Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jinzhong Zhao | -- | 2152 | 2022-11-25 07:40:15 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2152 | 2022-11-28 02:58:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gao, H.; Wang, L.; Jin, H.; Lin, Z.; Li, Z.; Kang, Y.; Lyu, Y.; Dong, W.; Liu, Y.; Shi, D.; et al. Role of Macrophages in Tendon-Bone Healing. Encyclopedia. Available online: https://encyclopedia.pub/entry/36508 (accessed on 11 January 2026).
Gao H, Wang L, Jin H, Lin Z, Li Z, Kang Y, et al. Role of Macrophages in Tendon-Bone Healing. Encyclopedia. Available at: https://encyclopedia.pub/entry/36508. Accessed January 11, 2026.
Gao, Haihan, Liren Wang, Haocheng Jin, Zhiqi Lin, Ziyun Li, Yuhao Kang, Yangbao Lyu, Wenqian Dong, Yefeng Liu, Dingyi Shi, et al. "Role of Macrophages in Tendon-Bone Healing" Encyclopedia, https://encyclopedia.pub/entry/36508 (accessed January 11, 2026).
Gao, H., Wang, L., Jin, H., Lin, Z., Li, Z., Kang, Y., Lyu, Y., Dong, W., Liu, Y., Shi, D., Jiang, J., & Zhao, J. (2022, November 25). Role of Macrophages in Tendon-Bone Healing. In Encyclopedia. https://encyclopedia.pub/entry/36508
Gao, Haihan, et al. "Role of Macrophages in Tendon-Bone Healing." Encyclopedia. Web. 25 November, 2022.
Copy Citation
Tendon-bone healing comprises three stages: the inflammatory stage, the repair stage, and the remodeling stage. Macrophages appear in substantial populations in the early stages of tendon-bone healing and persist throughout the healing process, regulating cell proliferation, differentiation, and extracellular matrix formation by secreting various inflammatory factors and growth factors. The importance of macrophages in tendon-bone healing has been progressively emphasized in recent years, and the processes by which they affect tendon-bone healing have gradually been uncovered.
macrophage
tendon-to-bone interface
tendon-bone healing
1. Macrophages Phenotypes and Spectrum
Macrophages can be categorized into M1 and M2 phenotypes, based on their pro-inflammatory and anti-inflammatory functions [1][2]. M1 macrophages are primarily responsible for the eradication of harmful bacteria and the stimulation of inflammatory reactions, while M2 macrophages are responsible for the regulation of immunosuppression or allergic responses [1][2]. Categorizing macrophages as merely M1 or M2 is simplistic and does not accurately reflect the heterogeneity of macrophages in vivo; besides, it does not allow for in-depth research into the role that they play in tissue regeneration and repair. It is currently suggested that macrophage phenotypes can be further characterized, based on inducing stimuli; this is widely accepted and is used to classify macrophages in terms of tissue regeneration and repair [3]. The phenotypes of macrophages can be influenced by different stimuli, and, subsequently, the induced phenotypes of macrophages are capable of reacting in terms of tissue regeneration and repair (Figure 1). Under the stimulation of TNF-α, IFN-γ, and lipopolysaccharide (LPS), M0 macrophages can polarize to M1 macrophages; these clear pathogenic microorganisms and cell debris, while producing high levels of reactive oxygen species (ROS) and inflammatory factors (e.g., IL-1β and IL-6) [4]. Macrophages can convert into the M2a type, in response to IL-4 and IL-13 stimulation, and release arginase-1 (Arg-1) and insulin-like growth factor-1 (IGF-1) to enhance vascularization and collagen formation. M2a macrophages are essential in the synthesis of an extracellular matrix, which is indispensable for tissue repair [4][5]. The formation of M2b macrophages can be induced by immune complexes and LPS and will express CD86, CD68, and MHCII, which can secrete IL-10 to suppress inflammation and produce high levels of inflammatory factors (e.g., TNF-α, and IL-1β) [6][7]. The M2c macrophages, which can be induced by glucocorticoids, IL-10, and TGF-β, are capable of secreting IL-10 and matrix metalloproteinase, to regulate extracellular matrix remodeling and fibrosis [4,[8]. The formation of M2d macrophages can be induced by IL-6 and adenosine, which secrete transforming growth factor β (TGF-β) and vascular endothelial growth factor (VEGF) to enhance granulation tissue formation [9]. Until now, the specific signaling and differentiation cascades that produce specific macrophage phenotypes have remained unknown, and the significance of different phenotypes in the context of tissue regeneration has not yet been fully elucidated.
