Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zi-Chao Wang | -- | 3919 | 2022-11-22 14:20:25 | | | |
| 2 | Sirius Huang | Meta information modification | 3919 | 2022-11-23 02:58:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yan, Y.; Nisar, T.; Fang, Z.; Wang, L.; Wang, Z.; Gu, H.; Wang, H.; Wang, W. Chemical Compositions and Biosynthesis of Black Goji Anthocyanins. Encyclopedia. Available online: https://encyclopedia.pub/entry/35857 (accessed on 08 February 2026).
Yan Y, Nisar T, Fang Z, Wang L, Wang Z, Gu H, et al. Chemical Compositions and Biosynthesis of Black Goji Anthocyanins. Encyclopedia. Available at: https://encyclopedia.pub/entry/35857. Accessed February 08, 2026.
Yan, Yuzhen, Tanzeela Nisar, Zhongxiang Fang, Lingling Wang, Zichao Wang, Haofeng Gu, Huichun Wang, Wenying Wang. "Chemical Compositions and Biosynthesis of Black Goji Anthocyanins" Encyclopedia, https://encyclopedia.pub/entry/35857 (accessed February 08, 2026).
Yan, Y., Nisar, T., Fang, Z., Wang, L., Wang, Z., Gu, H., Wang, H., & Wang, W. (2022, November 22). Chemical Compositions and Biosynthesis of Black Goji Anthocyanins. In Encyclopedia. https://encyclopedia.pub/entry/35857
Yan, Yuzhen, et al. "Chemical Compositions and Biosynthesis of Black Goji Anthocyanins." Encyclopedia. Web. 22 November, 2022.
Copy Citation
Lycium ruthenicum is a therapeutic plant and its fruits (black goji) are commonly used as a traditional Chinese medicine. Among the 39 identified black goji anthocyanins (BGAs), most are 3,5-diglycoside derivatives of petunidin (>95%) with an individual anthocyanin [petunidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside], accounting for 80% of the total BGAs. Due to their unique anthocyanin profile, BGAs possess various health benefits.
acylated anthocyanins
biosynthesis
Lycium ruthenicum
petunidin
1. Introduction
Lycium ruthenicum, a therapeutic plant that belongs to the family of Solanaceae and genus of Lycium, is mainly distributed in China, Mongolia, Central Asia and North Africa, and grows under the environment of special geography (high altitude) and arid climate (low temperature, less rain and strong sunlight) [1][2]. The fruit, known as black goji, is popular in traditional Chinese medicine for disease treatment, such as heart disease, abnormal menstruation, urethral and urethral stones, tinea and furuncle, hypertension and menopause, as highlighted in the Tibetan medical classics of “Jing Zhu Ben Cao” [2][3][4][5][6]. Modern pharmacological research has demonstrated its potential use as an antioxidant, immune-enhancer, hepatic-function protector and anti-tumor, antifatigue, antiaging, anti-atherosclerotic, hypolipidemic and hypoglycemic agent [5][6][7]. The biological functions of black goji are derived from its functional components such as anthocyanins, essential oils, organic acids, trace minerals and polysaccharides [8][9][10][11]. Black goji is also popular in the food industry for preparing functional foods such as black goji juices and drinks, not only owing to its biological functions but also its attractive black-bluish color [3]. Recently, black goji consumption has remarkably increased, and the demands for functional black goji products are expanding [7][12].
Anthocyanins are abundant in black goji and they are the principal functional compounds that exhibit a wide range of health benefits (e.g., antioxidant activities, anti-cardiovascular disease and anti-tumor effects) and thus are responsible for most of black goji’s biological functions [4][7][13][14][15][16][17]. The anthocyanins are also responsible for the attractive color of the fruit [18]. To date, many researchers have explored certain aspects of black goji anthocyanins (BGAs), e.g., extraction, chemical compositions, biosynthesis and health benefits [2][7][11][14][15][19][20][21][22][23].
