Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sundarrajan subramanian | -- | 1887 | 2022-11-27 07:07:37 | | | |
| 2 | Dean Liu | Meta information modification | 1887 | 2022-11-28 04:35:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Awasthi, G.; Sharma, R.; Sundarrajan, S.; Ramakrishna, S.; Kumar, P. Hybrid Material-Based Chemiresistive Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/36657 (accessed on 06 March 2026).
Awasthi G, Sharma R, Sundarrajan S, Ramakrishna S, Kumar P. Hybrid Material-Based Chemiresistive Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/36657. Accessed March 06, 2026.
Awasthi, Gaurav, Ritika Sharma, Subramanian Sundarrajan, Seeram Ramakrishna, Pawan Kumar. "Hybrid Material-Based Chemiresistive Sensors" Encyclopedia, https://encyclopedia.pub/entry/36657 (accessed March 06, 2026).
Awasthi, G., Sharma, R., Sundarrajan, S., Ramakrishna, S., & Kumar, P. (2022, November 27). Hybrid Material-Based Chemiresistive Sensors. In Encyclopedia. https://encyclopedia.pub/entry/36657
Awasthi, Gaurav, et al. "Hybrid Material-Based Chemiresistive Sensors." Encyclopedia. Web. 27 November, 2022.
Copy Citation
Development of hybrid materials, especially inorganic–organic materials, coordination polymers, conducting polymers, carbon materials, and many more, has produced breakthroughs in diverse applications. Various advance materials have been reported in the literature using metal organic frameworks (MOFs), which compensate for the limitations of sensors.
hybrid materials (HMs)
inorganic-organic materials
coordination polymers
1. Background of the Hybrid Materials
Hybrid materials, in accordance with the International Union of Pure and Applied Chemistry, are materials made up of a combination of inorganic and organic components, or a combination of both with sizes varying from a few nanometers to tens of nanometers, and they are developing into a very powerful and promising category of materials [1][2]. Although the development of organic-inorganic hybrid materials attracted both scientific and industrial attention in the early 1940s, it is worth noting that the study of hybrid materials underwent its first major evolution mostly between the 17th century and the modern era [3][4]. The earliest period, which proved the existence of hybrid materials, extended from prehistory (20,000 years ago) to the 10th century AD. It can be demonstrated with examples such as the bleaching agents that were based on clay and used in ancient Rome or Cyprus. The hybrid clays were utilized to shape and encase Chinese porcelain known as “egg shell”, and the Maya Blue or Prussian Blue pigments. In the case of natural materials, mostly, the organic component holds the inorganic constituents and/or soft tissue together, while the inorganic part gives them mechanical strength and a structure as a whole [5]. In actuality, these natural hybrid materials are frequently the most integrated intelligent systems, which are skilled in striking trade-offs between various tasks such as mechanical behavior, density, controlled permeability, color, and hydrophobicity [3]. In addition, man-made hybrid materials have existed since the beginning of time; for instance, an intercalated organic color compound called Maya blue found in clay minerals, demonstrates the utilization of hybrid materials in the old days [2]. Hybrid materials are divided into four categories [6]. They are
- (i)
-
Composites: matrix and micron-level dispersion constituting the material mixture.
- (ii)
-
Nanocomposites: combination of comparable types of materials at the sub-micron scale.
- (iii)
-
Hybrids: a sub-micron-scale combination of several materials.
- (iv)
-
Nanohybrids: composite, nanocomposite, hybrid, and non-hybrid materials that have been combined at the atomic or molecular level via chemical bonding.
Based on chemical bond strength, HMs are classified into Class 1 hybrids (hybrids with weak bonding such as van der Waals forces, weak electrostatic interactions, and hydrogen bonding) and Class 2 hybrids (strong interactions/Covalent bonding between components) (Figure 1). The organic–inorganic hybrids can be categorized based on their interfaces as Class 1 materials, which interact weakly and are linked by blends or interpenetrating networks. On the other hand, Class 2 materials interact through strong chemical bonds, and they are linked by covalently connected polymers. An example of a Class 1 hybrid material is a binary blend of poly (2,6-dimethyl-1,4-phenylene ether) (PPE)/poly (styrene-co-acrylonitrile) (SAN), and an example of a Class 2 hybrid material is obtained by the interaction of three organic components, PPE, SAN and SBM (polystyrene-block-polybutadiene-block-poly (methyl methacrylate)). HMs that are structural composites can be further classified as either single-layer (continuous or discontinuous fibers) or multilayer (laminates) [7]. Metal organic frameworks (MOFs) have been combined with other functional materials such as metal nanoparticles (NPs), polymers, and other MOFs to create MOF-based hybrid materials. Hybrid materials based on covalent organic frameworks (COF) have also been designed [8]. The introduction of MOFs with high electrical conductivity or intrinsic charge mobility presents the possibility for creation of new types of MOF-based sensing devices. In the new era, two-dimensional (2D) and three-dimensional (3D) types of MOFs are utilized in chemiresistive sensors. The highest conductivity values were found in 2D MOFs. It is due to the prolonged conjugation and in-plane charge delocalization in the 2D sheets, which were mediated through electronic communication via the metal nodes [9][10]. For the construction of reliable chemiresistive sensors, it is also important to have a hierarchical pore structure, higher thermal and chemical stability, and strong bonds between each of the analytes. All of these properties are found in MOFs as well as in COFs [11][12]. Considering their functional groups, holes, and highly organized porosity structure, COFs offer a huge active site in which to insert electroactive molecules. Additionally, the stability of electrochemical sensors is increased by their improved biocompatibility [12].