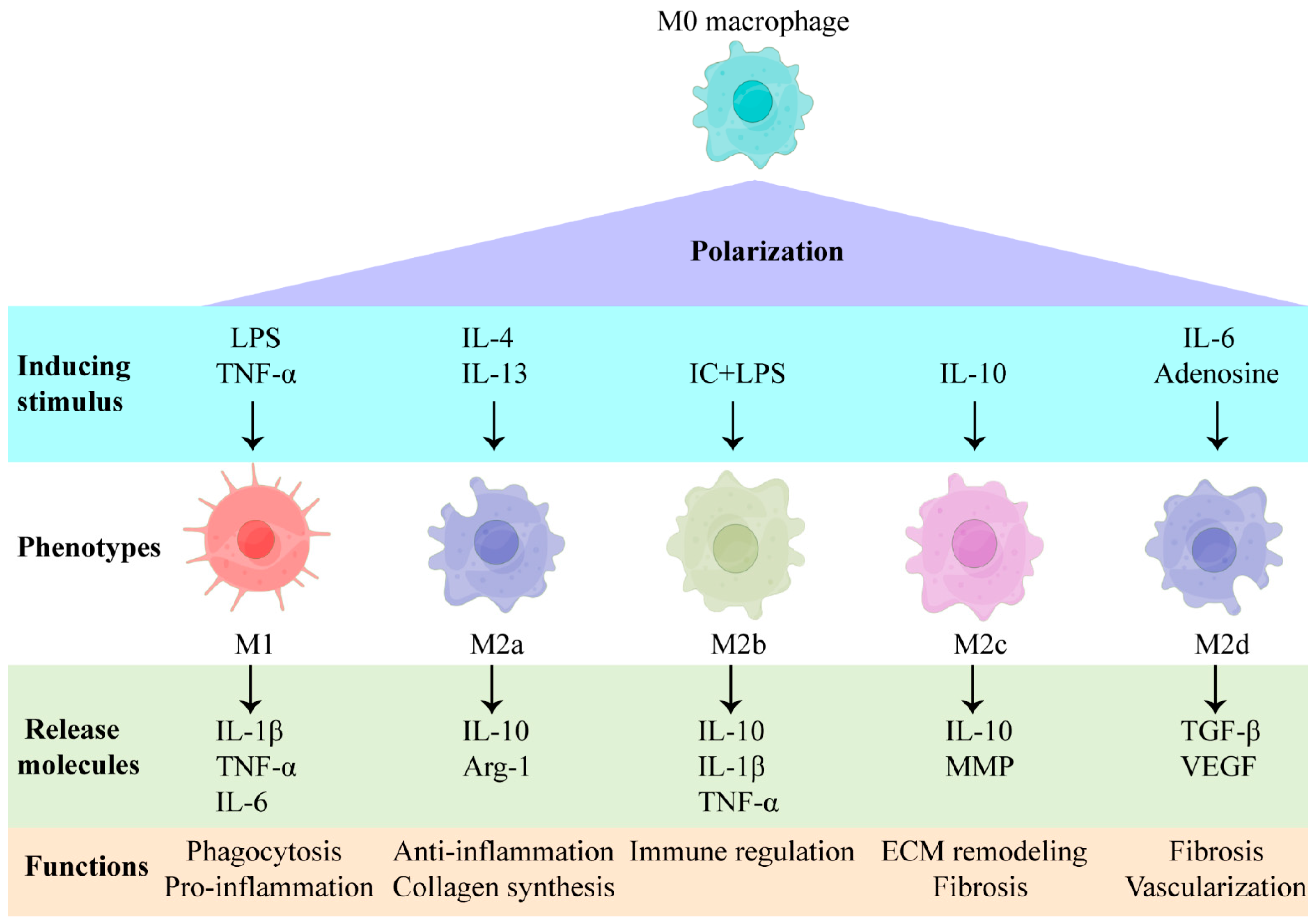
Figure 1. Inducing stimuli and the factors secreted by macrophages of different phenotypes. LPS: lipopolysaccharide; TNF-α: tumor necrosis factor-alpha; IL: interleukin; IC: immune complex; MMP: matrix metalloproteinase; Arg-1: arginase-1; TGF-β: transforming growth factor beta; VEGF: vascular endothelial growth factor.
