Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | shubai Liu | -- | 2236 | 2022-11-24 16:54:31 | | | |
| 2 | Peter Tang | Meta information modification | 2236 | 2022-11-25 02:49:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
He, Z.; Lin, J.; He, Y.; Liu, S. Polysaccharide-Peptide from Trametes versicolor for Colorectal Cancer Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/36404 (accessed on 12 January 2026).
He Z, Lin J, He Y, Liu S. Polysaccharide-Peptide from Trametes versicolor for Colorectal Cancer Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/36404. Accessed January 12, 2026.
He, Zhicheng, Jian Lin, Yingying He, Shubai Liu. "Polysaccharide-Peptide from Trametes versicolor for Colorectal Cancer Treatment" Encyclopedia, https://encyclopedia.pub/entry/36404 (accessed January 12, 2026).
He, Z., Lin, J., He, Y., & Liu, S. (2022, November 24). Polysaccharide-Peptide from Trametes versicolor for Colorectal Cancer Treatment. In Encyclopedia. https://encyclopedia.pub/entry/36404
He, Zhicheng, et al. "Polysaccharide-Peptide from Trametes versicolor for Colorectal Cancer Treatment." Encyclopedia. Web. 24 November, 2022.
Copy Citation
The incidence and mortality of colorectal cancer have shown an upward trend. Therefore, the prevention, diagnosis, and treatment of colorectal cancer still need our continuous attention. Finding compounds with strong anticancer activity and low toxicity is a good strategy for colorectal cancer (CRC) therapy. Trametes versicolor is a traditional Chinese medicinal mushroom with a long history of being used to regulate immunity and prevent cancer. Its extractions were demonstrated with strong cell growth inhibitory activity on human colorectal tumor cells, while the anticancer activity of them is not acted through a direct cytotoxic effect.
Trametes versicolor
polysaccharide peptide
colorectal cancer
natural Medicine
traditional Medicine
1. Introduction
Cancer is the second leading cause of mortality in the world and with 9.9 million deaths in 2020. Colorectal cancer (CRC) is the second leading cause of malignant tumor-related deaths and the third most diagnosed cancer in the world [1]. According to data from Globocan in 2020 [2], there are 1.93 million new cases of CRC and 935,175 people that die from CRC. Compared with 2012 [3], the case numbers were increased about 330,000 and 240,000 people, respectively. Therefore, the prevention, diagnosis, and treatment of colorectal cancer still need to be continuously attended. At present, the major clinical treatments for colorectal cancer are surgery and chemotherapy, while natural products and their derivatives play important roles in chemotherapy. Natural products are compounds that are extracted from plants, animals, and microorganisms. The diverse pharmacological activities of natural products are based on the complexity and diversity of their structures and also provide numerous lead compounds for the discovery of new drugs. Natural product researchers have been searching for compounds that are effective at killing tumor cells but more friendly to normal cells in last few decades due to most types of natural products inhibiting the proliferation of cancer cells by their highly cytotoxic nature, and their side effects affect patients’ quality of life.