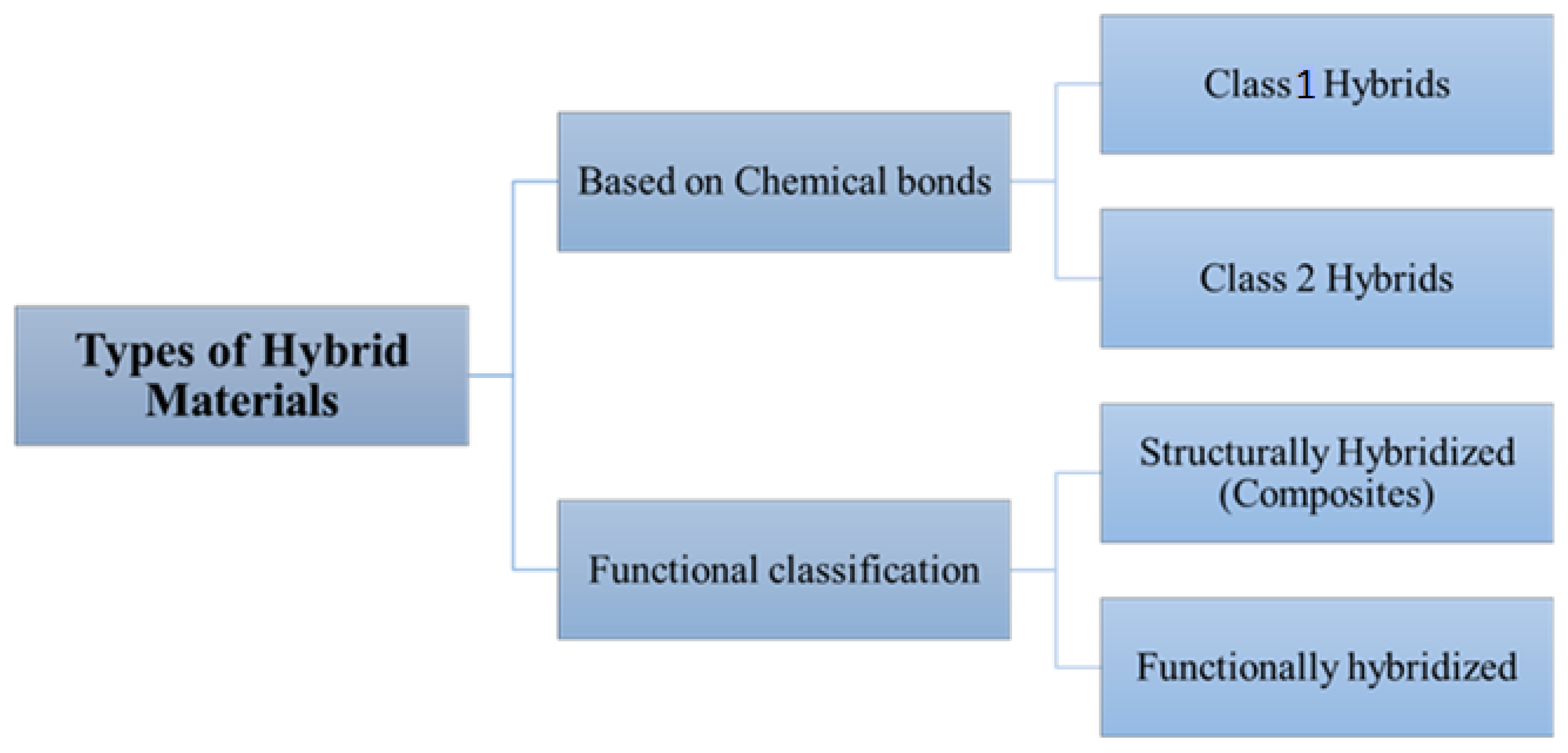
Figure 1. Types of hybrid materials.