2. The Impact of M1 Macrophages on Tendon-Bone Healing
M0 macrophages in circulation, bone marrow, and synovial membrane are recruited to the tendon-to-bone interface and polarize primarily into M1 macrophages during the inflammatory stage [10]. The expression levels of M1 macrophage markers at the tendon-to-bone interface peak on the third day after a rotator cuff injury and subsequently decrease gradually [11]. M1 macrophages can phagocytize cellular debris and eliminate foreign pathogens, as well as influence tendon-bone healing by secreting inflammatory factors (e.g., IL-1β, TNF-α, and IL-6).
IL-1β is rarely detected at native tendon-to-bone interfaces, but it is abundant in the fibrous scar tissue at the tendon-to-bone interface and in the surrounding joint fluid, which is assumed to be associated with poor tendon-bone healing [12][13]. IL-1β significantly reduces the expression of type-I collagen mRNA in tendon-derived progenitor cells and promotes the expression of cyclooxygenase-2, MMP1, MMP3, and prostaglandin E2 in human tendon cells, which correlates with the degradation of extracellular matrices [14][15]. Moreover, IL-1β impairs tendon-bone healing via the NF-κB pathway [16]. It activates the IκB kinase (IKK) complex, which phosphorylates and degrades the NF-κB dimer inhibitor, IκB, enabling NF-κB dimer transfer into the nucleus to increase the pro-inflammation factor gene expression [17]. The increased expression of IKK and NF-κB is detected in the tendon tissues of rotator cuff-tear patients and the tendon-to-bone interface tissues of acute rotator cuff-tear mice [16]. In IKK constitutively active mice, increased CD68+ macrophage infiltration and an apparent degeneration were observed in the tendon-to-bone interface, leading to a loss of the metachromasia at the fibrocartilage layer in the tendon-to-bone interface, and less bone volume in the humeral head [16]. The regeneration process after a tendon-to-bone interface injury was significantly improved in IKK conditional knock-out mice, compared to wild-type or IKK constitutively active mice, suggesting that activated NF-κB pathway impairs tendon-bone healing and that these changes may be related to an increase in pro-inflammatory cytokines and M1 macrophages (Figure 4) [16]. When the NF-κB pathway is activated, it leads to the decreased expression of Sox9, hindering the chondrogenic differentiation of MSCs, which may contribute to the poor regeneration of the fibrocartilage layer during tendon-bone healing [18][19]. IL-1β also leads to the excessive activity of osteoclasts around the tendon-to-bone interface, resulting in bone loss during tendon-bone healing and poor regeneration of the tendon-to-bone interface [20][21][22]. Hence, it can be concluded that IL-1β impedes tendon-bone healing, either directly or indirectly, by activating the NF-κB pathway, an important pathway that is implicated in poor tendon-to-bone interface regeneration.
