
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Évellin do Espirito Santo | -- | 2798 | 2022-11-24 17:02:54 | | | |
| 2 | Lindsay Dong | -2 word(s) | 2796 | 2022-11-25 03:04:43 | | |
Video Upload Options
In nature, interactions between bacteria and microalgae play an indispensable role in maintaining the integrity of the aquatic ecosystem through networks of interactions such as competition and mutualism. In fact, in the wild, the growth of algae is consistently associated with the growth of other microorganisms, especially bacteria. Axenic culture systems and sterilization of culture media in large-scale production of microalga are not economically feasible. Therefore, the characterization of associated heterotrophs in algae culture systems is an important step since bacteria may use compounds excreted by algae, increasing the availability of trace elements and solubility of nutrients, making them more bioavailable for microalgae. In addition, they can help to reduce the saturation of dissolved O2. In microalgae cultivation, it is well known that dissolved O2 can attain inhibitory levels.
1. Microalgae-Bacteria Interaction
1.1. Microbial Interactions through Quorum Sensing
1.2. Oxygen and Carbon Dioxide Exchanges
2. Effects of Interactions
2.1. Inhibitory Effect by Metabolites on Algae and Bacteria
2.2. Bacteria That Promote the Growth of Microalgae
2.3. Supply of Nutrients
2.4. Modification of the Composition of Microalgae in Co-Culture
The association of Rhizobium sp. KB10 with B. braunii increased algae growth by nine times and improved the oleate content, used to produce biodiesel [29]. Inoculation of the bacterial strain Rhizobium 10II in the cultivation of Ankistrodesmus sp. strain SP2-15 increased by 30% the chlorophyll content in the microalgae biomass, and the lipid productivity was up to 112 g.m−2d−1 on the sixth day of cultivation [30]. The co-cultivation of Chlamydomonas reinhardtii with Bradyrhizobium japonicum improved the growth of the microalgae by 3.9 times, reaching lipid contents 26% higher and increasing Fe-hydrogenase activity and H2 production [31].
