
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nassima Radouane | -- | 1737 | 2022-11-27 13:01:31 | | | |
| 2 | Dean Liu | Meta information modification | 1737 | 2022-11-28 04:53:37 | | |
Video Upload Options
To overcome the long-standing disadvantages of PCMs, for instance, small values of thermal conductivity, liquid leakage, separation of phase, and the problem of supercooling, advanced phase change composites (PCCs) manufactured by chemical modifications or the incorporation of functional additives are essential to overcome these disadvantages and promote the large-scale application of PCMs.
1. Introduction
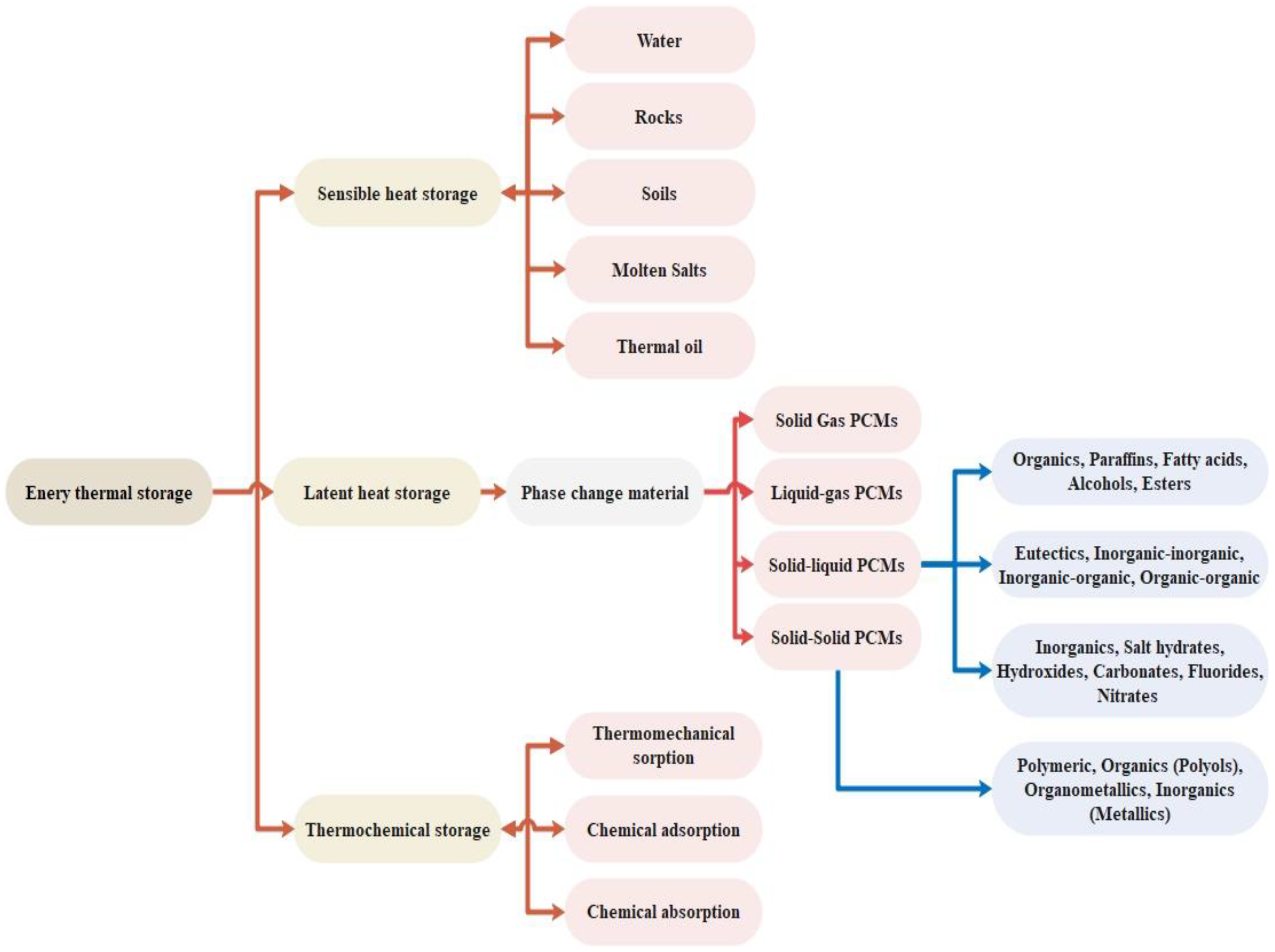
| Advantages | Disadvantages | |
|---|---|---|
| Organic |
|
|
| Inorganic |
|
|
| Eutectic |
|
|
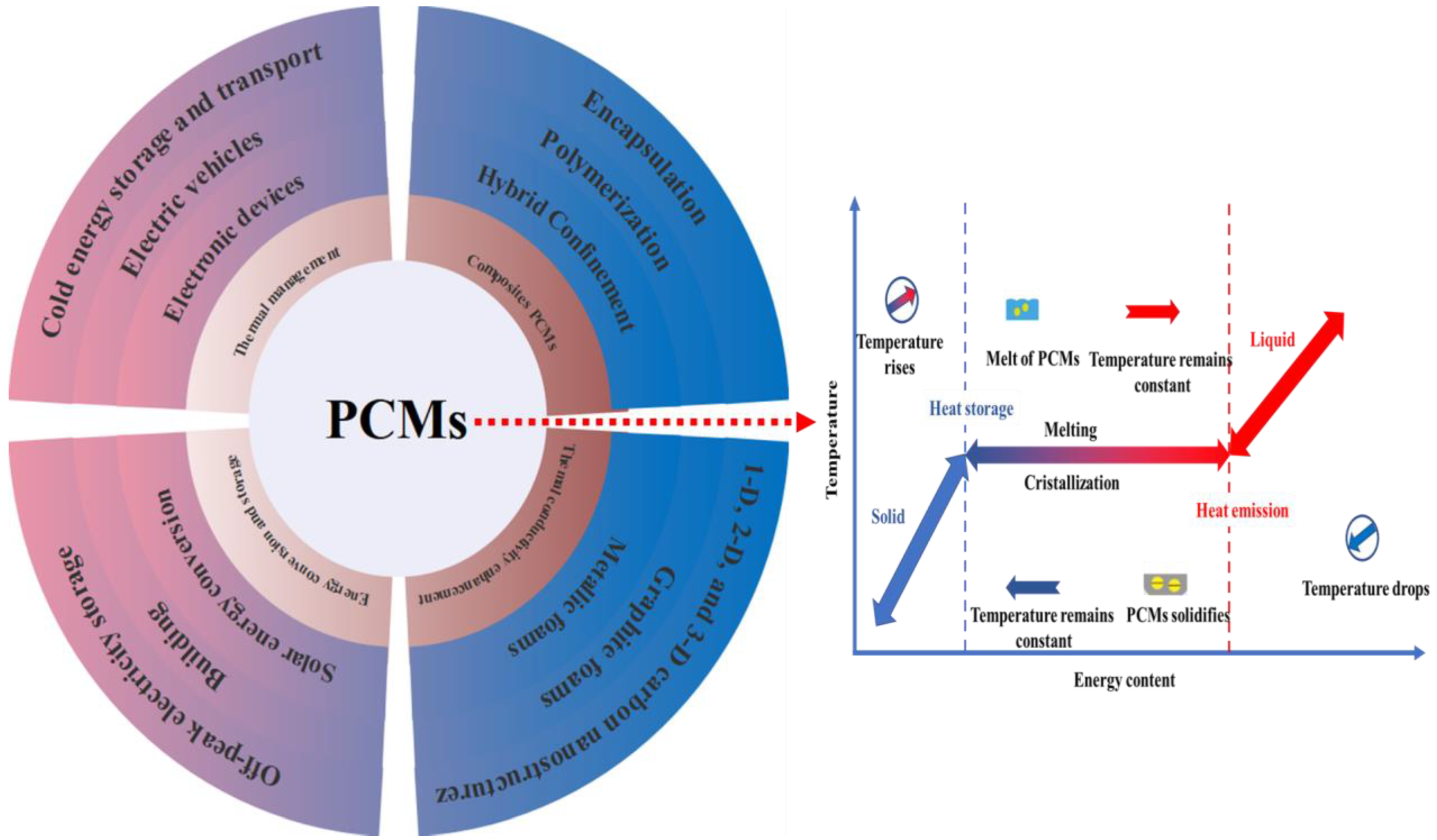
2. Thermal Conductivity Improvement of PCCs
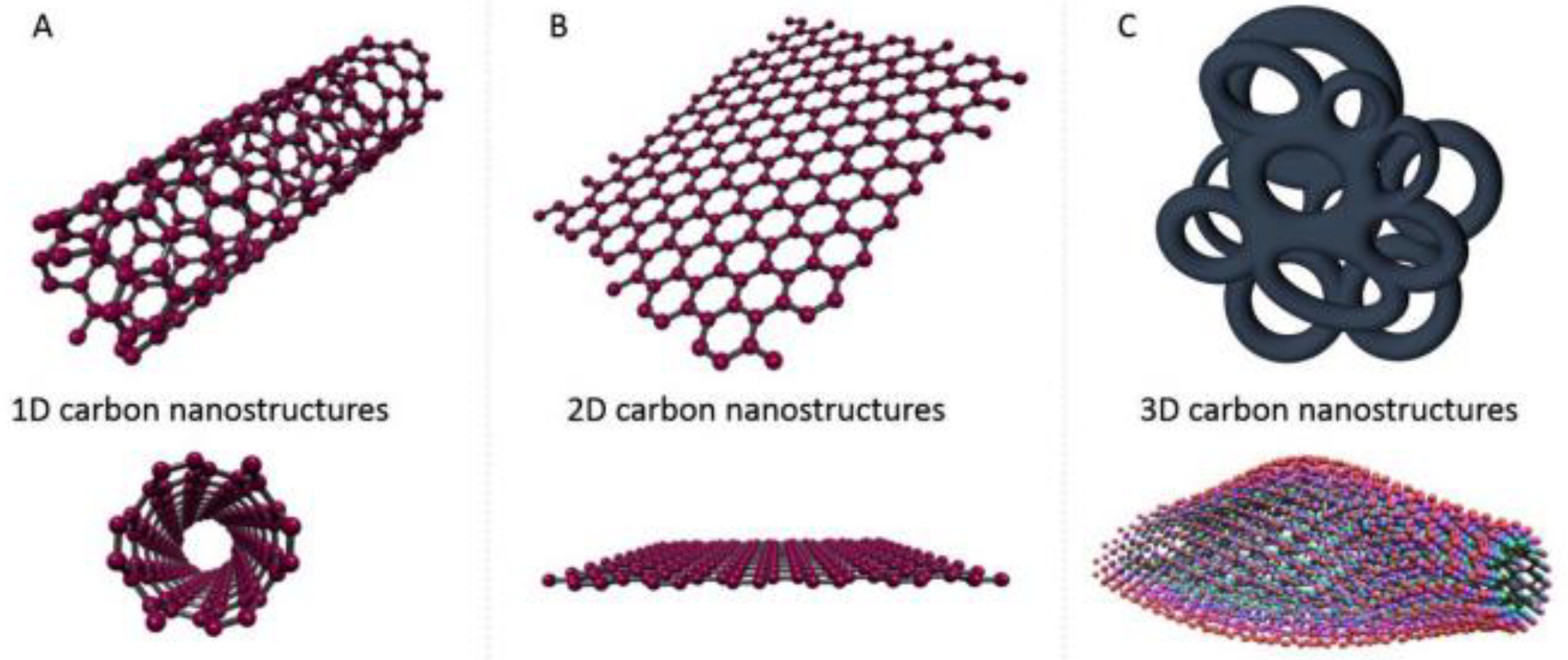
To overcome the problem of low thermal conductivity of PCCs, many solutions have been explored and can be divided into two categories. The first solution consists of adding fins to the heat storage system to intensify the heat transfer during the solid–liquid phase change [20][21]. The second type of remedy is to improve the thermal conductivity of PCCs. This is achieved by adding conductive carbon-based additives such as carbon nanotubes, carbon fibers, and graphite or by adding conductive metal particles (TiO2, aluminum nitride…) [20][22]. Improving PCC thermal conductivity can also be accomplished by impregnating PCCs with conductive porous materials [20][23].