2. History of Chemiresistive Sensors
Chemiresistive sensors are conductive materials with a built-in resistance or conductance that changes in response to analyte binding. The resistance change occurs due to either electron or hole transfer induced by the surface reaction between the analyte and the sensing substance. The analyte interacts with the sensing material via covalent bonding, hydrogen bonding or molecular recognition. Wohltjen and co-workers were the first to coin the term “chemiresistor” in 1985 [13]. They investigated copper phthalocyanine complex as a chemiresistive material. At room temperature, it was found that the resistance of the complex decreased when ammonia vapor was present. In 1970, a carbon monoxide detector using powdered SnO2 became the first commercialized metal oxide chemiresistive sensor [14]. Since the mid 1990s, mixed-metal oxide chemiresistor sensors have been commercially available for medical, industrial, and air quality monitoring applications [15]. Metal oxide-based chemiresistive sensors are generally gas sensors that can detect oxidizing as well as reducing gases. Metal oxide chemiresistive sensors require high operating temperature, i.e., 200 °C or higher in order to remove an activation energy barrier for resistivity to change [16]. After metal oxides, conductive polymers are the second most researched material as chemiresistive sensors. The most cited chemiresistive materials include metal oxides (MOx), metallic nanoparticles, conductive polymers, and carbon-based nanomaterials such as carbon nanotubes and graphene [17]. Composite chemiresistive sensors have recently been developed by integrating two high-performance materials. These composite materials exhibit a significant boost in sensing properties when compared to pristine materials. A schematic diagram of a chemiresistor sensor is displayed in Figure 2 [16].
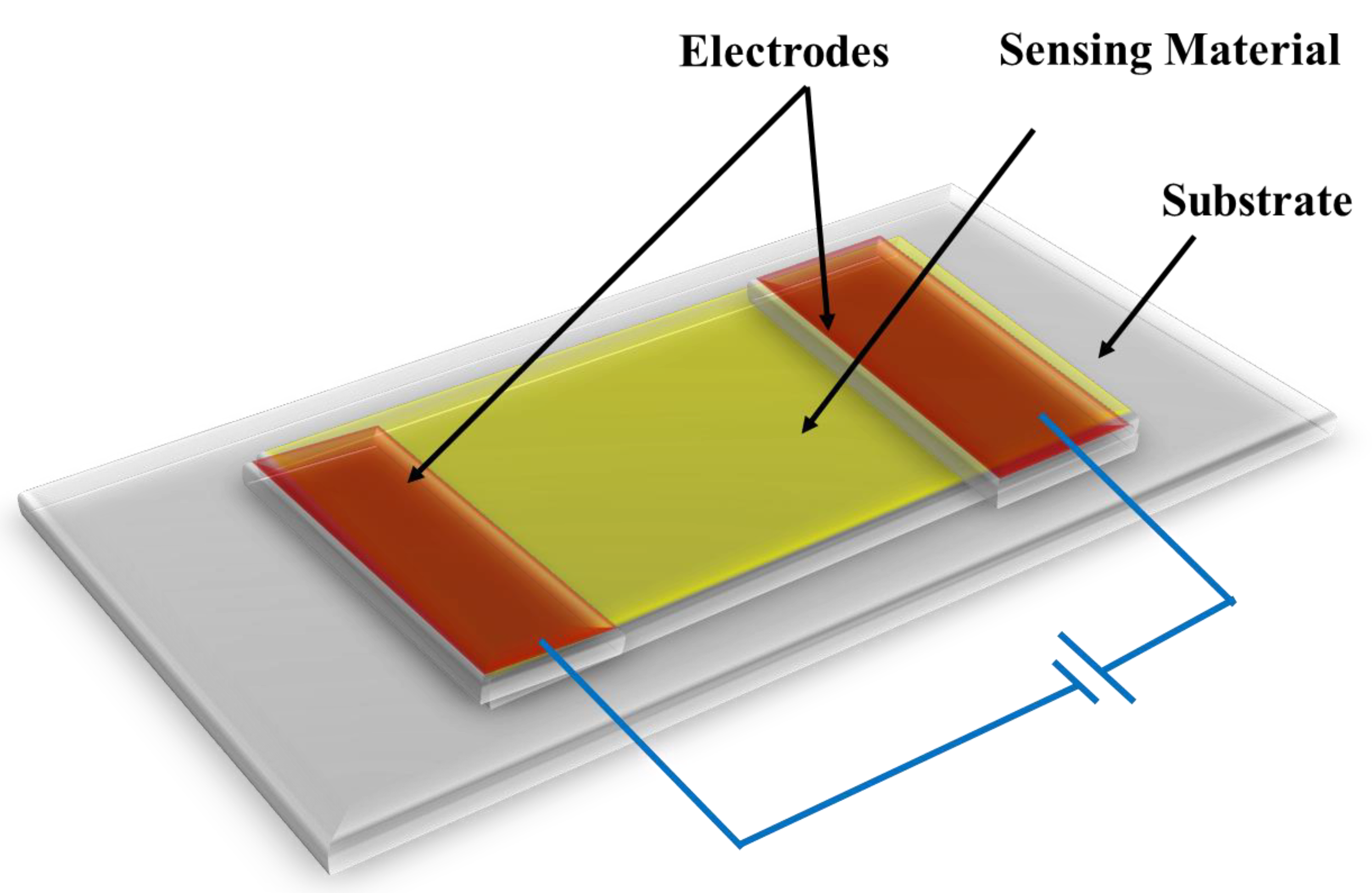
Figure 2. Schematic representation of chemiresistor sensor.