Traditional Chinese medicine uses Trametes versicolor (formerly Coriolus versicolor) for its longevity-enhancing and health-promoting properties. Polysaccharide Krestin (PSK) or polysaccharide peptide (PSP) are two natural products extracted from Trametes versicolor, and their main components are a highly heterogeneous mixture of β-glucan macromolecules that possess a molecular weight of approximately 100 kDa and contain various moieties, including peptides, bound to β-glucan backbones [4][5][6]. They have been used as adjuvant therapy for cancer in Japan and China [7] and are commonly considered as nontoxic in addition to having no adverse effects [8]. The complexity and high molecular weight of PSK and PSP, however, make it difficult to study their mechanism of action [9], and for a long time, they have only been used as adjuvant to supplement chemotherapy and radiation therapy rather than as an anti-cancer drug for clinical treatment. In 1992, Yang et al. [10] isolated a small polypeptide with a molecular weight of around 10 KDa from the crude extraction of Coriolus versicolor (Cov-1) polysaccharide peptide, and its anti-cancer activities were significantly higher than PSP and PSK. A polysaccharide–peptide complex with a molecular mass of approximately 15.5 KDa was discovered by Wang et al. in 1996 [11] isolated, and it possessed the activities of inhibiting the growth of mice-implanted sarcoma 180 cells. The results of this study advanced the research on Trametes versicolor glycopeptide. However, the structure of these small molecular weight glycopeptides was still unclear at the time due to the technical limitations, and no more studies on pharmacological mechanisms were conducted. Musarin, a novel 12 kDa polysaccharide peptide isolated from Trametes versicolor powder, was recently found and described by He et al. [12]. Its protein sequence was verified, and its 3D structure was predicted in this study. Studies on the associated mechanism have shown that musarin has potent anti-colon cancer activity without being harmful to healthy colon cells in both in vivo and in vitro tests.
2. PSK and PSP: Mainly Polysaccharide Peptides in Trametes versicolor
Due to its wide range of adhibition as a food supplement in western countries, Trametes versicolor has experienced growth in popularity. The primary products are polysaccharide Krestin (PSK) and polysaccharide peptide (PSP), which were extracted from different strains of Trametes versicolor, including “CM-101” (PSP in China) and “Cov-1” (PSP Krestin or PSK in Japan) [8]. Their main components are a highly heterogeneous mixture of β-glucan macromolecules with a molecular weight of about 100 kDa that contain various moieties, including peptides, bound to their backbones [4][5][6]; the contents of other sugar components such as fucose, galactose, mannose, and xylose were different [7][13]. Their differences are mainly in peptide content and glycan composition; PSP contains about 10–30 percent peptides, while PSK contains up to 90 percent peptide [14]. Due to their complex compositions, its precise structure cannot be clarified. All that can be determined is the main molecular structure of the polysaccharide component [13]. Additionally, they have received medical approval primarily for use as adjuvants in the treatment of cancer in China and Japan. The immunomodulatory effects of polysaccharide peptides may be responsible for this. PSK was approved for marketing in Japan in 1977 after a long period of clinical trials. In 1987, PSK had annual sales of $357 million in Japan [4]. The PSP was applicated around the 1990s, a decade after the PSK. Studies have shown that PSP has a variety of physiological functions such as enhancing immunity, anti-tumor, protecting the liver, anti-oxidation, and lowering blood lipids. PSP has been clinically used to treat cancer, hepatitis, hyperlipidemia, chronic bronchitis, and other diseases. Moreover, PSP has low cytotoxicity and no serious adverse reactions [8], but also can enhance the therapeutic effect of chemoradiotherapy drugs [15], alleviate the toxicity and side effects caused by chemoradiotherapy in cancer patients, and improve the quality of life of patients [16].
3. Anti-Tumor Activity of Trametes versicolor Extracts
Previous studies indicated that both PSK and PSP have a variety of anti-cancer activities. They are effective whether administered by orally, intravenously, or intraperitoneally, and are frequently regarded as nontoxic as well as free of adverse effects [8]. Numerous studies have showed the potent and widespread antitumor activities of PSP and PSK. PSK exhibited anti-leukemic [17], anti-hepatoma [18], anti-ovarian cancer [19], anti-breast cancer [20], and anti-colorectal cancer cell [21][22][23] activities, according to in vitro experiments. Additionally, PSP has anti-glioma [24], anti-HL-60 cells [25], anti-prostate cancer [26], anti-breast cancer [27], and anti-HepG2 cells [28] activities. Furthermore, PSK and PSP also exhibit anti-tumor potential in vivo, as seen in cases of prostate cancer [22][26], hepatoma [29], and mammary gland tumors [30].