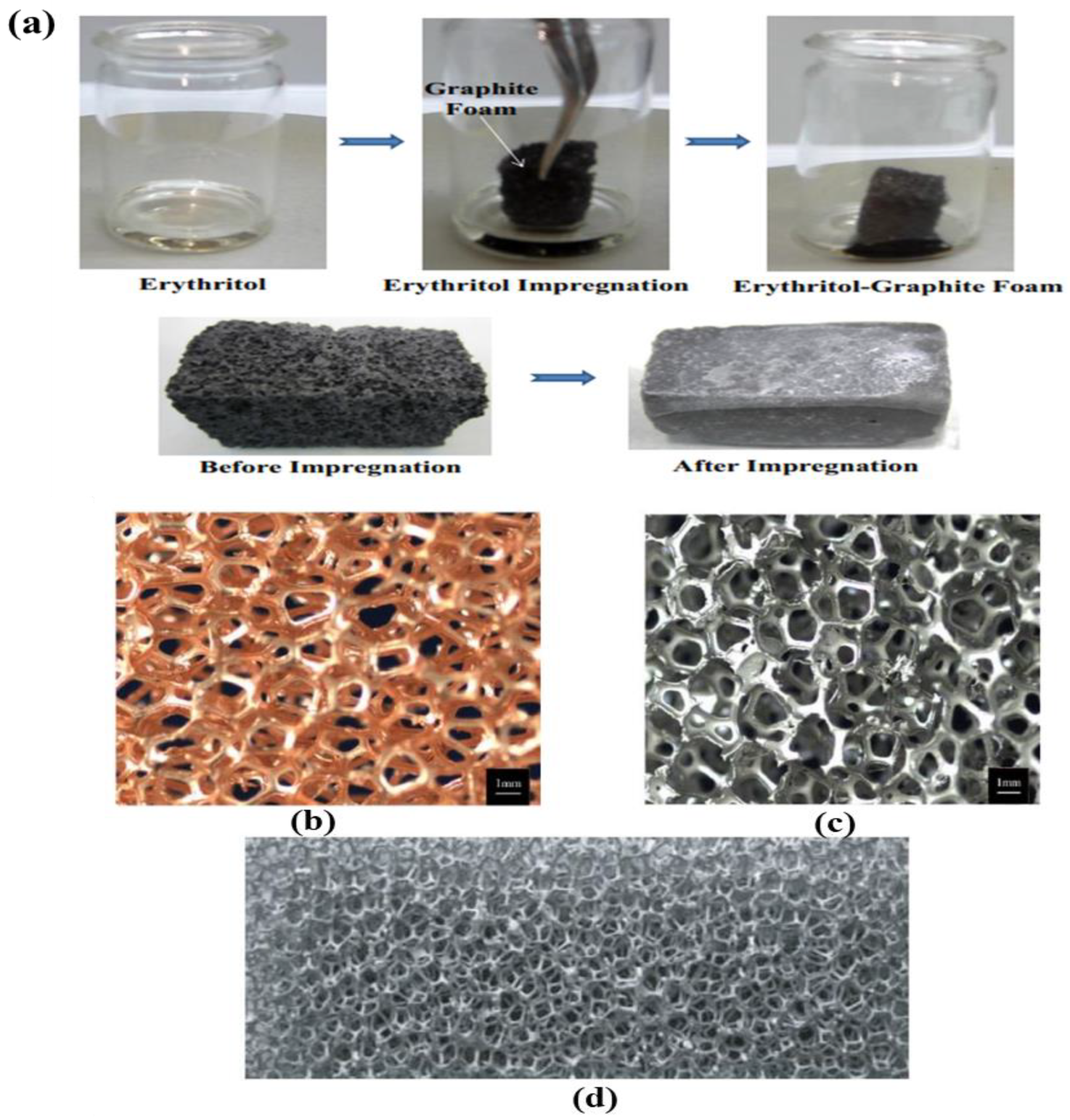
2.1. Improvement of the Thermal Conductivity by Graphite Foams
2.2. Impregnation of PCMs in Metallic Foams
References
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990.
- Rehman, A.; Ma, H.; Ozturk, I.; Ulucak, R. Sustainable development and pollution: The effects of CO2 emission on population growth, food production, economic development, and energy consumption in Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 17319–17330.
- Rahman, A.; Farrok, O.; Haque, M.M. Environmental impact of renewable energy source based electrical power plants: Solar, wind, hydroelectric, biomass, geothermal, tidal, ocean, and osmotic. Renew. Sustain. Energy Rev. 2022, 161, 112279.