3. Broadening of Nitroaromatic Compounds
Nitroaromatic compounds (NACs) are one of the most prevalent and significant classes of industrial chemicals currently in use. The chemical structure of NACs includes one or more nitro groups, and they are aromatic in nature. These compounds are synthetic as well as naturally occurring compounds. Nitroaromatic compounds can occur in both aqueous and atmospheric conditions. Beginning in the early 19th century, the chemistry of nitro compounds was developed, and in the 20th century, it was combined with organic chemistry [18]. Nitro compounds are crucial as synthetic intermediates and building blocks for the synthesis of frameworks for medicines, agrochemicals, dyes, and explosives [18]. Nitro groups have high electronegativity that causes delocalization of the π-electron to accomplish its own charge deficiency [19]. The primary reaction step is nitration, which is generally used to synthesize nitroaromatic compounds. When sulfuric and nitric acids are mixed, nitronium ions (NO2+) are produced. These ions are then used to react with aromatic substrates in an electrophilic substitution [19]. Several nitroaromatic compounds have been used in high-energy explosives due to the nitro group’s peculiar chemistry. The nitrogen atom rapidly accepts electrons in this oxidation state (III), allowing explosives based on nitroarene to serve as self-oxidants. As a result, due to detonation of an explosive charge, energy is quickly released from the molecules. HNO3 alone or in combination with H2SO4 is used in traditional nitration procedures, and this method has remained unchallenged for more than 150 years [18].
The most commonly used NACs include 2,4,6-Trinitrobenzene (TNB), 2,4,6-Trinitrophenol (TNP), 2,4,6-Trinitrotoluene (TNT), Dinitrobenzene (DNB), Dinitrotoluene (DNT), Dinitronaphthalene (DNN), 1,3,5-Trinitroso-1,3,5-triazinane (RDX), Dinitrophenol (DNP), Nitrobenzene (NB), Nitroaniline (NA), and Nitrocatechol (NC). NACs can be used as starting materials in the chemical synthesis of a wide range of substances, including explosives, pesticides, dyes, drugs, cosmetics, preservatives, paints, corrosion inhibitors, gasoline additives, and other industrial chemicals. The nitro group has some special properties that make it useful in this regard [20].
Picric acid or 1,3,5-trinitrophenol (TNP) was initially developed as a yellow fabric dye in 1771, and it is now used in explosive shells [21]. Toluene and nitric acid are combined to create dinitrotoluene (DNT). Although DNT exists with six isomers, most of the information is relevant to 2,4-DNT and 2,6-DNT [22]. Mononitrotoluene, DNT, and TNT are the products sequentially produced by the nitration of toluene. 2,4-DNT is synthesized through the nitration of 4-nitrotoluene [23]. Explosive DNT is used to make smokeless powders, as a rocket propellant plasticizer, and as a gelatinizing and waterproofing agent [24].
A chronology of nitro compounds as explosive is presented in Figure 3. In 1867, dynamite was invented. TNT was used as a weapon in World War I in 1914 as it had more advantages over dynamite since the shock waves produced by TNT could rupture the steel on armor-plated vehicles. During World War II, two new explosives were introduced, RDX and PETN (penta erythrito tetranitrate). RDX was renamed as Composition Four or C-4 explosive. In 1945, ammonium nitrate, as an inexpensive fertilizer, was manufactured and shipped to Europe for enriching depleted farm soil. In 1957, ammonium nitrate fuel oil was developed as an explosive [25].
