
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beatrix Zheng | -- | 1933 | 2022-11-24 01:44:11 |
Video Upload Options
Ozone (O3) is a trace gas of the troposphere, with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer exists which is located between 10 and 50 kilometers above the earths surface. The troposphere is the lowest layer of the Earth's atmosphere. It extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground level or tropospheric ozone is created by chemical reactions between oxides of nitrogen (NOx gases) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming. Photochemical and chemical reactions involving ozone drive many of the chemical processes that occur in the troposphere by day and by night. At abnormally high concentrations (the largest source being emissions from combustion of fossil fuels), it is a pollutant, and a constituent of smog. Its levels have increased significantly since the industrial revolution, as NOx gasses & VOCs are some of the byproducts of combustion. With more heat and sunlight in the summer months, more ozone is formed which is why regions often experience higher levels of pollution in the summer months. Although the same molecule, ground level ozone can be harmful to our health, unlike stratospheric ozone that protects the earth from excess UV radiation. Photolysis of ozone occurs at wavelengths below approximately 310–320 nanometres. This reaction initiates the chain of chemical reactions that remove carbon monoxide, methane, and other hydrocarbons from the atmosphere via oxidation. Therefore, the concentration of tropospheric ozone affects how long these compounds remain in the air. If the oxidation of carbon monoxide or methane occur in the presence of nitrogen monoxide (NO), this chain of reactions has a net product of ozone added to the system.
1. Measurement
Ozone in the atmosphere can be measured by remote sensing technology, or by in-situ monitoring technology. Because ozone absorbs light in the UV spectrum, the most common way to measure ozone is to measure how much of this light spectrum is absorbed in the atmosphere.[1][2] Because the stratosphere has higher ozone concentration than the troposphere, it is important for remote sensing instruments to be able to determine altitude along with the concentration measurements. The TOMS-EP instrument aboard a satellite from NASA is an example of an ozone layer measuring satellite,[3] and TES is an example of an ozone measuring satellite that is specifically for the troposphere.[4] Lidar is a common ground based remote sensing technique to measure ozone. TOLnet is the network of ozone observing lidars across the United States.[5]
Ozonesondes are a form of in situ, or local measurements. An ozonesonde is an ozone measuring instrument attached to a meteorological balloon, so that the instrument can directly measure ozone concentration at the varying altitudes along the balloon's upward path. The information collected from the instrument attached to the balloon is transmitted back using radiosonde technology.[1] NOAA has worked to create a global network of tropospheric ozone measurements using ozonesondes.[6]
Ozone is also measured in air quality environmental monitoring networks. In these networks, in-situ ozone monitors based on ozone's UV-absorption properties are used to measure ppb-levels in ambient air.
Total atmospheric ozone (sometimes seen in weather reports) is measured in a column from the surface to the top of the atmosphere, and is dominated by high concentrations of stratospheric ozone. Typical units of measure for this purpose include the Dobson unit and millimoles per square meter (mmol/m2).
2. Formation
The majority of tropospheric ozone formation occurs when nitrogen oxides (NOx), carbon monoxide (CO) and volatile organic compounds (VOCs), react in the atmosphere in the presence of sunlight, specifically the UV spectrum. NOx, CO, and VOCs are considered ozone precursors.[7][8] Motor vehicle exhaust, industrial emissions, and chemical solvents are the major anthropogenic sources of these ozone precursors.[8] Although the ozone precursors often originate in urban areas, winds can carry NOx hundreds of kilometers, causing ozone formation to occur in less populated regions as well. Methane, a VOC whose atmospheric concentration has increased tremendously during the last century, contributes to ozone formation but on a global scale rather than in local or regional photochemical smog episodes. In situations where this exclusion of methane from the VOC group of substances is not obvious, the term Non-Methane VOC (NMVOC) is often used.
The chemical reactions involved in tropospheric ozone formation are a series of complex cycles in which carbon monoxide and VOCs are oxidised to water vapour and carbon dioxide. The reactions involved in this process are illustrated here with CO but similar reactions occur for VOC as well. The oxidation begins with the reaction of CO with the hydroxyl radical (•OH).[9] The radical intermediate formed by this reacts rapidly with oxygen to give a peroxy radical HO2•
An outline of the chain reaction that occurs in oxidation of CO, producing O3:[9][10]
The reaction begins with the oxidation of CO by the hydroxyl radical (•OH). The radical adduct (•HOCO) is unstable and reacts rapidly with oxygen to give a peroxy radical, HO2•:
- •OH + CO → •HOCO
- •HOCO + O2 → HO2• + CO2
Peroxy-radicals then go on to react with NO to produce NO2, which is photolysed by UV-A radiation to give a ground-state atomic oxygen, which then reacts with molecular oxygen to form ozone.[11]
- HO2• + NO → •OH + NO2
- NO2 + hν → NO + O(3P) , λ<400 nm
- O(3P) + O2 → O3
- note that these three reactions are what forms the ozone molecule, and will occur the same way in the oxidation of CO or VOCs case.