- Sayed, E.T.; Wilberforce, T.; Elsaid, K.; Rabaia, M.K.H.; Abdelkareem, M.A.; Chae, K.J.; Olabi, A.G. A critical review on environmental impacts of renewable energy systems and mitigation strategies: Wind, hydro, biomass and geothermal. Sci. Total Environ. 2021, 766, 144505.
- Yu, M.; Hong, S.H. Supply-demand balancing for power management in smart grid: A Stackelberg game approach. Appl. Energy 2016, 164, 702–710.
- Yu, Q.; Jiang, Z.; Cong, L.; Lu, T.; Suleiman, B.; Leng, G.; Wu, Z.; Ding, Y.; Li, Y. A novel low-temperature fabrication approach of composite phase change materials for high temperature thermal energy storage. Appl. Energy 2019, 237, 367–377.
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123.
- Schmit, H.; Rathgeber, C.; Hoock, P.; Hiebler, S. Critical review on measured phase transition enthalpies of salt hydrates in the context of solid-liquid phase change materials. Thermochim. Acta 2020, 683, 178477.
- Devaux, P.; Farid, M. Benefits of PCM Underfloor Heating with PCM Wallboards for Space Heating in Winter. In Thermal Energy Storage with Phase Change Materials; CRC Press: Boca Raton, FL, USA, 2021; pp. 310–327.
- Liu, D.; Xin-Feng, L.; Bo, L.; Si-quan, Z.; Yan, X. Progress in thermochemical energy storage for concentrated solar power: A review. Int. J. Energy Res. 2018, 42, 4546–4561.
- Zhang, L.; Liu, W.; Wen, X.; Chen, J.; Zhao, C.; Castillo-Rodríguez, M.; Yang, L.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Electrospun submicron NiO fibers combined with nanosized carbon black as reinforcement for multi-functional poly(lactic acid) composites. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105662.
- Zhang, S.; Li, Z.; Yao, Y.; Tian, L.; Yan, Y. Heat transfer characteristics and compatibility of molten salt/ceramic porous composite phase change material. Nano Energy 2022, 100, 107476.
- Sarbu, I.; Dorca, A. Review on heat transfer analysis in thermal energy storage using latent heat storage systems and phase change materials. Int. J. Energy Res. 2019, 43, 29–64.
- Zhang, P.; Xiao, X.; Ma, Z.W. A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl. Energy 2016, 165, 472–510.
- Sobolčiak, P.; Mrlik, M.; Popelka, A.; Minařík, A.; Ilcikova, M.; Srnec, P.; Nogellova, Z.; Ouederni, M.; Krupa, I. Foamed Phase Change Materials Based on Recycled Polyethylene/Paraffin Wax Blends. Polymers 2021, 13, 1987.
- Bott, T.R.; Gudmundsson, J.S. Deposition of paraffin wax from kerosene in cooled heat exchanger tubes. Can. J. Chem. Eng. 1977, 55, 381–385.
- Yao, Y.; Wu, H.; Liu, Z. A new prediction model for the effective thermal conductivity of high porosity open-cell metal foams. Int. J. Therm. Sci. 2015, 97, 56–67.
- Qian, T.; Li, J.; Min, X.; Guan, W.; Deng, Y.; Ning, L. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536.
- Kim, H.S.; Kim, S.H.; Jeon, Y.M.; Na, Y.S.; Bae, Y.S.; Hahn, S.H.; Joung, M.; Lee, K.D.; Han, H.S.; Woo, M.H.; et al. Feasibility experiment of physics-based global electron temperature profile control in KSTAR. Fusion Eng. Des. 2018, 135, 1–8.
- Al-Maghalseh, M.; Mahkamov, K. Methods of heat transfer intensification in PCM thermal storage systems: Review paper. Renew. Sustain. Energy Rev. 2018, 92, 62–94.
- Zayed, M.E.; Zhao, J.; Li, W.; Elsheikh, A.H.; Elbanna, A.M.; Jing, L.; Geweda, A.E. Recent progress in phase change materials storage containers: Geometries, design considerations and heat transfer improvement methods. J. Energy Storage 2020, 30, 101341.
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856.
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295.
- Kim, J.H.; Jeong, E.; Lee, Y.S. Preparation and characterization of graphite foams. J. Ind. Eng. Chem. 2015, 32, 21–33.
