
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ruirui wang | -- | 2190 | 2022-11-26 05:15:32 | | | |
| 2 | Lindsay Dong | Meta information modification | 2190 | 2022-11-28 04:25:49 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2190 | 2022-11-28 04:26:30 | | |
Video Upload Options
The recycling, development, and application of keratin-containing waste (e.g., hair, wool, feather, and so on) provide an important means to address related environmental pollution and energy shortage issues. The extraction of keratin and the development of keratin-based functional materials are key to solving keratin-containing waste pollution. Keratin-based biofilms are gaining substantial interest due to their excellent characteristics, such as good biocompatibility, high biodegradability, appropriate adsorption, and rich renewable sources, among others. At present, keratin-based biofilms are a good option for various applications, and the development of keratin-based biofilms from keratin-containing waste is considered crucial for sustainable development.
1. Introduction
2. Novel Extraction Methods of Keratin
2.1. Thermal Hydrolysis
2.2. Ultrasonic Technology
2.3. Eco-Friendly Solvent System
2.4. Microbial Decomposition
3. Preparation Method of Keratin-Based Biofilms
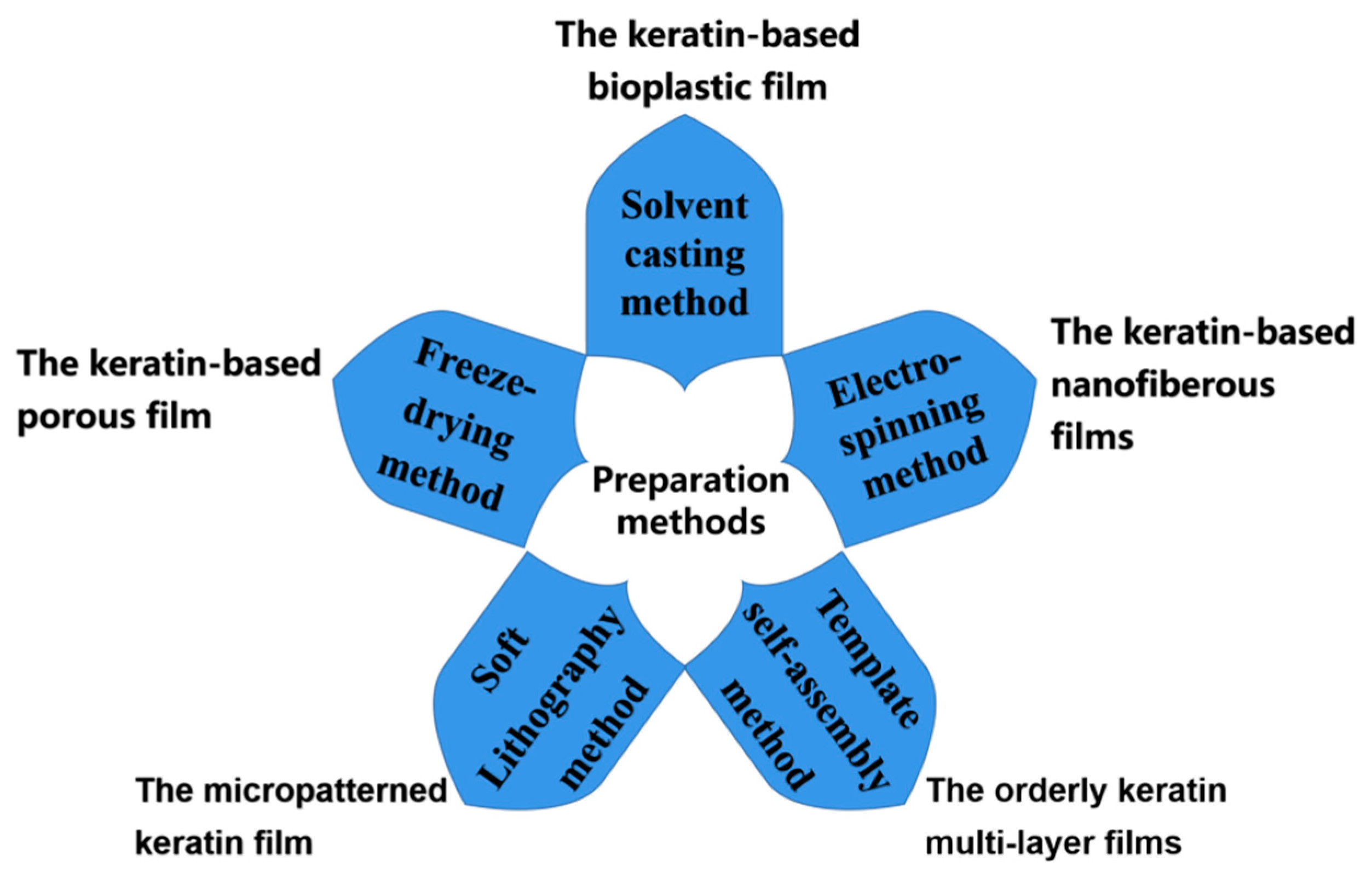
3.1. Solvent Casting Method
Solvent casting is an effective, low-cost, and simple-to-operate method for the development of keratin-based functional films. However, as the backbone of the natural fur and hair is heavily damaged during extraction, pure keratin films prepared using the solvent casting method may have poor mechanical properties, seriously hindering the application of the pure keratin film. To improve the mechanical properties of keratin-based biofilms, it is necessary to introduce other chemicals to modify the keratin during casting. At present, the main modification methods include grafting, blending, and cross-linking modification.
3.2. Electrospinning Method
3.3. Template Self-Assembly Method
3.4. Freeze-Drying Method
3.5. Soft Lithography Method
4. Functional Properties of Keratin-Based Films
4.1. Biocompatibility
4.2. Biodegradability
4.3. Hygroscopic Nature
4.4. Adsorption
References
- Tasaki, K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manag. 2020, 104, 33–41.