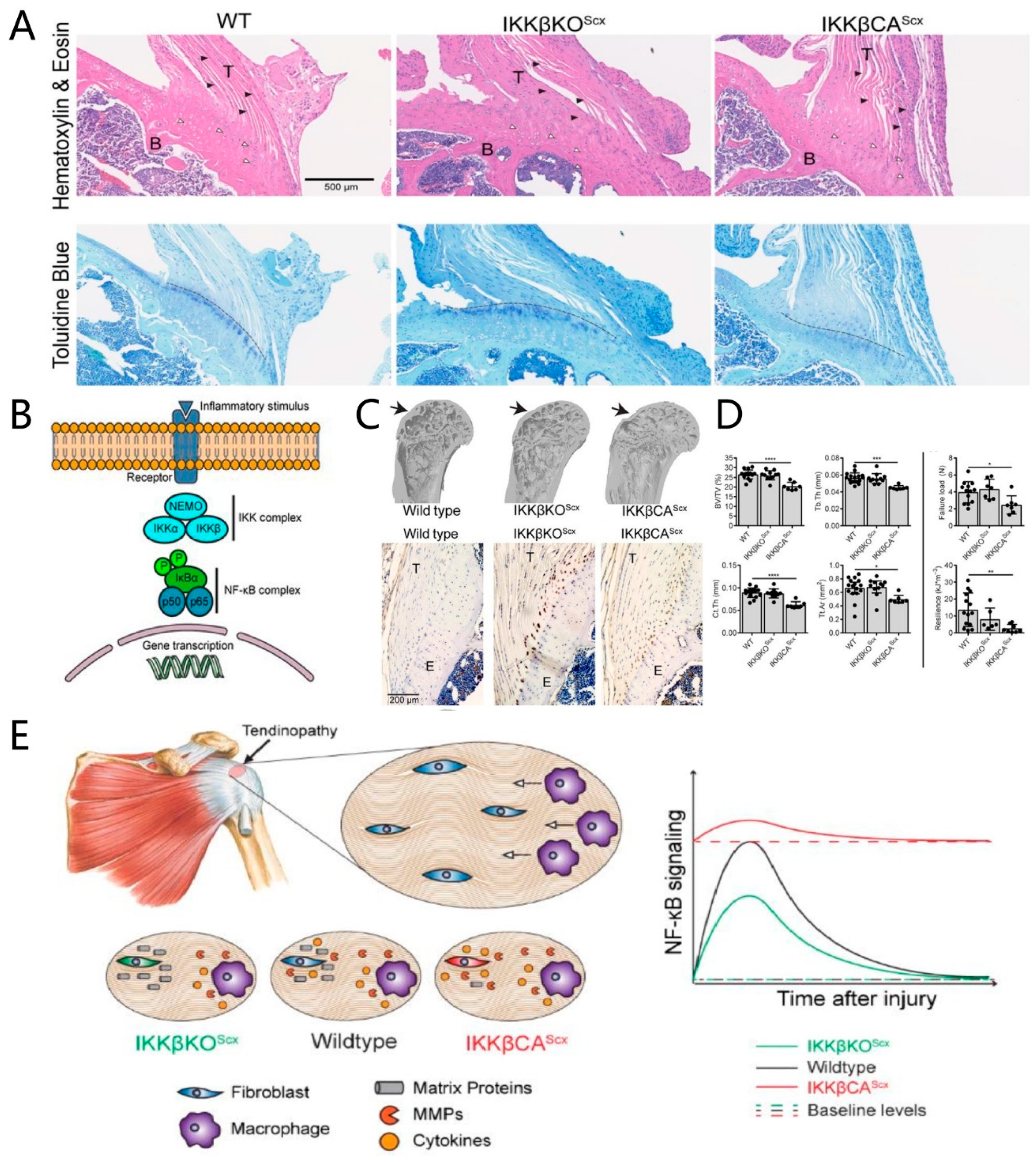
Figure 2. Activated IKK/NF-κB is detrimental to the tendon-to-bone interface and impedes tendon-bone healing. (A) Toluidine blue stain shows the loss of metachromasia at the fibrocartilage layer of the tendon-to-bone interface. Black arrowheads: spindle shaped tendon fibroblasts, white arrowheads: enthesis chondrocytes. Metachromasia demonstrating fibrocartilage interface can be seen below the dashed line in Toluidine blue stained sections; (B) schematic of NF-κB signaling and gene transcription; (C) microcomputed tomography (μCT) three-dimensional reconstruction shows the bone loss in activated IKK/NF-κB mice, while immunolabeling for CD68 shows more macrophage infiltration in activated IKK/NF-κB mice; (D) the quantification of bone morphometry and the quantification of mechanical properties show that IKK/NF-κB is detrimental to the tendon-to-bone interface. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05; (E) schematic of how IKK/NF-κB drives chronic tendinopathy and impairs tendon-bone healing. WT: wild-type; IKKβKOScx: IκB kinase, knocked out in the tendon fibroblast; IKKβCAScx: IκB kinase, overexpressed in the tendon fibroblast; T: tendon; B: bone; E: enthesis (tendon-to-bone interface). Copyright 2019, The American Association for the Advancement of Science.