Human leukemia HL60 cell is the most commonly utilized to examine the anti-tumor effect of PSP because they can inhibit the proliferation of tumor cells by disrupting the malignant cell cycle [25][31] and inducing apoptosis [14]. The anti-apoptotic proteins Bcl-2 and Survivin were shown to be decreased during these effects, while Bax and cytochrome C are increased. A number of phosphatase and kinase genes are also activated, along with caspase-3, -8, and -9 [32]. PSP significantly enhanced radiation-induced in vitro damage to C6 rat glioma cells [24]. According to results of Luk et al. [26], PSP treatment of prostate cancer PC-3 cell lines led to a time- and dose-dependent downregulation of CSC (cancer stem cell) markers (CD133 and CD44). In addition to inhibiting PC-3 cells to form prostatic spheres, PSP treatment significantly reduced the tumorigenicity of the cells in vivo. Furthermore, it was demonstrated that oral administration of PSP significantly reduced prostate tumor formation in TgMAP mice and inhibited prostate cancer tumor stem cell proliferation. PSP inhibit the growth and metastasis of various tumors in animal models in addition to preventing tumor formation brought on by different chemical carcinogens [15]. PSP inhibited cancer cell migration and significantly reduced the production of the matrix metalloproteinase MMP-9 in a time- and dose-dependent manner, according to in vitro cell migration assays of breast cancer cells from 4T1 mice with PSP. When given PSP therapy in vivo, mice injected with 4T1 cells showed inhibited proliferation in the lung, preventing the development of liver metastases [33]. Cancer cells treated with PSP had longer DNA synthesis time (Ts), while Doxo and VP-16 indicated stronger pro-apoptotic effect [27]. PSP enhanced the plasma half-life of anti-cancer drugs and decreased the clearance rate of cyclophosphamide, while increasing the cytotoxic effect of cyclophosphamide on the HepG2 cancer cell line [28].
These findings indicated that polysaccharide peptides in Trametes versicolor had potent antitumor activity, provide a wealth of references for PSK and PSP research on anticancer studies, and highlight their potential for thorough research and cancer treatment.
4. Anti-Colorectal Cancer Activity of Trametes versicolor Extraction
PSK was found to have an inhibitory effect on colon cancer cell lines (HT29 and SW480) by Hirahara et al. [21][34], but additional research was not done. According to Roca’s results [23], LoVo and HT-29 human colon cancer cells were prevented from proliferation, migration, and invasion by treatment of polysaccharide-rich extracts from Trametes versicolor. They suggested that the antitumor activity may be caused by elevating the expression of E-cadherin protein and suppressing the activity of MMP-2. Additionally, Knezevic [35] discovered that the extraction (basidiocarps and mycelium) from Trametes versicolor exhibited the activity against the proliferation of human colon carcinoma (LS174). Aside from these studies, few studies have addressed the anti-colorectal cancer activity of PSK and PSP. This may be attributed to the large molecular weight and complexity of PSP, which makes the future of its research prospects not very promising.
5. Polysaccharide Peptides with Smaller Molecular Weight from Trametes versicolor
Researchers have also tried to determine whether there are smaller polypeptides that exert anti-cancer activity potential given the large molecular weight of PSP. In 1992, Yang et al. [10] isolated a small polypeptide known as SPCV with about 10 KDa molecular weight from the crude extraction of polysaccharide peptide of Coriolus versicolor (Cov-1). Additionally, in vitro experiments indicated that the proliferation of leukemia cells and SCG-7901 were significantly more inhibited in SPCV treated group than that in PSP and PSK groups. In nude mice inoculated with tumor cells, pretreatment of SPCV for two weeks significantly decreased the incidence of tumor mass. From the mycelial culture of Tricholoma mongolicum, Wang et al. [11] also isolated a polysaccharide-peptide complex with a molecular mass of 15.5 kDa in 1996. This complex possessed the activities of activating macrophages, stimulating macrophage antigen-presenting, which in turn enhanced proliferation of T-cells, and inhibiting the growth of sarcoma 180 cells that had been implanted in mice.