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509.
- Jin, E.; Reddy, N.; Zhu, Z.; Yang, Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59, 1729–1738.
- Dou, Y.; Huang, X.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and characterization of a dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174.
- Oluba, O.M.; Osayam, E.; Shoyombo, A.O. Production and characterization of keratin-starch bio-composite film from chicken feather waste and turmeric starch. Biocatal. Agric. Biotechnol. 2021, 33, 101996–102002.
- Pan, F.; Lu, Z.; Tucker, I.; Sarah, H.; Jordan, P.; Lu, J. Surface active complexes formed between keratin polypeptides and ionic surfactants. J. Colloid Interface Sci. 2016, 484, 125–134.
- Ye, W.; Qin, M.; Qiu, R.; Li, J. Keratin-based wound dressings: From waste to wealth. Int. J. Biol. Macromol. 2022, 211, 183–197.
- Jaiswar, G.; Modak, S.; Singh, R.; Dabas, N. Functionalization of biopolymer keratin-based biomaterials and their absorption properties for healthcare application. Woodhead Publ. Ser. Polym. Biomater. 2022, 2022, 257–270.
- Esparza, Y.; Bandara, N.; Ullah, A.; Wu, J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater. Sci. Eng. C 2018, 90, 446–453.
- Rajabi, M.; Ali, A.; Mcconnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612–110634.
- Bertini, F.; Canetti, M.; Patroccu, A.; Zoccola, M. Wool keratin-polypropylene composites: Properties and thermal degradation. Polym. Degrad. Stab. 2013, 98, 980–987.
- Gaidau, C.; Epure, D.G.; Enascuta, C.E.; Carsote, C.; Sendrea, C.; Proietti, N.; Chen, W.; Gu, H. Wool keratin total solubilisation for recovery and reintegration-an ecological approach. J. Cleaner Prod. 2019, 236, 117586–117598.
- Wu, W.; Ma, S.; Chen, R.; Huang, Y.; Deng, Y. Genome-wide analysis of keratinibaculum paraultunense strain KD-1 T and its key genes and metabolic pathways involved in the anaerobic degradation of feather keratin. Arch. Microbiol. 2022, 204, 634–648.
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318.
- Ramya, K.R.; Thangam, R.; Madhan, B. Comparative analysis of the chemical treatments used in keratin extraction from red sheep’s hair and the cell viability evaluations of this keratin for tissue engineering applications. Process Biochem. 2020, 90, 223–232.