The net reaction in this case is then:
- CO + 2O2 → CO2 + O3
The amount of ozone produced through these reactions in ambient air can be estimated using a modified Leighton relationship. The limit on these interrelated cycles producing ozone is the reaction of •OH with NO2 to form nitric acid at high NOx levels. If nitrogen monoxide (NO) is instead present at very low levels in the atmosphere (less than 10 approximately ppt), the peroxy radicals (HO2• ) formed from the oxidation will instead react with themselves to form peroxides, and not produce ozone. [11]
3. Health Effects
Health effects depend on ozone precursors, which is a group of pollutants, primarily generated during the combustion of fossil fuels. Ground-Level Ozone is created by nitrous oxides reacting with organic compounds in the presence of sunlight.[12] There are many man-made sources of these organic compounds including vehicle and industrial emissions, along with several other sources.[12] Reaction with daylight ultraviolet (UV) rays and these precursors create ground-level ozone pollution (Tropospheric Ozone). Ozone is known to have the following health effects at concentrations common in urban air:
- Irritation of the respiratory system, causing coughing, throat irritation, and/or an uncomfortable sensation in the chest. Ozone affects people with underlying respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and lung cancer as well those who spend a lot of time being active outdoors.[13]
- Reduced lung function, making it more difficult to breathe deeply and vigorously. Breathing may become more rapid and more shallow than normal, and a person's ability to engage in vigorous activities may be limited. Ozone causes the muscles in the airways to constrict which traps air in the alveoli leading to wheezing and shortness of breath.[13]
- Aggravation of asthma. When ozone levels are high, more people with asthma have attacks that require a doctor's attention or use of medication. One reason this happens is that ozone makes people more sensitive to allergens, which in turn trigger asthma attacks.
- Increased susceptibility to respiratory infections.
- Inflammation and damage to the lining of the lungs. Within a few days, the damaged cells are shed and replaced much like the skin peels after a sunburn. Animal studies suggest that if this type of inflammation happens repeatedly over a long time period (months, years, a lifetime), lung tissue may become permanently scarred, resulting in permanent loss of lung function and a lower quality of life.
- More recent data suggests that ozone can also have harmful effects via the inflammatory pathway leading to heart disease, type 2 diabetes, and other metabolic disorders.[14]
It was observed in the 1990s that ground-level ozone can advance death by a few days in predisposed and vulnerable populations.[15] A statistical study of 95 large urban communities in the United States found significant association between ozone levels and premature death. The study estimated that a one-third reduction in urban ozone concentrations would save roughly 4000 lives per year (Bell et al., 2004). Tropospheric Ozone causes approximately 22,000 premature deaths per year in 25 countries in the European Union. (WHO, 2008)
4. Problem Areas
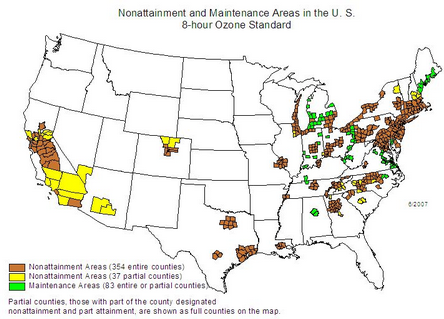
US-ozone-non-attainment-2007-06. https://handwiki.org/wiki/index.php?curid=1548027
The United States Environmental Protection Agency has developed an Air Quality index to help explain air pollution levels to the general public. 8-hour average ozone mole fractions of 76 to 95 nmol/mol are described as "Unhealthy for Sensitive Groups", 96 nmol/mol to 115 nmol/mol as "unhealthy" and 116 nmol/mol to 404 nmol/mol as "very unhealthy" [1]. The EPA has designated over 300 counties of the United States, clustered around the most heavily populated areas (especially in California and the Northeast), as failing to comply with the National Ambient Air Quality Standards.
The North Front Range of Colorado has been out of compliance with the Federal Air Quality standards. The U.S. EPA designated Fort Collins as part of the ozone non-attainment area in November 2007.[16] This means that the U.S.’s environmental law considers the air quality to be worse than the National Ambient Air Quality Standards, which are defined in the Clean Air Act Amendments.[17] In 2018, the Lung Association ranked Larimer county 19th in the nation for high ozone days.[18] Fort Collins was also ranked 24 for high ozone days out of 228 metropolitan areas, 52 for 24-hour particle pollution out of 217 metropolitan areas, and156 for annual particle pollution out of 203 metropolitan areas.[18]
In monitoring air quality, Boulder County, CO is classified by the EPA as part of a nine-county group that includes the Denver metro area and North Front Range region. This nine-county zone has recorded ozone levels that exceed the EPA’s ozone standard since 2004.[19] Attempts have been made under the Early Action Compact to bring the area’s air quality up to the EPA’s standards. However since 2004 ozone pollution in Boulder County has regularly failed to meet federal standards set by the Environmental Protection Agency.[20] The County of Boulder continues trying to alleviate some of the ozone pollution through programming that encourages people to drive less, and stop ozone polluting activities during the heat of the day.[21]
5. Climate Change
Melting of sea ice releases molecular chlorine, which reacts with UV radiation to produce chlorine radicals. Because chlorine radicals are highly reactive, they can expedite the degradation of methane and tropospheric ozone and the oxidation of mercury to more toxic forms.[22] Ozone production rises during heat waves, because plants absorb less ozone. It is estimated that curtailed ozone absorption by plants could be responsible for the loss of 460 lives in the UK in the hot summer of 2006.[23] A similar investigation to assess the joint effects of ozone and heat during the European heat waves in 2003, concluded that these appear to be additive.[24]
References
- "How is ozone measured in the atmosphere?". https://www.esrl.noaa.gov/csd/assessments/ozone/2010/twentyquestions/Q5.pdf.