2.5. Flocculant Activity by Bacteria
2.6. Microalgae Co-Immobilization by Bacteria
3. Microalgae as Potential Raw Material for Bioproducts
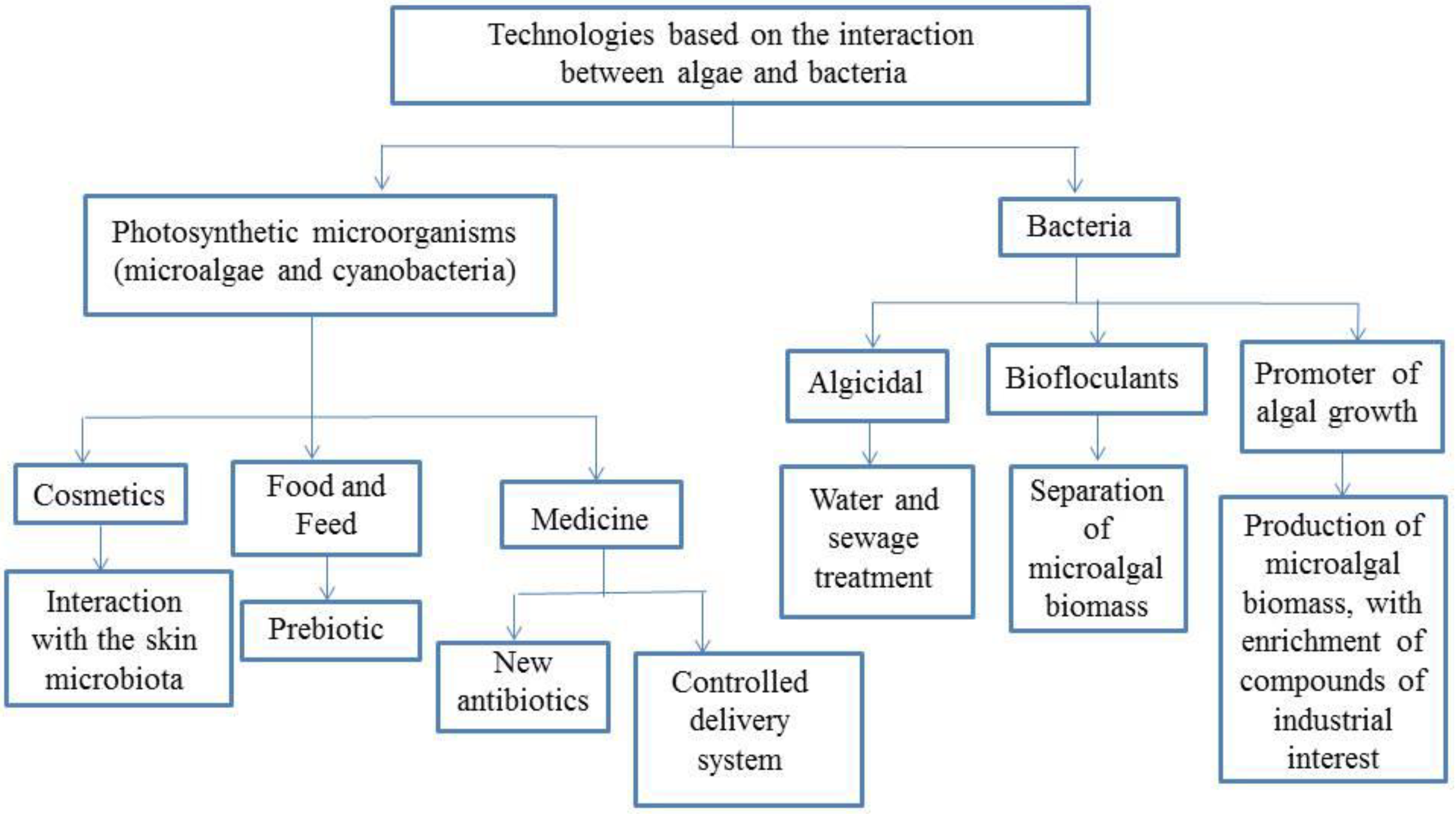
3.1. Extracts for Microbial Growth
3.2. Bioproducts
3.2.1. Microalgae for Developing Prebiotic Products
3.2.2. Animal Diet
3.3. Cosmetics
3.4. Pharmaceuticals
References
- Papenfort, K.; Bassler, B.L. Quorum Sensing Signal-Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588.
- Santos, C.A.; Almeida, F.A.; Quecán, B.X.V.; Pereira, P.A.P.; Gandra, K.M.B.; Cunha, L.R.; Pinto, U.M. Bioactive Properties of Syzygium Cumini (L.) Skeels Pulp and Seed Phenolic Extracts. Front. Microbiol. 2020, 11, 990.
- Lima, E.M.F.; Quecán, B.X.V.; Cunha, L.R.; Franco, B.D.G.M.; Pinto, U.M. Cell-Cell Communication in Lactic Acid Bacteria: Potential Mechanisms; Albuquerque, M.A.C., LeBlanc, A.M., LeBlanc, J.G.J., Salvio, R.B., Eds.; Lactid Aci.; CRC Press: Boca Raton, FL, USA, 2020.
- De Almeida, F.A.; Carneiro, D.G.; Mendes, T.A.D.O.; Barros, E.; Pinto, U.M.; De Oliveira, L.L.; Vanetti, M.C.D. N-Dodecanoyl-Homoserine Lactone Influences the Levels of Thiol and Proteins Related to Oxidation-Reduction Process in Salmonella. PLoS ONE 2018, 13, e0204673.
- Kim, C.S.; Gatsios, A.; Cuesta, S.; Lam, Y.C.; Wei, Z.; Chen, H.; Russell, R.M.; Shine, E.E.; Wang, R.; Wyche, T.P.; et al. Characterization of Autoinducer-3 Structure and Biosynthesis in E. Coli. ACS Cent. Sci. 2020, 6, 197–206.
- Brencic, A.W.S.C. Detection of and Response to Signals Involved in Host-Microbe Interactions by Plant-Associated Bacteria. Microbiol. Mol. Biol. Rev. 2016, 69, 155–194.
- Dow, L. How Do Quorum-Sensing Signals Mediate Algae–Bacteria Interactions? Microorganisms 2021, 9, 1391.
- Zhou, J.; Lyu, Y.; Richlen, M.L.; Anderson, D.M.; Cai, Z. Quorum Sensing Is a Language of Chemical Signals and Plays an Ecological Role in Algal-Bacterial Interactions. CRC. Crit. Rev. Plant Sci. 2016, 35, 81–105.
- Natrah, F.M.I.; Bossier, P.; Sorgeloos, P.; Yusoff, F.M.; Defoirdt, T. Significance of Microalgal-Bacterial Interactions for Aquaculture. Rev. Aquac. 2014, 6, 48–61.