- Ye, J.P.; Gong, J.S.; Su, C.; Liu, Y.; Jiang, M.; Pan, H.; Li, R.; Geng, Y.; Xu, Z.; Shi, J. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloids Surf. B 2020, 194, 111158–111197.
- Posati, T.; Giuri, D.; Nocchetti, M.; Sagnella, A.; Gariboldi, M.; Ferroni, C.; Sotgiu, G.; Varchi, G.; Zamboni, R.; Aluigi, A. Keratin-hydrotalcites hybrid films for drug delivery applications. Eur. Polym. J. 2018, 105, 177–185.
- Zuniga, K.; Isaac, A.; Christy, S.; Wrice, N.; Mangum, L.; Natesan, S.; Burnett, L.; Kowalczewski, C. Characterization of a human platelet lysate-loaded keratin hydrogel for wound healing applications in vitro. Int. J. Mol. Sci. 2022, 23, 4100.
- Oluba, O.M.; Obokare, O.; Bayo-Olorunmeke, O.A.; Ojeaburu, S.I.; Ogunlowo, O.M.; Irokanulo, E.O.; Akpor, O.B. Fabrication, characterization and antifungal evaluation of polyphenolic extract activated keratin starch coating on infected tomato fruits. Sci. Rep. 2022, 12, 4340–4351.
- Shi, Y.; Huang, L.; Wang, X.; Li, Y.; Shen, R. Intelligent drug delivery system based on silk fibroin/wool keratin. Math. Probl. Eng. 2022, 2022, 6748645.
- Azmi, N.A.; Idris, A.; Yusof, N.S.M. Ultrasonic technology for value added products from feather keratin. Ultrason. Sonochem. 2018, 47, 99–107.
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186.
- Zhong, X.; Li, R.; Wang, Z. Eco-fabrication of antibacterial nanofibrous membrane with high moisture permeability from wasted wool fabrics. Waste Manag. 2020, 102, 404–411.
- Wang, Y.; Cao, X. Extracting keratin from chicken feathers by using a hydrophobic ionic liquid. Process Biochem. 2012, 47, 896–899.
- Ghosh, A.; Clerens, S.; Deb-Choudhury, S.; Dyer, J. Thermal effects of ionic liquid dissolution on the structures and properties of regenerated wool keratin. Polym. Degrad. Stab. 2014, 108, 108–115.
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass valorization using natural deep eutectic solvents: What’s new in France? Molecules 2021, 26, 6556.
- Yeo, I.; Lee, Y.J.; Song, K.; Jin, H.; Lee, J.; Kim, D.; Lee, D.; Kang, N. Low-molecular weight keratins with anti-skin aging activity produced by anaerobic digestion of poultry feathers with Fervidobacterium islandicum AW-1. J. Biotechnol. 2018, 271, 17–25.
- Fontoura, R.; Daroit, D.J.; Corrêa, A.P.F.; Moresco, K.S.; Santi, L.; Beys-da-Silva, W.O.; Yates, J.R.; Moreira, J.C.F.; Brandelli, A. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 2019, 49, 71–76.
- Shen, B.; Zhang, D.; Wei, Y.; Zhao, Z.; Ma, X.; Zhao, X.; Wang, S.; Yang, W. Preparation of Ag doped keratin/PA6 nanofiber membrane with enhanced air filtration and antimicrobial properties. Polymers 2019, 11, 1511.
- Schifino, G.; Gasparini, C.; Drudi, S.; Giannelli, M.; Sotgiu, G.; Posati, T.; Zamboni, R.; Treossi, E.; Maccaferri, E.; Giorgini, L.; et al. Keratin/polylactic acid/graphene oxide composite nanofibers for drug delivery. Int. J. Pharm. 2022, 623, 121888.
- Guidotti, G.; Soccio, M.; Bondi, E.; Posati, T.; Sotgiu, G.; Zamboni, R.; Torreggiani, A.; Corticelli, F.; Lotti, N.; Aluigi, A. Effects of the blending ratio on the design of keratin/poly (butylene succinate) nanofibers for drug delivery applications. Biomolecules 2021, 11, 1194.