These findings imply that smaller molecular weight polypeptides in PSP might be the active substances. Additionally, they have a greater chance of being used as a drug in the world than PSK and PSP, which have heavier molecular weights. Physical and chemical properties revealed them to be mixtures with smaller molecular weight peptides, but their sequences and structures were not clearly clarified, possibly as a result of the technical constraints of the period. This makes it challenging to perform further research on the pharmacological mechanisms that exert their anticancer activity. Additionally, it restricts its ability to be used clinically in the treatment of colorectal cancer.
6. Musarin: A Novel Polysaccharide Peptide from Trametes versicolor
PSK and PSP’s complicated composition and high molecular weight make mechanistic research difficult, which hinders their adoption into Western medicine’s pharmacopeia [9]. Therefore, determining PSK and PSP’s active components is very important for further study because of this. Recent research has shed new light on the polysaccharide peptide from Trametes versicolor. In this research, a novel 12 kDa protein named musarin was discovered and characterized, which was isolated from Trametes versicolor powder [12]. Multiple colorectal cancer cell lines and functional assays were used to assess the anti-cancer bioactivity of musarin. The findings indicated that musarin inhibits the proliferation of multiple colon cancer cell lines, while it did not influence the growth of normal colon cells. Additionally, it did not cause cancer cells to undergo apoptosis and necrosis. It is suggested that musarin prevents proliferation of colorectal cancer cells without having lethal effects. Furthermore, colorectal cancer stem cells and a NOD/SCID murine xenograft model were used to assess its anti-cancer effectiveness. The size and weight of tumor xenografts in NOD/SCID mice were significantly suppressed in vivo after receiving musarin orally for 14 days. Additionally, oral administration of musarin had similar impact to gefitinib in clearing tumors. Moreover, none of the musarin-treated mice had the skin rash and hair loss side effects that some gefitinib-treated mice did.
Musarin’s mechanism of action has also been studied due to its clear structure and strong anti-tumor activity. Firstly, musarin indicated dose-dependent EGFR tyrosine kinase inhibitor activity, with an IC50 value of 1.39 μM. Musarin is worked to function as tyrosine kinase inhibitor to suppress the growth of colorectal cancer stem-like cells and does not have the same adverse effects as gefitinib. Musarin treatment downregulated the EGFR-Ras signaling pathway (Figure 1A) in colorectal cancer stem-like cells, including EGFR, p-Akt (Ser473), and cyclin D1, as well as p-EGFR-Y1173 and p-EGFR-Y1045. As a result, the EGFR signaling pathway is quenched by musarin-modulated phosphorylation of specific EGFR sites, which inhibits overall EGFR activity limiting proliferation of the CSC-like CD24+CD44+ HT29 subpopulation. Musarin is expected to inhibit proliferation and epithelial to mesenchymal transition of colon cancer cells via altering the EGFR phosphorylation selectivity and differential expression of other related signaling molecules. This is the first time the molecular mechanism of Trametes versicolor-derived polysaccharide peptides’ inhibitory effect on colorectal cancer has been properly reported. This research not only provides the groundwork for further research into the specific structure of the Trametes versicolor polysaccharide peptide, but it also demonstrates that it has significant therapeutic promise in the treatment of colorectal cancer.
