Enormous amounts of keratin-containing waste (e.g., hair, wool, feather, and so on) are produced by various industries, such as textiles, food, leather, technology, and so on. According to statistics, the world produces over 2.5 million tonnes of wool, greater than 40 million tons of hair, and more than 65 million tonnes of feathers every year [
1,
2,
3,
4]. This waste fur and hair, which contains about 90% keratin, remains a rich, yet unexploited bio-resource for bio-film production [
5].
Keratin is an important fibrous structural protein present in waste fur and hair [
13], which possesses unique physicochemical characteristics. Keratin is a natural biopolymer that is distinguished from other proteins by its high cysteine content (7–13%) [
14,
15]. Keratin is a type of intermediate filament found in the cytoskeleton of eukaryotic cells and contains high levels of certain amino acids, such as alanine, glycine, serine, and valine while having lower levels of lysine, tryptophan, and methionine [
16,
17,
18,
19,
20]. Its molecular structure contains many active groups (e.g., carboxyl, amino, thiol, and hydroxyl groups) which can be modified. These chemical groups on keratin molecules act with each other to produce non-covalent intra- and inter-molecular bonds, thus forming macromolecular aggregates of keratin. The non-covalent bonds mainly include disulphide bonds, hydrogen bonds, hydrophobic bonds, and ionic bonds. Keratin is divided into two categories: alpha-keratin (α-helix) and beta-keratin (β-sheets) [
21]. Both alpha- and beta-keratin are found in birds and reptiles while alpha-keratin is primarily seen in mammals [
22]. As a primary component of wool, feathers, hair, and other materials, keratin is frequently used in the biomedical field, due to its biodegradability and compatibility with living tissue [
23,
24,
25]. Keratin extracted from keratin-containing waste was studied for cosmetics, feed additives, and biomedical applications, among other fields. As disulphide bonds can promote intermolecular cross-linking, keratin is considered an excellent raw material for biofilms [
26]. Keratin-based biofilm provides an effective alternative to conventional polystyrene film. Keratin-based biofilms are gaining substantial interest due to their unique molecular composition and excellent characteristics, such as good biocompatibility, high biodegradability, appropriate adsorption, rich renewable sources, and so on [
27]. At present, keratin-based biofilms are widely considered a good option for various applications and the development of keratin-based biofilms from keratin-containing waste is crucial for sustainable development.
2. Novel Extraction Methods of Keratin
2.1. Thermal Hydrolysis
Tasaki [
1] has proposed a novel method for keratin extraction from hog hairs using a two-step heating thermal hydrolysis process (THP). The first heating temperature is at or above keratin’s denaturing temperature. The first stage can sufficiently dilate and loosen the network structure composed of keratin molecular chains. Meanwhile, the hydrogen bonds maintaining the keratin structure are disrupted. The second heating temperature is between 100 and 220 °C. At this stage, water molecules are further diffused into the keratin fibre network structure, in order to cleave the disulphide bonds; thus, the keratins are dissolved into the water. The action of the second stage is consistent with that of the chemical reductant 2-mercaptoethanol on hairs. This THP process does not require chemicals and will not promote environmental and health risks. The thermal hydrolysis technology is clean, safe, and eco-friendly; however, the extraction conditions are harsh and the keratin obtained through this method has low molecular weight and is a mixture of amino acids and polypeptides. Avoiding excessive hydrolysis of the keratin during extraction and improving the stability of the extraction process is key to ensuring the scalable production of thermal hydrolysis technology.
2.2. Ultrasonic Technology
Ultrasonic technology is widely used in the biomedical field. Strong mechanical vibration and acoustic cavitation disrupt the intra- and inter-molecular interactions of keratin, causing the accelerated dissolution of keratin during ultrasound extraction. Meanwhile, acoustic cavitation produces a transient high temperature in the extraction medium, which temperature promotes the production of secondary reducing radicals, thus enhancing the mass transfer efficiency. Azmi et al. [
73] investigated feather keratin extraction by combining ultrasound with [BMIM]Cl Ionic liquids, where the intervention of ultrasound significantly shortened the extraction time. The test indicated that ultrasonication does not disrupt the backbone structure of keratin. Ultrasonic techniques can shorten the extraction time and reduce the extraction temperature. As an eco-friendly and promising method to extract keratin, it can be controlled according to the ultrasonic irradiation time and the acoustic power. Ultrasonic technology is simple to operate, fast, and efficient; however, to implement the extraction process, special equipment is required. Therefore, at present, it can only be used as an auxiliary technology to conventional keratin extraction methods. In this regard, reducing the cost of the related equipment is key to ensuring the scalable production of ultrasonic technology.
