; Bhadraray Barman, D.; Mittal, R.B.; Lal, M.; et al. Effect of increasing temperature on yield of some winter crops in northwest India. Curr. Sci. 2008, 94, 82–88.
- Monneveux, P.; Ribaut, J.-M. Secondary traits for drought tolerance improvement in cereals. In Drought Adaptation in Cereals; Ribaut, J.-M., Ed.; The Haworth Press Inc.: Binghamton, NY, USA, 2006; pp. 97–143.
- Shah, T.M.; Imran, M.; Atta, B.M.; Ashraf, M.Y.; Hameed, A.; Waqar, I.; Shafiq, M.; Hussain, K.; Naveed, M.; Aslam, M.; et al. Selection and screening of drought tolerant high yielding chickpea genotypes based on physio-biochemical indices and multi-environmental yield trials. BMC Plant Biol. 2020, 20, 171.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
29 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