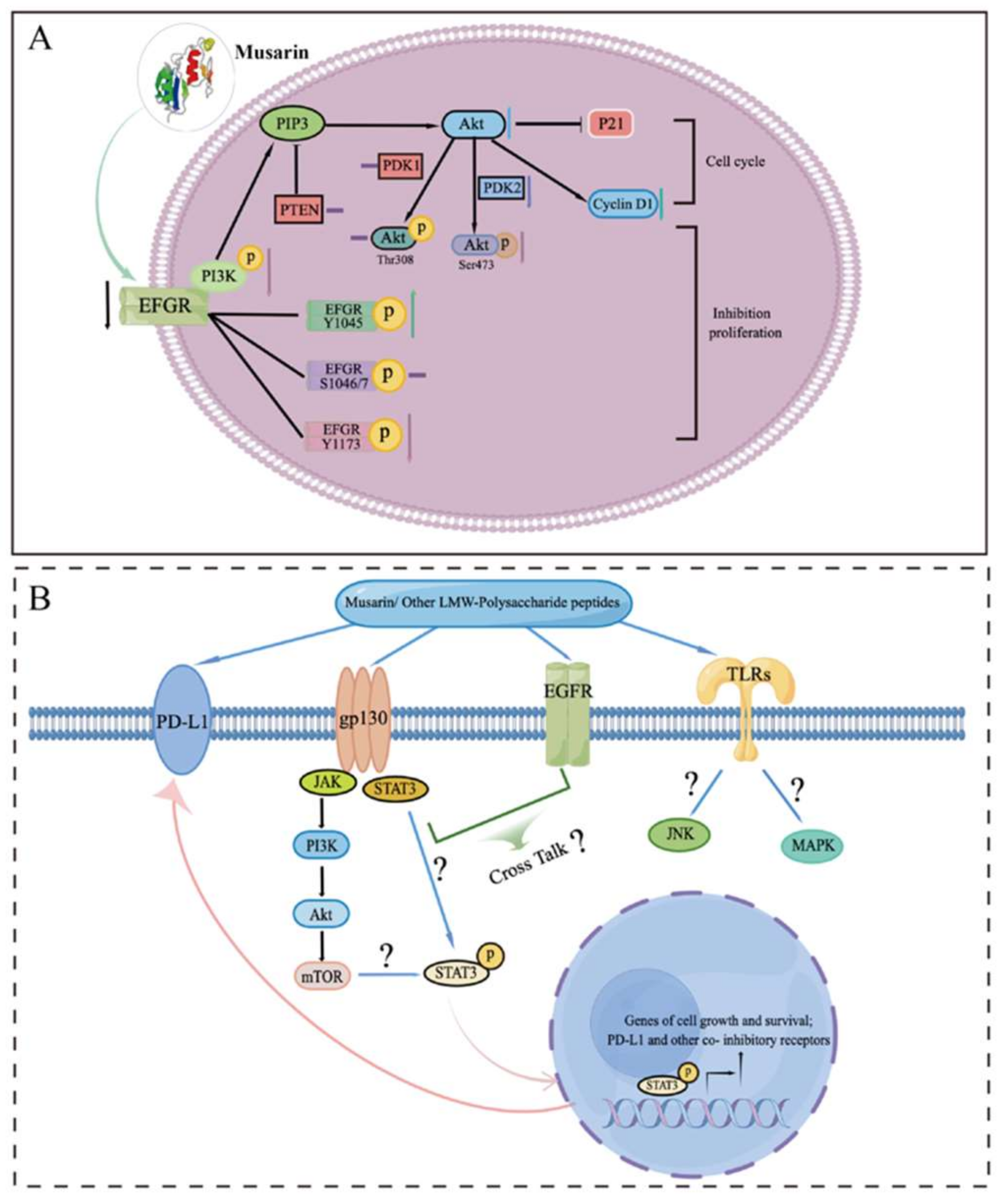
Figure 1. (A) Proposed molecular mechanism of Trametes versicolor-derived polysaccharide peptides’ musarin inhibitory effect on colorectal cancer. (B) Potential anti-proliferation molecular mechanism of musarin and other identified low molecular weight polysaccharide peptides (LMW-polysaccharide peptides) on CRC cells. (“?” Represents a mechanism that is currently unclear and need to be studied in the future).
References
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- Ng, T. A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (basidiomycetes: Polyporaceae). Gen. Pharmacol. Vasc. Syst. 1998, 30, 1–4.