- Zhang, C.; Li, Q.; Fu, L.; Zhou, D.; Crittenden, J.C. Quorum Sensing Molecules in Activated Sludge Could Trigger Microalgae Lipid Synthesis. Bioresour. Technol. 2018, 263, 576–582.
- Das, S.; Das, S.; Ghangrekar, M.M. Quorum-Sensing Mediated Signals: A Promising Multi-Functional Modulators for Separately Enhancing Algal Yield and Power Generation in Microbial Fuel Cell. Bioresour. Technol. 2019, 294, 122138.
- Amin, S.A.; Hmelo, L.R.; Van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and Signalling between a Cosmopolitan Phytoplankton and Associated Bacteria. Nature 2015, 522, 98–101.
- Segev, E.; Castañeda, I.S.; Sikes, E.L.; Vlamakis, H.; Kolter, R. Bacterial Influence on Alkenones in Live Microalgae. J. Phycol. 2016, 52, 125–130.
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of Cyanobacteria/Microalgae and Bacteria: Biotechnological Potential. Biotechnol. Adv. 2011, 29, 896–907.
- Mujtaba, G.; Lee, K. Advanced Treatment of Wastewater Using Symbiotic Co-Culture of Microalgae and Bacteria. Appl. Chem. Eng. 2016, 27, 1–9.
- Carvalho, J.C.M.; Morocho-Jacome, A.L.; Mejia-Silva, L.C.; Pérez-Mora, L.S.; Bresaola, M.D.; Ishii, M. No Processo Para Preparação de Uma Solução Salina Partir Da Vinhaça e Seus Usos 2021. BR10202100104, 19 January 2021.
- Astafyeva, Y.; Gurschke, M.; Qi, M.; Bergmann, L.; Indenbirken, D.; de Grahl, I.; Katzowitsch, E.; Reumann, S.; Hanelt, D.; Alawi, M.; et al. Microalgae and Bacteria Interaction-Evidence for Division of Diligence in the Alga Microbiota. Microbiol. Spectr. 2022, 10, e0063322.
- Paul, C.; Pohnert, G. Interactions of the Algicidal Bacterium Kordia algicida with Diatoms: Regulated Protease Excretion for Specific Algal Lysis. PLoS ONE 2011, 6, e21032.
- Guo, Z.; Tong, Y.W. The Interactions between Chlorella vulgaris and Algal Symbiotic Bacteria under Photoautotrophic and Photoheterotrophic Conditions. J. Appl. Phycol. 2014, 26, 1483–1492.
- Rivas, M.O.; Vargas, P.; Riquelme, C.E. Interactions of Botryococcus braunii Cultures with Bacterial Biofilms. Microb. Ecol. 2010, 60, 628–635.
- Le Chevanton, M.; Garnier, M.; Bougaran, G.; Schreiber, N.; Lukomska, E.; Bérard, J.B.; Fouilland, E.; Bernard, O.; Cadoret, J.P. Screening and Selection of Growth-Promoting Bacteria for Dunaliella Cultures. Algal Res. 2013, 2, 212–222.
- Sforza, E.; Pastore, M.; Santeufemia Sanchez, S.; Bertucco, A. Bioaugmentation as a Strategy to Enhance Nutrient Removal: Symbiosis between Chlorella protothecoides and Brevundimonas diminuta. Bioresour. Technol. Rep. 2018, 4, 153–158.
- Lee, C.; Jeon, M.S.; Kim, J.Y.; Lee, S.H.; Kim, D.G.; Roh, S.W.; Choi, Y.E. Effects of an Auxin-Producing Symbiotic Bacterium on Cell Growth of the Microalga Haematococcus Pluvialis: Elevation of Cell Density and Prolongation of Exponential Stage. Algal Res. 2019, 41, 101547.
- De-Bashan, L.E.; Antoun, H.; Bashan, Y. Involvement of Indole-3-Acetic Acid Produced by the Growth-Promoting Bacterium Azospirillum Spp. in Promoting Growth of Chlorella vulgaris. J. Phycol. 2008, 44, 938–947.
- Palacios, O.A.; Lopez, B.R.; Bashan, Y.; de -Bashan, L.E. Early Changes in Nutritional Conditions Affect Formation of Synthetic Mutualism Between Chlorella sorokiniana and the Bacterium Azospirillum brasilense. Microb. Ecol. 2019, 77, 980–992.
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae Acquire Vitamin B12 through a Symbiotic Relationship with Bacteria. Nature 2005, 438, 90–93.