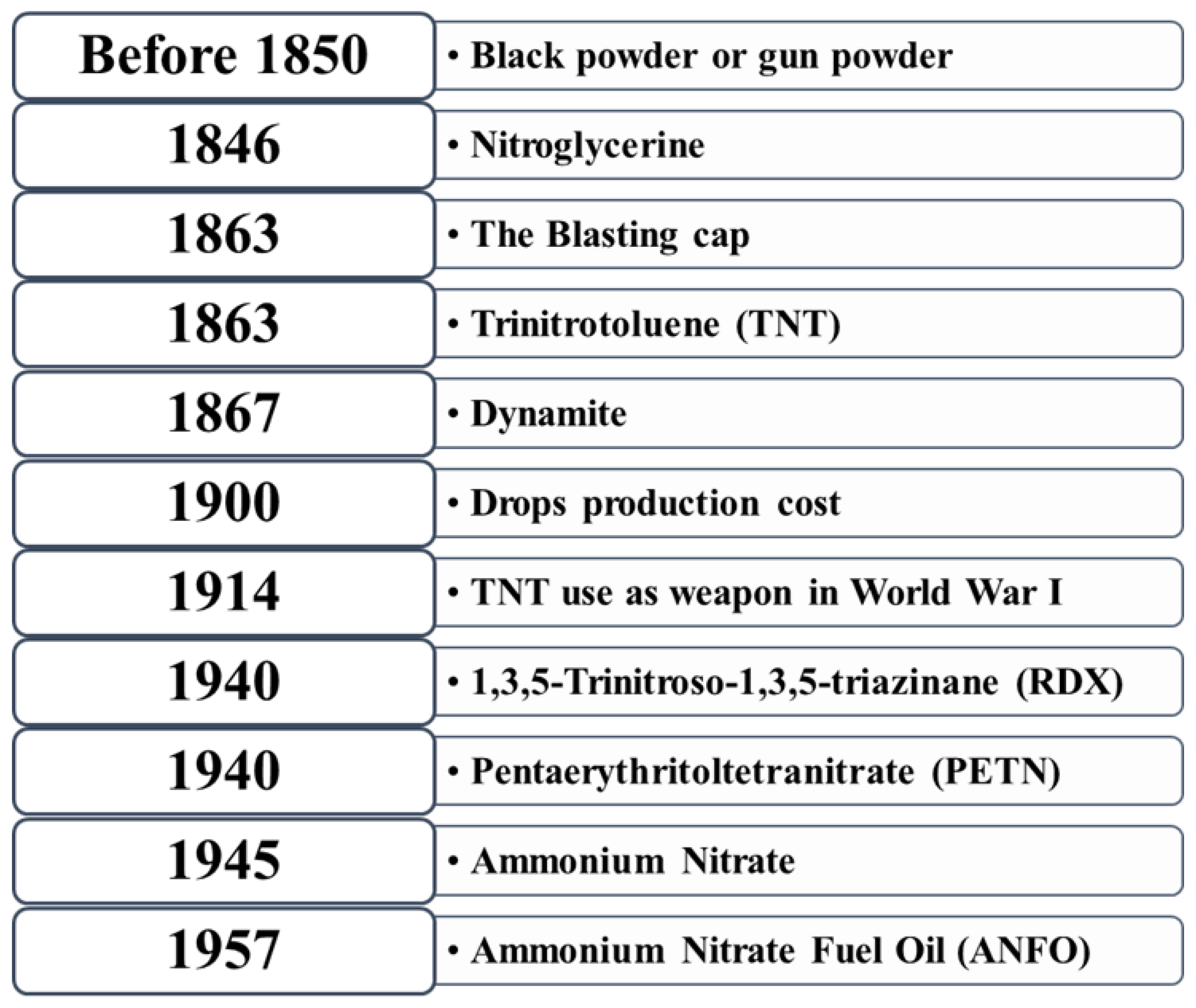
Figure 3. Chronological history of explosives [25].
4. Prerequisites for Chemiresistive Sensors
A simple chemiresistor is made up of a sensing material that coats a group of interdigitated electrodes or fills the space between two electrodes. It measures the resistance lying between the electrodes. As analytes interact with the sensing material, the intrinsic resistance of the sensing material can be altered in their presence. These interactions result in changes in measured electric properties such as resistance, which can be used to determine if an analyte is present or not, as well as its quantity if present.
It is advantageous to generate π-stacking complexes with electron-rich fluorophores due to NACs’ electron-deficient characteristic. It can be used to detect them using chromo-fluorogenic probes. Chemical sensors offer unique techniques for the fast detection of ultratrace NACs in explosives and can be integrated with small microelectronic systems [26]. Single-walled carbon nanotubes (SWCNTs) are a desirable type of chemiresistor due to their low-cost synthesis, ability to operate at ambient temperature, and extremely low power needs. The principle of a chemiresistor formed on chemically sensitive conducting polymers for the specific detection of chemical sensing substances is schematically illustrated in Figure 4 [27].