2. Chemical Compositions and Biosynthesis of BGAs
2.1. Extraction and Purification
As a class of water-soluble polyphenols, BGAs are generally extracted using an aqueous solution [24][25]. However, organic reagents including methanol, ethanol and acetone were found to be more efficient in the extraction of polyphenols [26]. Therefore, BGAs are commonly extracted using organic reagents [2][6][11][18][27] or aqueous solutions with different concentrations of organic reagents (50–80%) [1][4][14][15][16][19][20][21][22][28][29][30][31][32][33]. Ethanol is the most common reagent in BGA extraction due to its edible and safe properties [34]. The ethanol concentration, solution to black goji ratio, and extraction time show a significant effect on the extraction yield of BGAs [14]. The extractants were mostly adjusted to pH 2 or 2.5 using hydrochloric, formic or trifluoroacetic acid, and the extractions were carried out at a temperature less than 50 °C in the dark to protect BGAs from degradation.
An ultrasound-assisted system is widely adopted in BGA extraction [1][14][27][33][35][36][37], because particle collisions and cell wall disruption produced by ultrasound cavitation can promote solvent penetration into the sample matrix, enhancing the anthocyanin extraction rate [38]. The extract yield of BGAs by an optimized ultrasound-assisted extraction system, i.e., with extraction power of 348 W, extractant (90% ethanol) and material ratio of 25 mL/g, at temperature 42 °C for 29 min, was 17.92%, higher than the yield of microwave-assisted extraction and soaking extraction (16.85% and 16.34%, respectively) [35]. The efficiency of ultrasonic extraction is related to its power, as a lower extraction yield of BGAs (7.12%) was observed when the extraction power was lower, i.e., 300 W, with other parameters the same as described above [36].
Some novel techniques are also employed to enhance the efficiency of BGA extraction. A subcritical water extraction with the subcritical water flow speed of 3 mL/min at 170 °C for 55 min recovered higher amounts of BGAs (up to 26.33%) than a hot water extraction (15.76%) and a methyl alcohol extraction (21.35%) [39]. Pectinase extraction at 38 °C for 37 min using pectinase with a concentration of 52.04 mg/100 g dried black goji rendered the BGAs at 19.51 mg/g dry weight (DW) [40], which is much higher than the extraction using aqueous two-phase assisted by ultrasound (4.71 mg/g DW) [37]. β-cyclodextrin (β-CD, 1.65%) extraction with the liquid/solid ratio of 15:1 at 50 °C for 30 min produced higher extraction yields of both major [petunidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside, Pt3R5G] and total BGAs than pure water, aqueous hydroxypropyl-β-CD and ethanol and methanol solutions [41]. In addition, yeast fermentation for 2 h before the extraction by 77.8% ethanol was found to increase the total BGA content by 51% in the extract [42].
Anthocyanin-rich fruits generally contain abundant other types of phenolic compounds, which complicates the qualitative and quantitative analysis [43] and influences the stability, bioactivity and color properties of anthocyanins [44]. Thus, purification is a critical factor in BGA analysis. The crude extracts of BGAs were purified by SPE C-18 column [11][27][32], YMC-Pack ODS-A column [31], Oasis MCX column [32] and macroporous resin columns including AB-8 [1][4][14][16][19][25][28][29][30][31][36], Diaion HP2 MGL [15], Amberlite XAD-7HP [6][20][29], D-101 [18][37] and XAD-6 [40]. The columns were successively rinsed with water to remove sugars and other interfering substances and then methanol or ethanol was used to elute the anthocyanin fraction. The purification technologies removed the phenolic compounds from the crude extract, reducing the complexity of the matrix. For example, MCX purification increased the purity of BGAs to 65% in contrast to 43% in crude extract and 50% in a C-18 cartridge-purified sample [32]. Particularly, XAD-7HP purification led to the purity of Pt3R5G reaching >97% [20][29].