- Lu, H.; Yang, Y.; Gad, E.; Inatsuka, C.; Wenner, C.A.; Disis, M.L.; Standish, L.J. TLR2 Agonist PSK Activates Human NK Cells and Enhances the Antitumor Effect of HER2-Targeted Monoclonal Antibody Therapy. Clin. Cancer Res. 2011, 17, 6742–6753.
- Sullivan, R.; Smith, J.E.; Rowan, N.J. Medicinal Mushrooms and Cancer Therapy: Translating a traditional practice into Western medicine. Perspect. Biol. Med. 2006, 49, 159–170.
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135.
- Cui, J.; Chisti, Y. Polysaccharopeptides of Coriolus versicolor: Physiological activity, uses, and production. Biotechnol. Adv. 2003, 21, 109–122.
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332.
- Yang, M.M.P.; Chen, Z.; Kwok, J.S.L. The Anti-tumor Effect of a Small Polypeptide from Coriolus versicolor (SPCV). Am. J. Chin. Med. 1992, 20, 221–232.
- Wang, H.X.; Ooi, V.E.C.; Chang, S.T.; Ng, T.B.; Liu, W.K. A polysaccharide–peptide complex from cultured mycelia of the mushroom Tricholoma mongolicum with immunoenhancing and antitumor activities. Biochem. Cell Biol. 1996, 74, 95–100.
- He, Y.; Liu, S.; Newburg, D.S. Musarin, a novel protein with tyrosine kinase inhibitory activity from Trametes versicolor, inhibits colorectal cancer stem cell growth. Biomed. Pharmacother. 2021, 144, 112339.
- Rau, U.; Kuenz, A.; Wray, V.; Nimtz, M.; Wrenger, J.; Cicek, H. Production and structural analysis of the polysaccharide secreted by Trametes (Coriolus) versicolor ATCC 200801. Appl. Microbiol. Biotechnol. 2009, 81, 827–837.
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087.
- Dou, H.; Chang, Y.; Zhang, L. Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 361–381.
- Zhong, L.; Yan, P.; Lam, W.C.; Yao, L.; Bian, Z. Coriolus Versicolor and Ganoderma Lucidum Related Natural Products as an Adjunct Therapy for Cancers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2019, 10, 703.
- Kim, F.; Sakagami, H.; Tanuma, S.; Konno, K. Stimulation of interferon-gamma-induced human myelogenous leukemic cell differentiation by high molecular weight PSK subfraction. Anticancer. Res. 1990, 10, 55–58.
- Kobayashi, Y.; Kariya, K.; Saigenji, K.; Nakamura, K. Suppression of Cancer Cell Growth In Vitro by the Protein-Bound Polysaccharide of Coriolus versicolor QUEL (PS-K) with SOD Mimicking Activity. Cancer Biotherapy 1994, 9, 63–69.
- Kobayashi, Y.; Kariya, K.; Saigenji, K.; Nakamura, K. Enhancement of Anti-Cancer Activity of Cisdiaminedichloroplatinum by the Protein-Bound Polysaccharide of Coriolus versicolor QUEL (PS-K) in vitro. Cancer Biotherapy 1994, 9, 351–358.
- Aoyagi, H.; Lino, Y.; Takeo, T.; Horii, Y.; Morishita, Y.; Horiuchi, R. Effects of OK-432 (Picibanil) on the Estrogen Receptors of MCF-7 Cells and Potentiation of Antiproliferative Effects of Tamoxifen in Combination with OK-432. Oncology 1997, 54, 414–423.
- Hirahara, N.; Edamatsu, T.; Fujieda, A.; Fujioka, M.; Wada, T.; Tajima, Y. Protein-bound polysaccharide-K induces apoptosis via mitochondria and p38 mitogen-activated protein kinase-dependent pathways in HL-60 promyelomonocytic leukemia cells. Oncol. Rep. 2013, 30, 99–104.