2.3. Eco-Friendly Solvent System
The use of eco-friendly, recyclable solvent systems has opened new perspectives for the extraction of keratin. Using an eco-friendly solvent system to disrupt the inter- and intra-molecular interactions and disulphide bonds in native keratin allows for keratin extraction. The eco-friendly solvent system must provide a high enough yield and maximally retain the secondary structure of keratin; that is, the entire extraction process should not cause excessive hydrolysis of keratin.
Ionic liquids (ILs) are substances made up of organic/inorganic anions and bulky cations in liquid form at relatively low temperatures [
2]. The structures of cations or anions in ILs determine their properties. In principle, up to 10
18 ILs can be designed by changing cations and anions [
75]. As designer solvents, ILs are considered green solvents for keratin extraction. ILs have high thermal stability and low vapor pressure [
76,
77,
78]. ILs can disrupt the hydrogen bonds and disulphide bonds in keratin molecules, thus promoting keratin dissolution.
Deep eutectic solvents (DESs) possess similar physical properties to ILs, such as low vapor pressure, incombustibility, and so on. DESs are mainly composed of hydrogen bond donors (e.g., organic acids, polyols, amides, urea, and sugars) and hydrogen bond receptors (e.g., quaternary ammonium salts, amino acids, and metal ions), constituting a networked system of hydrogen bonds. The most significant physical property of DESs is their lower melting point than their components. DESs possess high extraction ability and good solubilisation strength [
86].
2.4. Microbial Decomposition
The use of aerobic and anaerobic micro-organisms to catalyse the decomposition of natural fur and hair is a new idea for extracting keratin. Yeo et al. [
94] digested chicken feathers with an extremely thermophilic bacterium
Fervidobacterium islandicum AW-1, in order to extract keratin. The strain possesses a variety of proteolytic enzymes that can decompose hair. The extracted keratin inhibited the expression of UVB-induced MMP-1 and MMP-13 in human dermal fibroblasts; thus, the extracted keratin can be used as a cosmeceutical peptide to prevent skin aging. Microbial decomposition requires mild extraction conditions. The whole extraction process of microbial catalytic decomposition is simple, safe, and pollution-free, making it a promising method for extracting bioactive keratin. Keratin resulting from the microbial decomposition of fur and hair has antioxidant properties and the ability to inhibit ACE and dipeptidyl peptidase IV activities [
70]; however, microbial decomposition can easily destroy the peptide bonds in the keratin structure, resulting in low molecular weight of the extracted keratin.
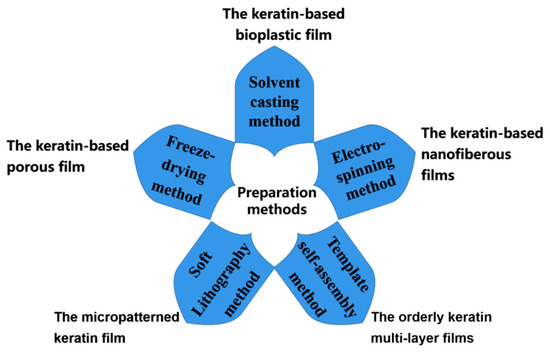
3. Preparation Method of Keratin-Based Biofilms
This section mainly reviews the main preparation methods for keratin-based biofilms, as shown in Figure 2.
Figure 2. Keratin-based biofilm preparation methods.
3.1. Solvent Casting Method
Solvent casting is an effective, low-cost, and simple-to-operate method for the development of keratin-based functional films. However, as the backbone of the natural fur and hair is heavily damaged during extraction, pure keratin films prepared using the solvent casting method may have poor mechanical properties, seriously hindering the application of the pure keratin film. To improve the mechanical properties of keratin-based biofilms, it is necessary to introduce other chemicals to modify the keratin during casting. At present, the main modification methods include grafting, blending, and cross-linking modification.