- Karthik, M.; Faik, A.; Blanco-Rodríguez, P.; Rodríguez-Aseguinolaza, J.; D’Aguanno, B. Preparation of erythritol–graphite foam phase change composite with enhanced thermal conductivity for thermal energy storage applications. Carbon 2015, 94, 266–276.
- Tao, Z.; Wang, H.; Liu, J.; Zhao, W.; Liu, Z.; Guo, Q. Dual-level packaged phase change materials – thermal conductivity and mechanical properties. Sol. Energy Mater. Sol. Cells 2017, 169, 222–225.
- Karthik, M.; Faik, A.; D’Aguanno, B. Graphite foam as interpenetrating matrices for phase change paraffin wax: A candidate composite for low temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 172, 324–334.
- Liu, Z.; Yao, Y.; Wu, H. Numerical modeling for solid-liquid phase change phenomena in porous media: Shell-and-tube type latent heat thermal energy storage. Appl. Energy 2013, 112, 1222–1232.
- Yang, H.; Li, Y.; Yang, Y.; Chen, D.; Zhu, Y. Effective thermal conductivity of high porosity open-cell metal foams. Int. J. Heat Mass Transf. 2020, 147, 118974.
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366.
- Tauseef-ur-Rehman; Ali, H.M. Experimental investigation on paraffin wax integrated with copper foam based heat sinks for electronic components thermal cooling. Int. Commun. Heat Mass Transf. 2018, 98, 155–162.
- Hussain, A.; Tso, C.Y.; Chao, C.Y.H. Experimental investigation of a passive thermal management system for high-powered lithium ion batteries using nickel foam-paraffin composite. Energy 2016, 115, 209–218.
- El Idi, M.M.; Karkri, M.; Abdou Tankari, M. A passive thermal management system of Li-ion batteries using PCM composites: Experimental and numerical investigations. Int. J. Heat Mass Transf. 2021, 169, 120894.
- Kamath, P.M.; Balaji, C.; Venkateshan, S.P. Convection heat transfer from aluminium and copper foams in a vertical channel - An experimental study. Int. J. Therm. Sci. 2013, 64, 1–10.
- Alhusseny, A.; Turan, A.; Nasser, A. Rotating metal foam structures for performance enhancement of double-pipe heat exchangers. Int. J. Heat Mass Transf. 2017, 105, 124–139.
- Zhang, Y.; Du, Y.; Shum, C.; Cai, B.; Le, N.C.H.; Chen, X.; Duck, B.; Fell, C.; Zhu, Y.; Gu, M. Efficiently-cooled plasmonic amorphous silicon solar cells integrated with a nano-coated heat-pipe plate. Sci. Rep. 2016, 6, 24972.
- Chen, P.; Gao, X.; Wang, Y.; Xu, T.; Fang, Y.; Zhang, Z. Metal foam embedded in SEBS/paraffin/HDPE form-stable PCMs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 149, 60–65.
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2012, 6, 628–647.
- Yuan, K.; Liu, J.; Fang, X.; Zhang, Z. Novel facile self-assembly approach to construct graphene oxide-decorated phase-change microcapsules with enhanced photo-to-thermal conversion performance. J. Mater. Chem. A 2018, 6, 4535–4543.
- Sheng, N.; Nomura, T.; Zhu, C.; Habazaki, H.; Akiyama, T. Cotton-derived carbon sponge as support for form-stabilized composite phase change materials with enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 192, 8–15.
- Mendes, R.G.; Wróbel, P.S.; Bachmatiuk, A.; Sun, J.; Gemming, T.; Liu, Z.; Rümmeli, M.H. Carbon Nanostructures as a Multi-Functional Platform for Sensing Applications. Chemosensors 2018, 6, 60.
- Wang, Y.; Tang, B.; Zhang, S. Single-Walled Carbon Nanotube/Phase Change Material Composites: Sunlight-Driven, Reversible, Form-Stable Phase Transitions for Solar Thermal Energy Storage. Adv. Funct. Mater. 2013, 23, 4354–4360.
- Hipólito-Valencia, B.J.; Rubio-Castro, E.; Ponce-Ortega, J.M.; Serna-González, M.; Nápoles-Rivera, F.; El-Halwagi, M.M. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640.
- Chen, L.; Zou, R.; Xia, W.; Liu, Z.; Shang, Y.; Zhu, J.; Wang, Y.; Lin, J.; Xia, D.; Cao, A. Electro- and photodriven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892.