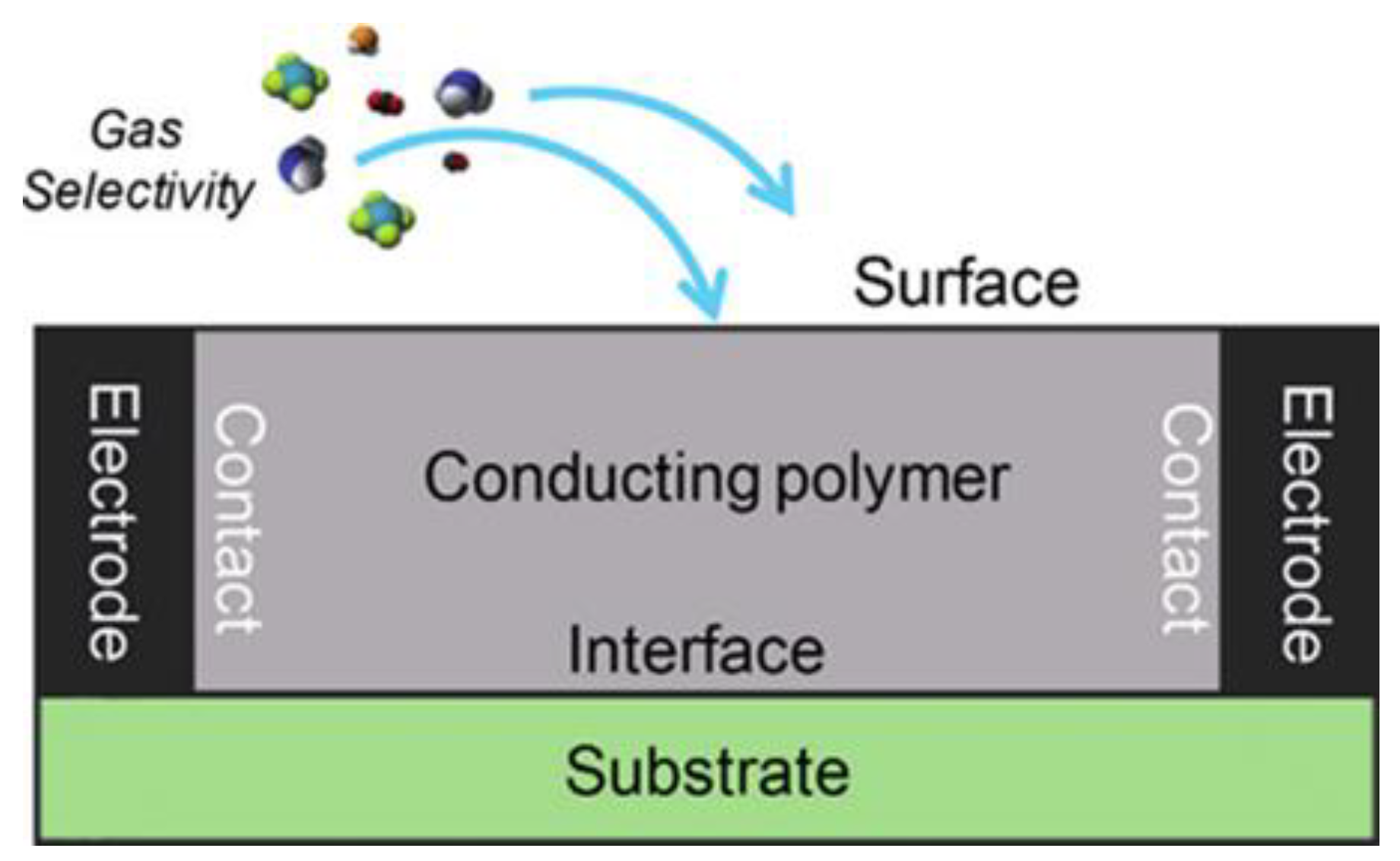
Figure 4. The chemiresistor sensor idea is illustrated schematically for the purpose of selectively detecting chemical sensing materials using chemically sensitive CPs. Reprinted from [27].
More recently, research on chemiresistor sensors based on MOFs has been gaining more attention due to their excellent properties, including high porosity, high surface area, chemical and thermal stability, and stable luminescent/electrochemical nature [28]. All of these properties have been summarized in Figure 5. Importantly, MOFs constructed from periodic table Group IV elements, i.e., metals such as cerium, zirconium, and hafnium, have become of particular interest. It is due to their notable chemical stability in water and their operation in aqueous media. Many research reports have been published on similar MOFs for chemosensors since 2013 [29]. Additionally, adding MOFs to a composite of active sensing materials will enhance the performance of chemiresistive sensors. Numerous research initiatives have been implemented in this regard to develop different electrically conductive MOFs as well as materials generated from MOFs for their usage in a variety of applications, including chemiresistive sensors [28]. For instance, chemically resistive sensors for the detection of CO2, NO2, and SO2 gases can be produced with UiO-66 and its derivatives. Due to their low electrical conductivities, the resistive responses of such Zr-MOFs are still within the range of 10−10 Ohm [30].