3.2. Electrospinning Method
In electrospinning, the keratin solution is sprayed under a strong electric field to obtain keratin filaments. The keratin filaments are then deposited and solidified onto a collecting device to form nanofibrous films. These keratin nanofibrous films have a large specific area, high aspect ratio, and high porosity. At the same time, they are uniform and their surface treatment is simple. As the keratin solution is sprayed into keratin filaments under a strong electric field during electrospinning, accurate control of the viscosity of the keratin solution is key to the success of electrospinning. The lower viscosity of the keratin solution will decrease the strength of the spinning, resulting in spinning failure; in contrast, the higher viscosity of the keratin solution will increase the resistance of spinning, also resulting in spinning failure. Keratin solution alone is difficult to spin, due to its poor viscosity [
109]; as such, it is usually necessary to add polymers or prepare high molecular weight keratin, in order to adjust the viscosity of the spinning fluid. Researchers have investigated the compatibility of keratin with various polymers in co-spinning, and successfully developed various types of keratin-based nanofibrous films [
110,
111].
3.3. Template Self-Assembly Method
The stability of keratin depends on certain factors, such as the degree of ionisation, amino acid sequence, the density of charges, and their distribution along the peptide chain. A large number of cysteine residues containing sulphur endow keratin with good self-assembly ability. Keratin is a desirable building block for template self-assembly. When the charged keratin solution is coated on the substrate surface, the cation and anion will be alternately deposited on the substrate surface due to electrostatic attraction. By inducing and regulating the self-assembly arrangements, regular and orderly keratin multi-layer films can be produced on the substrate surface.
3.4. Freeze-Drying Method
Keratin solution forms a distinctly interconnected porous network structure when freeze-dried. The freeze-drying process only relies on the sublimation of ice crystals in the keratin solution [
117]; as such, there is no need to introduce toxic chemical reagents in the freeze-drying process, making the operation process clean and facile.
3.5. Soft Lithography Method
Soft lithography is a facile, environmentally friendly, and functionally diverse patterned technology. The “Top-down” etching technology promotes the construction of protein micro- and nanostructures. Unlike traditional photolithography methods, soft lithography does not destroy the original structure and function of keratin. Zhu et al. [
127] developed a soft lithography approach to fabricate high-precision spatially patterned keratin film. In order to gain the photoactive wool keratin precursor, the wool keratin was modified using the chemical reagent 2-isocyanatoethyl methacrylate (IEM). Then, the wool keratin film with patterned microstructures was obtained by soft lithography. Their results showed that soft lithography was a facile method to acquire wool keratin film with surface microstructures and good optical properties.
4. Functional Properties of Keratin-Based Films
4.1. Biocompatibility
Cell binding motifs, such as leucine–aspartic acid–valine (LDV), glutamic acid–aspartic acid–serine (EDS), and arginine–glycine–aspartic acid (RGD) binding residues are generally present in keratin [
130,
131]. Furthermore, the extracted keratin has a strong self-assembly ability and can form regular and ordered structures for the regulation of cell behaviour [
132]. This can promote cell–matrix interactions and provide a good living environment for cells. The keratin-based biofilm can create a favourable 3D matrix displaying strong cytocompatibility or may be used as a culture dish to promote the adhesion, proliferation, and migration of cells in biomedicine.
4.2. Biodegradability
Keratin is a smart protein with high biodegradability, high sulphur content, and high nitrogen content, compared to other proteins. The proteins and amino acids in keratin-based films can be used as nutrients for plant growth [
139].
4.3. Hygroscopic Nature
A large number of hydrophilic groups (e.g., amino, carboxyl, and hydroxyl groups) are present in keratin. The random coil and α-helical structures are predominant in the keratin film, and the keratin film has a highly hydrophilic nature. The hydrogen and hydroxyl ions (H
+ and OH
−) formed by water dissociation move under the action of an applied electric field. Keratin chains tend to form a crystalline α-helical structure and yield an H-bond water network with amino acid residues on its side chain. This is also accompanied by proton transfer under high humidity conditions [
145]. Meanwhile, the structure of the keratin film is porous and rough. Therefore, the keratin film can be used as a humidity-sensing material for electrochemical sensors.
4.4. Adsorption
A large number of active groups, such as amino, hydroxyl, and carboxyl groups, on the molecular chain and the special backbone of keratin, confer a more appropriate adsorption capacity to keratin-based biofilm, compared with other low-cost absorbing materials [
152]. The polar amino acid residues contained in keratin can bind to cationic substances (e.g., heavy metals or dyes) [
153,
154], such as aspartic acid, glutamic acid, arginine, cystine, cysteine, and other amino acids, which account for more than 38% of the total amino acids [
155]. Modified keratin has shown strong adsorption capacity for the metals As, Cd, Cu, V
V, K, Co, Ni, Zn, and Cr
VI [
156]. Toxic water pollutants, such as heavy metals, dyes, and antibiotics, can be fixed in the network structure of the keratin-based film through complex reactions [
118,
157,
158,
159].