TNF-α is also an inflammatory factor that is produced substantially by M1 macrophages during tendon-bone healing [23]. TNF-α expression is considerably higher in torn tendons, following rotator-cuff damage, than in intact tendons, which leads to the increased apoptosis of tendon stem cells, increased matrix metalloproteinase synthesis, and decreased type-I collagen synthesis, resulting in the degradation of the extracellular matrix [24][25]. TNF-α can also activate the NF-κB pathway, leading to the large-scale production of pro-inflammatory factors that exacerbate the inflammatory response during tendon-bone healing [17]. Under the stimuli of TNF-α, monocytes can be induced into forming osteoclasts, resulting in bone loss during tendon-bone healing [26]. Reducing TNF-α levels in Lewis rats decreases the number of M1 macrophages during tendon bone-healing and stimulates the regeneration of the fibrocartilage layer, ultimately improving biomechanical strength [27].
IL-6 is a downstream product of the NF-κB pathway and is abundant in torn tendons after rotator cuff tears, wherein it may play a dual role in tendon-bone healing [28][29]. IL-6 has both pro-inflammatory and anti-inflammatory properties, with the membrane-bound IL-6R mediating its anti-inflammatory effects and the soluble IL-6R mediating its pro-inflammatory effects [30]. While IL-6 can recruit monocytes and promote their differentiation into osteoclasts, they also enhance the release of the IL-1 receptor antagonist and IL-10 to suppress inflammation [28][30]. The level of TNF-α was higher in IL-6 knock-out mice than in wild-type mice after a patellar tendon injury, with the ratio of type-III collagen to type-I collagen in their injured tendons also being increased, suggesting that IL-6 is involved in the inflammation process and the remodeling of extracellular matrices during tendon repair [31]. Surprisingly, however, biomechanical tests showed that the maximum stress of an injured tendon was lower in IL-6 knock-out mice than in wild-type mice [31]. The above findings indicate that the role of IL-6 in tissue regeneration is complicated, with the influence of IL-6 on tendon-bone healing needing further research.
M1 macrophages not only regulate tissue regeneration by secreting inflammatory factors but they also release chemokines (e.g., CCL2, CXCL8, and SDF-1) to recruit mesenchymal stem cells (MSCs) to the site of damage [32][33]. After a rotator cuff injury, bone marrow MSCs can infiltrate through the bone tunnel on the greater tuberosity of the humerus to the tendon-to-bone interface, to promote tendon-bone healing [34]. Yang et al. (2019) used second near-infrared fluorescence imaging to track the distribution of exogenous MSCs in mice. After a supraspinatus tendon tear, exogenous MSCs were injected into the joint cavity immediately and appeared in large numbers at the injured tendon-to-bone interface on day 3 after injection, which facilitated the tendon-bone healing [35]. All these findings demonstrate that endogenous or exogenous MSCs can be recruited to the tendon-to-bone interface to facilitate tendon-bone healing. The chemokines released by M1 macrophages may play an essential role in MSCs recruitment during tendon-bone healing.
In general, during the early stages of tendon-bone healing, a large infiltration of M1 macrophages can phagocytize the cellular debris and foreign pathogens, while also recruiting MSCs to the tendon-to-bone interface by secreting chemokines, which has a beneficial effect on tendon-bone healing. Regrettably, once the inflammatory factors are secreted in excess, the inflammatory response of the local microenvironment will be exacerbated, which is detrimental to tendon-bone healing.
3. The Impact of M2 Macrophages on Tendon-Bone Healing
M1 macrophages account for the majority of macrophages during the inflammatory phase of tendon-bone healing, while M2 macrophages that are characterized by CD206 and Arg-1 are predominant during the subsequent repair and remodeling stages [11][36][37]. Due to the plasticity of macrophages, M2 macrophage accumulation during tissue healing may be derived from the repolarization of M1 macrophages or the induction of M0 macrophages under IL-4, IL-13, and other stimuli [38].