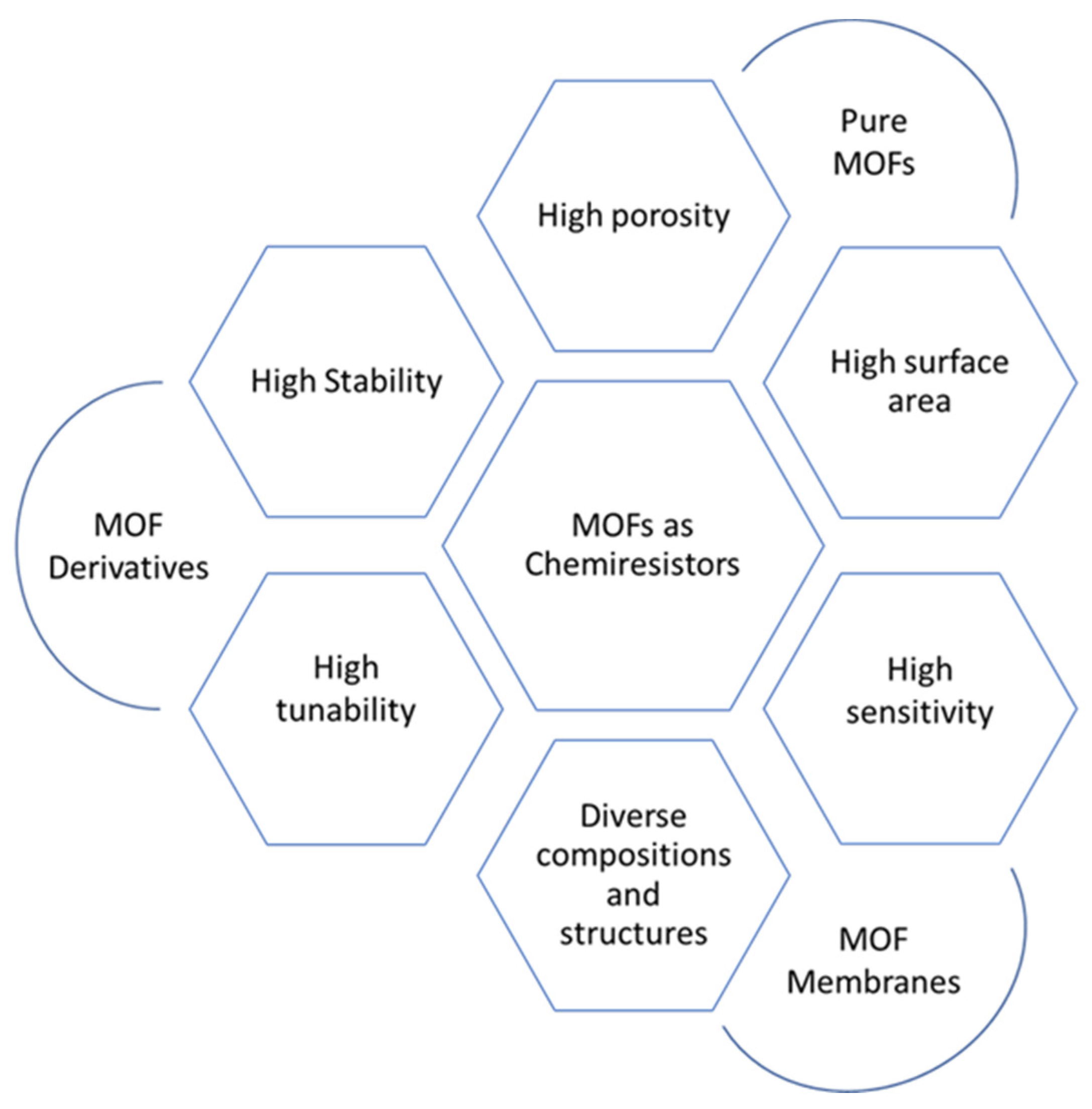
Figure 5. MOFs as chemiresistor sensors, with their properties and different forms.
References
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of hybrid materials-the place of inorganics-in-organics in it, their composition and applications. Front. Chem. 2019, 7, 179.
- Rejab, M.R.B.M.; Hamdan, M.H.B.M.; Quanjin, M.; Siregar, J.P.; Bachtiar, D.; Muchlis, Y. Historical Development of Hybrid Materials. Encycl. Renew. Sustain. Mater. 2020, 4, 445–455.
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158.
- Zhu, Y.-P.; Yuan, Z.-Y. History and Classification of Non-Siliceous Hybrid Materials; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-45634-7.