- Fukami, K.; Nishijima, T.; Ishida, Y. Stimulative and Inhibitory Effects of Bacteria on the Growth of Microalgae. Hydrobiologia 1997, 358, 185–191.
- Heo, J.; Kim, S.; Cho, D.H.; Song, G.C.; Kim, H.S.; Ryu, C.M. Genome-Wide Exploration of Escherichia coli Genes to Promote Chlorella vulgaris Growth. Algal Res. 2019, 38, 101390.
- Oh, H.M.; Ahn, C.Y.; Lee, Y.K.; Ko, S.R.; Kim, H.S. Novel Microorganism Rhizobium sp. Publication Calssifcation KB 10 Having Properties of Promoting Growth of Botryococcus braunii and Increased Fatty Acid Content. US20140087420A1, 11 August 2015.
- Do Nascimento, M.; Dublan, M.; de Los, A.; Ortiz-Marquez, J.C.F.; Curatti, L. High Lipid Productivity of an Ankistrodesmus-Rhizobium Artificial Consortium. Bioresour. Technol. 2013, 146, 400–407.
- Wu, S.; Li, X.; Yu, J.; Wang, Q. Increased Hydrogen Production in Co-Culture of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Bioresour. Technol. 2012, 123, 184–188.
- González-Fernández, C.; Ballesteros, M. Microalgae Autoflocculation: An Alternative to High-Energy Consuming Harvesting Methods. J. Appl. Phycol. 2013, 25, 991–999.
- Carvalho, J.C.M.; Tanaka, A.E.; Morocho-Jacome, A.L.; Cezare-Gomes, E.A.; Santo, E.E.; Pérez-Mora, L.S.; Ishii, M. Processo para Obtenção de Extratos Promotores do Crescimento Microbiano De Origem Vegetal E De Origem Microbiana, Extratos Promotores do Crescimento Microbiano, Processo para Obtenção de Biomassa de Microrganismos Fotossintetizantes, Biomassa de Microrganismos Fotossintetizantes, E Usos Da Biomassa De Microrganismos Fotossintetizantes. BR1020210100524, 8 September 2021.
- Lee, J.; Cho, D.H.; Ramanan, R.; Kim, B.H.; Oh, H.M.; Kim, H.S. Microalgae-Associated Bacteria Play a Key Role in the Flocculation of Chlorella vulgaris. Bioresour. Technol. 2013, 131, 195–201.
- Moreno-Garrido, I. Microalgae Immobilization: Current Techniques and Uses. Bioresour. Technol. 2008, 99, 3949–3964.
- de Medeiros, V.P.B.; Salgaço, M.K.; Pimentel, T.C.; da Silva, T.C.R.; Sartoratto, A.; dos Santos Lima, M.; da Costa Sassi, C.F.; Mesa, V.; Magnani, M.; Sivieri, K. Spirulina platensis Biomass Enhances the Proliferation Rate of Lactobacillus acidophilus 5 (La-5) and Combined with La-5 Impact the Gut Microbiota of Medium-Age Healthy Individuals through an in Vitro Gut Microbiome Model. Food Res. Int. 2022, 154, 110880.
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Morinigo, M.Á.; Esteban, M.Á. Histological Alterations and Microbial Ecology of the Intestine in Gilthead Seabream (Sparus aurata L.) Fed Dietary Probiotics and Microalgae. Cell Tissue Res. 2012, 350, 477–489.
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of Microbial Products in Cosmetic Industry. Nat. Prod. Bioprospect. 2019, 9, 267–278.
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic Attributes of Algae—A Review. Algal Res. 2017, 25, 483–487.
- Lemoine, V.; Bernard, C.; Leman-Loubière, C.; Clément-Larosière, B.; Girardot, M.; Boudesocque-Delaye, L.; Munnier, E.; Imbert, C. Nanovectorized Microalgal Extracts to Fight Candida albicans and Cutibacterium acnes Biofilms: Impact of Dual-Species Conditions. Antibiotics 2020, 9, 279.
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially Important Bioproducts from Microalgae and Their Current Applications-A Review. Energy Procedia 2019, 160, 752–760.
- Shchelik, I.S.; Sieber, S.; Gademann, K. Green Algae as a Drug Delivery System for the Controlled Release of Antibiotics. Chem. A Eur. J. 2020, 26, 16644–16648.
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Smaoui, S.; Abdelkafi, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780.