The ability of M2 macrophages to phagocytose pathogens and produce pro-inflammatory factors is limited, compared to that of the M1 macrophages, while their ability to release anti-inflammatory factors, including IL-4 and IL-10, is significantly increased [11][39]. These anti-inflammatory factors provide a suitable regenerative environment for subsequent tissue regeneration [40]. An M2 macrophage-conditioned medium promoted in vitro MSC osteogenesis; nevertheless, this positive effect was diminished with the addition of IL-10 neutralizing antibodies, suggesting that IL-10 is an important factor for M2 macrophages, to regulate tissue regeneration [41].
M2 macrophages also produce growth factors, such as TGF-β and VEGF, to regulate tissue regeneration [42][43]. TGF-β is highly attractive to macrophages and can form a positive feedback regulation of TGF-β and other growth factors via stimulating the macrophages [44][45]. The isoforms and concentrations of TGF-β play a decisive role in scar formation and tissue regeneration [46][47][48]. During tendon-bone healing after an acute rotator cuff injury in rats, TGF-β1 reaches the peak level at 10 days and is abundant in fibrous scar tissue, the main component of which is type-III collagen, indicating that TGF-β1 may be responsible for fibrous scar tissue formation at the injured tendon-to-bone interface [49]. When using exogenous TGF-β1 to promote tendon-bone healing after an acute rotator cuff injury in a rat model, although biomechanical strength was improved, TGF-β1 can elevate the transcriptional level of Col3a1 and enhance the formation of fibrous scar tissue [50]. In contrast, TGF-β3, which promotes fibrocartilage formation, is rarely expressed during tendon-bone healing [49][51]. Histological analysis revealed that the addition of TGF-β3 promoted the regeneration of fibrocartilage at the injured tendon-to-bone interface in the rotator cuff, thereby enhancing biomechanical strength after healing, with limited scar formation [52].
The growth factors released by macrophages, such as VEGF and BMP-2, play a significant role in tissue regeneration as well [53][54]. M2 macrophages were induced by sulfated chitosan in a mouse model, which subsequently facilitated endogenous VEGF production to induce the vascularization of ischemic disease [55]. Bone marrow MSC-derived exosomes stimulate the polarization of M2 macrophages and increase the expression of VEGF, which promotes vascularization around the injured tendon-to-bone interface in rats, beneficial for tendon-bone healing [56]. The regulation of M2 macrophage polarization through a surface topography design of honeycomb-like TiO2 can facilitate macrophages to release more BMP-2, to promote osteogenesis in a rat tibia implantation model [57].
M2 macrophages are believed to gradually replace M1 macrophages after the inflammatory stage to regulate tissue regeneration by secreting various anti-inflammatory and growth factors. Although the evidence that M2 macrophages promote tendon-bone healing by suppressing the inflammatory response is quite strong, the role of growth factors secreted by M2 macrophages on tendon-bone healing remains vague and controversial. For example, TGF-β1 secreted by macrophages may be the main cause of fibrous scar formation. Further studies are needed to illustrate the intricated correlation between the various factors secreted by macrophages and tendon-bone healing.
References
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709.
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13.
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13.
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419.
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097.
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396.
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545.
- Garash, R.; Bajpai, A.; Marcinkiewicz, B.M.; Spiller, K.L. Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp. Biol. Med. 2016, 241, 1054–1063.
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 58.
- Kawamura, S.; Ying, L.; Kim, H.; Dynybil, C.; Rodeo, S. Macrophages accumulate in the early phase of tendon–bone healing. J. Orthop. Res. 2005, 23, 1425–1432.
- Sunwoo, J.Y.; Eliasberg, C.D.; Carballo, C.B.; Rodeo, S.A. The role of the macrophage in tendinopathy and tendon healing. J. Orthop. Res. 2020, 38, 1666–1675.
- Gotoh, M.; Hamada, K.; Yamakawa, H.; Tomonaga, A.; Inoue, A.; Fukuda, H.O. Significance of granulation tissue in torn supraspinatus insertions: An immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J. Orthop. Res. 1997, 15, 33–39.
- Ma, J.; Piuzzi, N.S.; Muschler, G.F.; Iannotti, J.P.; Ricchetti, E.T.; Derwin, K.A. Biomarkers of Rotator Cuff Disease Severity and Repair Healing. JBJS Rev. 2018, 6, e9.