- Radulović, J. Hybrid filament-wound materials: Tensile characteristics of (aramide fiber/glass fiber)-epoxy resins composite and (carbon fibers/glass fiber)-epoxy resins composites. Sci. Tech. Rev. 2020, 70, 36–46.
- Nanko, M. Definitions and Categories of Hybrid Materials. Adv. Mater. Process. Technol. 2009, 11, 1–8.
- Das, P.P.; Chaudhary, V.; Kumar Singh, R.; Singh, D.; Aditya Bachchan, A. Advancement in hybrid materials, its applications and future challenges: A review. Mater. Today Proc. 2021, 47, 3794–3801.
- Peng, Y.; Zhao, M.; Chen, B.; Zhang, Z.; Huang, Y.; Dai, F.; Lai, Z.; Cui, X.; Tan, C.; Zhang, H. Hybridization of MOFs and COFs: A New Strategy for Construction of Core–Shell Hybrid Materials. Adv. Mater. 2018, 30, 1705454.
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal—Organic Framework for Chemiresistive Sensing. Angew. Chemie Int. Ed. 2015, 54, 4349–4352.
- Singh, T.; Rarotra, S.; Kumar, P.; Sharma, R.; Sridharan, V.; Sonne, C. Current perspectives on the environmental applications using conductive metal–organic frameworks (CMOFs). J. Porous Mater. 2022.
- Kolleboyina, J.; DMello, M.E.; Kesavan, K.; Schneemann, A.; Otyepka, M.; Kment, S.; Narayana, C.; Kalidindi, S.B.; Varma, R.S.; Zboril, R.; et al. A multifunctional covalently linked graphene–MOF hybrid as an effective chemiresistive gas sensor. J. Mater. Chem. A 2021, 9, 17434–17441.
- Chen, S.; Yuan, B.; Liu, G.; Zhang, D. Electrochemical Sensors Based on Covalent Organic Frameworks: A Critical Review. Front. Chem. 2020, 8, 1082.
- Andre, R.S.; Facure, M.H.M.; Schneider, R.; Migliorini, F.L.; dos Santos, D.M.; Mercante, L.A.; Correa, D.S. Sensing Materials: Nanofibers Produced by Electrospinning and Solution Blow Spinning. In Encyclopedia of Sensors and Biosensors, 1st ed.; Roger, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 521–541. ISBN 9780128225493.
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors. 2015, 3, 1–20.
- Wilson, D.M.; Hoyt, S.; Janata, J.; Booksh, K.; Obando, L. Chemical sensors for portable, handheld field instruments. IEEE Sens. J. 2001, 1, 256–274.
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453.
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554.
- Nishiwaki, N. A Walk through Recent Nitro Chemistry Advances. Molecules 2020, 25, 3680.
- Ju, K.-S.; Parales, R.E. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250.
- Tiwari, J.; Tarale, P.; Sivanesan, S.; Bafana, A. Environmental persistence, hazard, and mitigation challenges of nitroaromatic compounds. Environ. Sci. Pollut. Res. 2019, 26, 28650–28667.
- Lee, P.R. Explosives Development and Fundamentals of Explosives Technology. In Explosive Effects and Applications; Springer: Berlin/Heidelberg, Germany, 1998; pp. 23–45.
- Young, R.A. Dinitrotoluene. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 179–182.
- Booth, G. Nitro Compounds, Aromatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000.
- Pichtel, J. Distribution and fate of military explosives and propellants in soil: A review. Appl. Environ. Soil Sci. 2012, 2012, 617236.
- Graham, T. The Explosive History of Nitrogen. ChemMatters 2003, 8–10. Available online: https://highschoolenergy.acs.org/content/hsef/en/how-do-we-use-energy/history-of-nitrogen/_jcr_content/articleContent/columnsbootstrap/column1/acscontainer_0/containerPar/download/file.res/Explosive_History_Nitrogen.pdf (accessed on 25 October 2022).
- Zhang, L.; Kang, Z.; Xin, X.; Sun, D. Metal–organic frameworks based luminescent materials for nitroaromatics sensing. Cryst. Eng. Comm. 2015, 18, 193–206.
- Khanyile, N. Advances in Nanostructured Polyamide-Based Chemical Sensors. J. Nanomater. 2022, 2022, 5543283.
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963.
- Pal, S.; Yu, S.S.; Kung, C.W. Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors. Chemosensors 2021, 9, 306.
- DMello, M.E.; Sundaram, N.G.; Singh, A.; Singh, A.K.; Kalidindi, S.B. An amine functionalized zirconium metal–organic framework as an effective chemiresistive sensor for acidic gases. Chem. Commun. 2019, 55, 349–352.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
28 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