- Wenner, C.A.; Martzen, M.R.; Lu, H.; Verneris, M.R.; Wang, H.; Slaton, J.W. Polysaccharide-K augments docetaxel-induced tumor suppression and antitumor immune response in an immunocompetent murine model of human prostate cancer. Int. J. Oncol. 2012, 40, 905–913.
- Roca-Lema, D.; Martinez-Iglesias, O.; Portela, C.F.D.A.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In Vitro Anti-proliferative and Anti-invasive Effect of Polysaccharide-rich Extracts from Trametes Versicolor and Grifola Frondosa in Colon Cancer Cells. Int. J. Med. Sci. 2019, 16, 231–240.
- Mao, X.W.; Green, L.M.; Gridley, D.S. Evaluation of Polysaccharopeptide Effects against C6 Glioma in Combination with Radiation. Oncology 2001, 61, 243–253.
- Yang, X.; Sit, W.-H.; Chan, D.K.-O.; Wan, J.M.-F. The cell death process of the anticancer agent polysaccharide-peptide (PSP) in human promyelocytic leukemic HL-60 cells. Oncol. Rep. 2005, 13, 1201–1210.
- Luk, S.U.; Lee TK, W.; Liu, J.; Lee DT, W.; Chiu, Y.T.; Ma, S.; Ling, M.T. Chemopreventive Effect of PSP Through Targeting of Prostate Cancer Stem Cell-Like Population. PLoS ONE 2011, 6, e19804.
- Wan, J.M.-F.; Sit, W.-H.; Louie, J.C.Y. Polysaccharopeptide enhances the anticancer activity of doxorubicin and etoposide on human breast cancer cells ZR-75-30. Int. J. Oncol. 2008, 32, 689–699.
- Chan, S.-L.; Yeung, J.H. Effects of polysaccharide peptide (PSP) from Coriolus versicolor on the pharmacokinetics of cyclophosphamide in the rat and cytotoxicity in HepG2 cells. Food Chem. Toxicol. 2006, 44, 689–694.
- Hirose, K.; Hakozaki, M.; Matsunaga, K.; Yoshikumi, C.; Hotta, T.; Yanagisawa, M.; Yamamoto, M.; Endo, H. Cloning of sequences induced and suppressed by administration of PSK, antitumor protein-bound polysaccharide. Biochem. Biophys. Res. Commun. 1985, 126, 884–892.
- Fujii, T.; Saito, K.; Matsunaga, K.; Oguchi, Y.; Ikuzawa, M.; Furusho, T.; Taguchi, T. Prolongation of the survival period with the biological response modifier PSK in rats bearing N-methyl-N-nitrosourea-induced mammary gland tumors. In Vivo 1995, 9, 55–57.
- Hsieh, T.-C.; Wu, P.; Park, S.; Wu, J.M. Induction of cell cycle changes and modulation of apoptogenic/anti-apoptotic and extracellular signaling regulatory protein expression by water extracts of I’m-Yunity™ (PSP). BMC Complement. Altern. Med. 2006, 6, 30.
- Li, X.Y. Immunomodulating components from chinese medicines. Pharm. Biol. 2000, 38 (Suppl. 1), 33–40.
- Luo, K.-W.; Yue, G.G.-L.; Ko, C.-H.; Lee, J.K.-M.; Gao, S.; Li, L.-F.; Li, G.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.-S. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014, 21, 1078–1087.
- Hirahara, N.; Fujioka, M.; Edamatsu, T.; Fujieda, A.; Sekine, F.; Wada, T.; Tanaka, T. Protein-bound polysaccharide-K (PSK) induces apoptosis and inhibits proliferation of promyelomonocytic leukemia HL-60 cells. Anticance Res. 2011, 31, 2733–2738.
- Knežević, A.; Stajić, M.; Sofrenić, I.; Stanojković, T.; Milovanovic, I.; Tešević, V.; Vukojević, J. Antioxidative, antifungal, cytotoxic and antineurodegenerative activity of selected Trametes species from Serbia. PLoS ONE 2018, 13, e0203064.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
25 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