- Tsuzaki, M.; Guyton, G.; Garrett, W.; Archambault, J.M.; Herzog, W.; Almekinders, L.; Bynum, D.; Yang, X.; Banes, A.J. IL-1 beta induces COX2, MMP-1,-3 and-13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res. 2003, 21, 256–264.
- Zhang, K.; Asai, S.; Yu, B.; Enomoto-Iwamoto, M. IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem. Biophys. Res. Commun. 2015, 463, 667–672.
- Abraham, A.C.; Shah, S.A.; Golman, M.; Song, L.; Li, X.; Kurtaliaj, I.; Akbar, M.; Millar, N.L.; Abu-Amer, Y.; Galatz, L.M.; et al. Targeting the NF-κB signaling pathway in chronic tendon disease. Sci. Transl. Med. 2019, 11, eaav4319.
- Park, M.H.; Hong, J.T. Roles of NF-kappaB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15.
- Xu, Z.; Ke, T.; Zhang, Y.; Fu, C.; He, W. Agonism of GPR120 prevented IL-1β-induced reduction of extracellular matrix through SOX-9. Aging 2020, 12, 12074–12085.
- Ousema, P.H.; Moutos, F.T.; Estes, B.T.; Caplan, A.I.; Lennon, D.P.; Guilak, F.; Weinberg, J.B. The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds. Biomaterials 2012, 33, 8967–8974.
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8.
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009, 183, 1862–1870.
- Xu, J.; Su, W.; Chen, J.; Ye, Z.; Wu, C.; Jiang, J.; Yan, X.; Cai, J.; Zhao, J. The Effect of Antiosteoporosis Therapy with Risedronate on Rotator Cuff Healing in an Osteoporotic Rat Model. Am. J. Sports Med. 2021, 49, 2074–2084.
- Millar, N.L.; Murrell, G.A.; McInnes, I.B. Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122.
- Millar, N.L.; Wei, A.Q.; Molloy, T.J.; Bonar, F.; Murrell, G.A.C. Cytokines and apoptosis in supraspinatus tendinopathy. J. Bone Jt. Surg.-Br. Vol. 2009, 91, 417–424.
- Moqbel, S.A.A.; Xu, K.; Chen, Z.; Xu, L.; He, Y.; Wu, Z.; Ma, C.; Ran, J.; Wu, L.; Xiong, Y. Tectorigenin Alleviates Inflammation, Apoptosis, and Ossification in Rat Tendon-Derived Stem Cells via Modulating NF-Kappa B and MAPK Pathways. Front. Cell Dev. Biol. 2020, 8, 568894.
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-kappaB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44.
- Gulotta, L.V.; Kovacevic, D.; Cordasco, F.; Rodeo, S.A. Evaluation of tumor necrosis factor alpha blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy 2011, 27, 1351–1357.
- Abraham, A.C.; Shah, S.A.; Thomopoulos, S. Targeting Inflammation in Rotator Cuff Tendon Degeneration and Repair. Tech. Shoulder Elb. Surg. 2017, 18, 84–90.
- Nakama, K.; Gotoh, M.; Yamada, T.; Mitsui, Y.; Yasukawa, H.; Imaizumi, T.; Higuchi, F.; Nagata, K. Interleukin-6-induced activation of signal transducer and activator of transcription-3 in ruptured rotator cuff tendon. J. Int. Med. Res. 2006, 34, 624–631.
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888.
- Lin, T.W.; Cardenas, L.; Glaser, D.L.; Soslowsky, L.J. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J. Biomech. 2006, 39, 61–69.
- Lu, D.; Xu, Y.; Liu, Q.; Zhang, Q. Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 681171.
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89.
- Kida, Y.; Morihara, T.; Matsuda, K.; Kajikawa, Y.; Tachiiri, H.; Iwata, Y.; Sawamura, K.; Yoshida, A.; Oshima, Y.; Ikeda, T.; et al. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J. Shoulder Elb. Surg. 2013, 22, 197–205.
- Yang, Y.; Chen, J.; Shang, X.; Feng, Z.; Chen, C.; Lu, J.; Cai, J.; Chen, Y.; Zhang, J.; Hao, Y.; et al. Visualizing the Fate of Intra-Articular Injected Mesenchymal Stem Cells In Vivo in the Second Near-Infrared Window for the Effective Treatment of Supraspinatus Tendon Tears. Adv. Sci. 2019, 6, 1901018.