- "Measuring ozone from space". https://phys.org/news/2016-09-ozone-space.html.
- NASA. "total-ozone-mapping-spectrometer-earth-probe". https://eospso.nasa.gov/missions/total-ozone-mapping-spectrometer-earth-probe.
- NASA. "TES". https://tes.jpl.nasa.gov/.
- LaRC, Ali Aknan (2005-06-22). "NASA Tropospheric Chemistry Integrated Data Center". https://www-air.larc.nasa.gov/missions/TOLNet/.
- Laboratory, US Department of Commerce, NOAA, Earth System Research. "ESRL Global Monitoring Division - Ozone and Water Vapor Group". https://www.esrl.noaa.gov/gmd/ozwv/ozsondes/.
- "Tropospheric ozone | Climate & Clean Air Coalition". http://ccacoalition.org/en/slcps/tropospheric-ozone.
- "Ozone in the Troposphere | UCAR Center for Science Education". https://scied.ucar.edu/ozone-troposphere.
- Reeves, Claire E.; Penkett, Stuart A.; Bauguitte, Stephane; Law, Kathy S.; Evans, Mathew J.; Bandy, Brian J.; Monks, Paul S.; Edwards, Gavin D. et al. (2002-12-11). "Potential for photochemical ozone formation in the troposphere over the North Atlantic as derived from aircraft observations during ACSOE". Journal of Geophysical Research: Atmospheres 107 (D23): ACH 14–1–ACH 14–14. doi:10.1029/2002jd002415. ISSN 0148-0227. Bibcode: 2002JGRD..107.4707R. http://juser.fz-juelich.de/record/34366.
- "8.2 Tropospheric ozone". http://elte.prompt.hu/sites/default/files/tananyagok/AtmosphericChemistry/ch08s02.html.
- Warneck, Peter (1999). Chemistry of The Natural Atmosphere. Academic Press. ISBN 9780080529066.
- "Good Up High Bad Nearby - What is Ozone?". https://cfpub.epa.gov/airnow/index.cfm?action=gooduphigh.index.
- US EPA, OAR (2015-06-05). "Health Effects of Ozone Pollution". https://www.epa.gov/ground-level-ozone-pollution/health-effects-ozone-pollution.
- Adar, Sara Dubowsky (2012-09-25). "Childhood Exposures to Ozone". Circulation 126 (13): 1570–1572. doi:10.1161/circulationaha.112.133207. ISSN 0009-7322. PMID 23008468. https://dx.doi.org/10.1161%2Fcirculationaha.112.133207
- Schlink, Uwe; Herbarth, Olf; Richter, Matthias; Dorling, Stephen; Nunnari, Giuseppe; Cawley, Gavin; Pelikan, Emil (April 2006). "Statistical models to assess the health effects and to forecast ground-level ozone". Environmental Modelling & Software 21 (4): 547–558. doi:10.1016/j.envsoft.2004.12.002. ISSN 1364-8152. https://dx.doi.org/10.1016%2Fj.envsoft.2004.12.002
- "Ozone FAQs || Air Quality". https://www.fcgov.com/airquality/ozone.
- US EPA, OAR (2014-04-10). "NAAQS Table". https://www.epa.gov/criteria-air-pollutants/naaqs-table.
- "Fort Collins, CO". https://www.lung.org/our-initiatives/healthy-air/sota/city-rankings/msas/fort-collins-co.html.
- "Ozone". https://www.bouldercounty.org/environment/air/ozone/.
- "Simple Steps | Simple Steps. Better Air.". https://simplestepsbetterair.org/simple-steps-to-better-air/.
- "Ozone" (in en-US). 2020. https://www.bouldercounty.org/environment/air/ozone/.
- Jin Liao (January 2014). "High levels of molecular chlorine in the Arctic atmosphere". Nature Geoscience 7 (2): 91–94. doi:10.1038/ngeo2046. Bibcode: 2014NatGe...7...91L. https://dx.doi.org/10.1038%2Fngeo2046
- "It's not just the heat – it's the ozone: Study highlights hidden dangers". University of York. http://www.york.ac.uk/news-and-events/news/2013/research/heat-ozone/.
- Kosatsky T. (July 2005). "The 2003 European heat waves". Eurosurveillance 10 (7): 3–4. doi:10.2807/esm.10.07.00552-en. PMID 29208081. https://dx.doi.org/10.2807%2Fesm.10.07.00552-en