- Posati, T.; Sotgiu, G.; Varchi, G.; Ferroni, C.; Zamboni, R.; Corticelli, F.; Puglia, D.; Torre, L.; Terenzi, A.; Aluigi, A. Developing keratin sponges with tunable morphologies and controlled antioxidant properties induced by doping with polydopamine (PDA) nanoparticles. Mater. Des. 2016, 110, 475–484.
- Zhu, S.; Luo, W.; Zeng, W. Preparation of free-standing micropatterned keratin films by soft lithography. Acta Chim. Sin. 2019, 77, 533–538.
- Tan, H.B.; Wang, F.Y.; Ding, W.; Zhang, Y.; Ding, J.; Cai, D.X.; Yu, K.F.; Yang, J.; Yang, L.; Xu, Y.Q. Fabrication and evaluation of porous keratin/chitosan (KCS) scaffolds for effectively accelerating wound healing. Biomed. Environ. Sci. 2015, 28, 178–189.
- Mi, X.; Chang, Y.; Xu, H.; Yang, Y. Valorization of keratin from food wastes via crosslinking using non-toxic oligosaccharide derivatives. Food Chem. 2019, 300, 125181–125188.
- Lee, C.; Pant, B.; Kim, B.; Jang, R.; Park, S.; Park, M.; Park, S.; Kim, H. Carbon quantum dots incorporated keratin/polyvinyl alcohol hydrogels: Preparation and photoluminescent assessment. Mater. Lett. 2017, 207, 57–61.
- Teresa, K.; Justyna, B. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701.
- Natali, M.; Campana, A.; Posati, T.; Benvenuti, E.; Prescimone, F.; Ramirez, D.O.; Varesano, A.; Vineis, C.; Zamboni, R.; Muccini, M.; et al. Engineering of keratin functionality for the realization of bendable all biopolymeric micro-electrode array as humidity sensor. Biosens. Bioelectron. 2019, 141, 111480–111488.
- Song, K.; Qian, X.; Zhu, X.; Li, X.; Hong, X. Fabrication of mechanical robust keratin film by mesoscopic molecular network reconstruction and its performance for dye removal. J. Colloid Interface Sci. 2020, 579, 28–36.
- Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Jannoke, L. Study of methylene blue adsorption on keratin nanofibrous membranes. J. Hazard. Mater. 2014, 268, 156–165.
- Jin, X.; Wang, H.; Jin, X.; Wang, H.; Chen, L.; Wang, W.; Lin, T.; Zhu, Z. Preparation of keratin/PET nanofiber membrane and its high adsorption performance of Cr(VI). Sci. Total Environ. 2019, 710, 135546–135578.
- Aluigi, A.; Zoccola, M.; Vineis, C.; Tonin, C.; Ferrero, F.; Canetti, M. Study on the structure and properties of wool keratin regenerated from formic acid. Int. J. Biol. Macromol. 2007, 41, 266–273.
- Zahara, I.; Arshad, M.; Naeth, M.A.; Naeth, A.; Siddique, T.; Ullah, A. Feather keratin derived sorbents for the treatment of wastewater produced during energy generation processes. ACS Med. Chem. Lett. 2021, 273, 128545–128582.
- Posati, T.; Listwan, A.; Sotgiu, G.; Torreggiani, A.; Zamboni, R.; Aluigi, A. Keratin/Hydrotalcites hybrid sponges as promising adsorbents for cationic and anionic dyes. Front. Bioeng. Biotechnol. 2020, 8, 68–76.
- Zubair, M.; Roopesh, M.S.; Ullah, A. Nano-modified feather keratin derived green and sustainable biosorbents for the remediation of heavy metals from synthetic wastewater. Chemosphere 2022, 308, 136339.
- Olawale, S.A.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Okafor, C.C.; Sellaoui, L.; Badawi, M. Thermodynamics and mechanism of the adsorption of heavy metal ions on keratin biomasses for wastewater detoxification. Adsorpt. Sci. Technol. 2022, 2022, 7384924.
- David, P.S.; Karunanithi, A.; Fathima, N.N. Improved filtration for dye removal using keratin–polyamide blend nanofibrous membranes. Environ. Sci. Pollut. Res. 2020, 27, 45629–45638.