- Sugg, K.B.; Lubardic, J.; Gumucio, J.P.; Mendias, C.L. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J. Orthop. Res. 2014, 32, 944–951.
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2596–2606.
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969.
- Hou, J.; Yang, R.; Vuong, I.; Li, F.; Kong, J.; Mao, H.Q. Biomaterials strategies to balance inflammation and tenogenesis for tendon repair. Acta Biomater. 2021, 130, 1–16.
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.H.G.; et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 2020, 239, 119833.
- Fu, C.; Huang, A.H.; Galatz, L.M.; Han, W.M. Cellular and molecular modulation of rotator cuff muscle pathophysiology. J. Orthop. Res. 2021, 39, 2310–2322.
- Chu, C.; Deng, J.; Sun, X.; Qu, Y.; Man, Y. Collagen Membrane and Immune Response in Guided Bone Regeneration: Recent Progress and Perspectives. Tissue Eng. Part B Rev. 2017, 23, 421–435.
- Rappolee, D.A.; Werb, Z. Macrophage-derived growth factors. Curr. Top. Microbiol. Immunol. 1992, 181, 87–140.
- Wahl, S.M.; Hunt, D.A.; Wakefield, L.M.; McCartney-Francis, N.; Wahl, L.M.; Roberts, A.B.; Sporn, M.B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. USA 1987, 84, 5788–5792.
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222.
- Longaker, M.T.; Bouhana, K.S.; Harrison, M.R.; Danielpour, D.; Roberts, A.B.; Banda, M.J. Wound healing in the fetus: Possible role for inflammatory macrophages and transforming growth factor-beta isoforms. Wound Repair Regen. 1994, 2, 104–112.
- Lech, M.; Anders, H.J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 2013, 1832, 989–997.
- Galatz, L.M.; Sandell, L.J.; Rothermich, S.Y.; Das, R.; Mastny, A.; Havlioglu, N.; Silva, M.J.; Thomopoulos, S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J. Orthop. Res. 2006, 24, 541–550.
- Arimura, H.; Shukunami, C.; Tokunaga, T.; Karasugi, T.; Okamoto, N.; Taniwaki, T.; Sakamoto, H.; Mizuta, H.; Hiraki, Y. TGF-beta1 Improves Biomechanical Strength by Extracellular Matrix Accumulation Without Increasing the Number of Tenogenic Lineage Cells in a Rat Rotator Cuff Repair Model. Am. J. Sports Med. 2017, 45, 2394–2404.
- Qu, D.; Zhu, J.P.; Childs, H.R.; Lu, H.H. Nanofiber-based transforming growth factor-beta3 release induces fibrochondrogenic differentiation of stem cells. Acta Biomater. 2019, 93, 111–122.
- Kovacevic, D.; Fox, A.J.; Bedi, A.; Ying, L.; Deng, X.H.; Warren, R.F.; Rodeo, S.A. Calcium-phosphate matrix with or without TGF-beta3 improves tendon-bone healing after rotator cuff repair. Am. J. Sports Med. 2011, 39, 811–819.
- Xiao, H.; Chen, Y.; Li, M.; Shi, Q.; Xu, Y.; Hu, J.; Li, X.; Chen, C.; Lu, H. Cell-Free Book-Shaped Decellularized Tendon Matrix Graft Capable of Controlled Release of BMP-12 to Improve Tendon Healing in a Rat Model. Am. J. Sports Med. 2021, 49, 1333–1347.
- Prabhath, A.; Vernekar, V.N.; Sanchez, E.; Laurencin, C.T. Growth factor delivery strategies for rotator cuff repair and regeneration. Int. J. Pharm. 2018, 544, 358–371.
- Yu, Y.M.; Dai, K.; Gao, Z.H.; Tang, W.; Shen, T.; Yuan, Y.; Wang, J.; Liu, C.S. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7, eabd8217.
- Huang, Y.; He, B.; Wang, L.; Yuan, B.; Shu, H.; Zhang, F.; Sun, L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res. 2020, 11, 496.
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
28 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