2.2. Characterization
To date, researchers have isolated and identified 39 anthocyanins from the crude extracts or purified powder of BGAs by HPLC (e.g., HPLC-DAD and semipreparative HPLC), HPLC-MS (e.g., HPLC-DAD-ESI-MS and HPLC-ESI-ToF-MS) and NMR techniques (Table 1) [1][2][4][5][6][11][14][18][19][22][25][27][28][29][31][32][33][39]. In nature, the glycosylation of anthocyanins usually occurs at the third position in the C ring or at the third and fifth positions in the A ring (Figure 1A) through O-glycosidic bond forming mono- or di-glycosyl-anthocyanins [45], and anthocyanidin 3-O-glycosides (3-monoglylcosy-lanthocyanins) are the most common anthocyanins and twice as abundant as 3,5-O-diglycosides (3,5-diglycosyl-anthocyanins) [46]. However, as shown in Table 1, BGAs are all diglycosylated with sugar moieties, including galactoside, glucoside and rutinoside at the third and fifth positions of the aglycones, including petunidin (Pt), malvidin (Mv), delphinidin (Dp) and peonidin (Pn), and mostly monoacylated (at the sugar moieties of the third position) with acyl groups, including coumaric, caffeic, malic and ferulic acids. The existence of abundant 3,5-diglycosyl-anthocyanins is a unique feature of BGAs. This is confirmed by the reports in common berries including blue honeysuckle, grape, haskap and sweet cherry [3][47][48][49], where 3-monoglycosyl-anthocyanins are the primary anthocyanins, and 3,5-diglycosyl-anthocyanins are present only in a small proportion. The most common naturally occurring anthocyanidins are cyanidin (Cy, 50%), Dp (12%), Pn (12%), pelargonidin (Pg, 12%), Mv (7%) and Pt (7%) (Figure 1A) [46], and Pt is rarely present in berries [50]. However, Pt is the major component of black goji anthocyanidins, followed by Mv and Dp, with average contents of 5.71, 0.47 and 0.29 mg/g DW, respectively [1]. As shown in Table 1, eight Pt derivatives (petunidin 3-O-rutinoside-5-O-glucoside, petunidin 3-O-rutinoside (feruloyl)-5-O-glucoside, petunidin 3-O-rutinoside (trans-caffeoyl)-5-O-glucoside, petunidin 3-O-rutinoside (p-coumaroyl)-5-O-glucoside, petunidin 3-O-rutinoside (cis-p-coumaroyl)-5-O-glucoside, Pt3R5G, petunidin 3-O-rutinoside (glucosyl-cis-p-coumaroyl)-5-O-glucoside and petunidin 3-O-rutinoside (glucosyl-trans-p-coumaroyl)-5-O-glucoside), one Mv derivative (malvidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside) and one Dp derivative (delphinidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside) (in bold) are quantitatively abundant in black goji [22]. Consequently, Pt derivatives are the predominant BGAs and account for >95% of the total BGAs in fresh black goji [7][11][19]. Among all derivatives, Pt3R5G is the main component and accounts for almost 80% of the total BGAs [11][39]. The content of Pt3R5G in ripened black goji ranges from 8.29 to 31.51 mg/g DW, with an average value of 17.24 mg/g DW [1][35]. Due to the high concentration of Pt3R5G, the total BGA content of black goji is very high, and in fresh fruit, it reaches 24.04 mg/g fresh weight (FW), which is higher than other pigmented plants, such as blue honeysuckle (1.16–14.00 mg/g FW), blueberries (4.38–6.62 mg/g FW) and mulberry (0.12.73–3.83 mg/g FW) [15]. The dominance of Pt glycosides is also a unique feature of BGAs. In addition, there are many cis-trans isomeric anthocyanins in black goji. Acyl groups in acylated anthocyanins are typically trans configured, and only a few isomers are present in cis forms in nature [51]. The co-existence of both cis and trans anthocyanin isomers in the same source is another unique feature of BGAs.
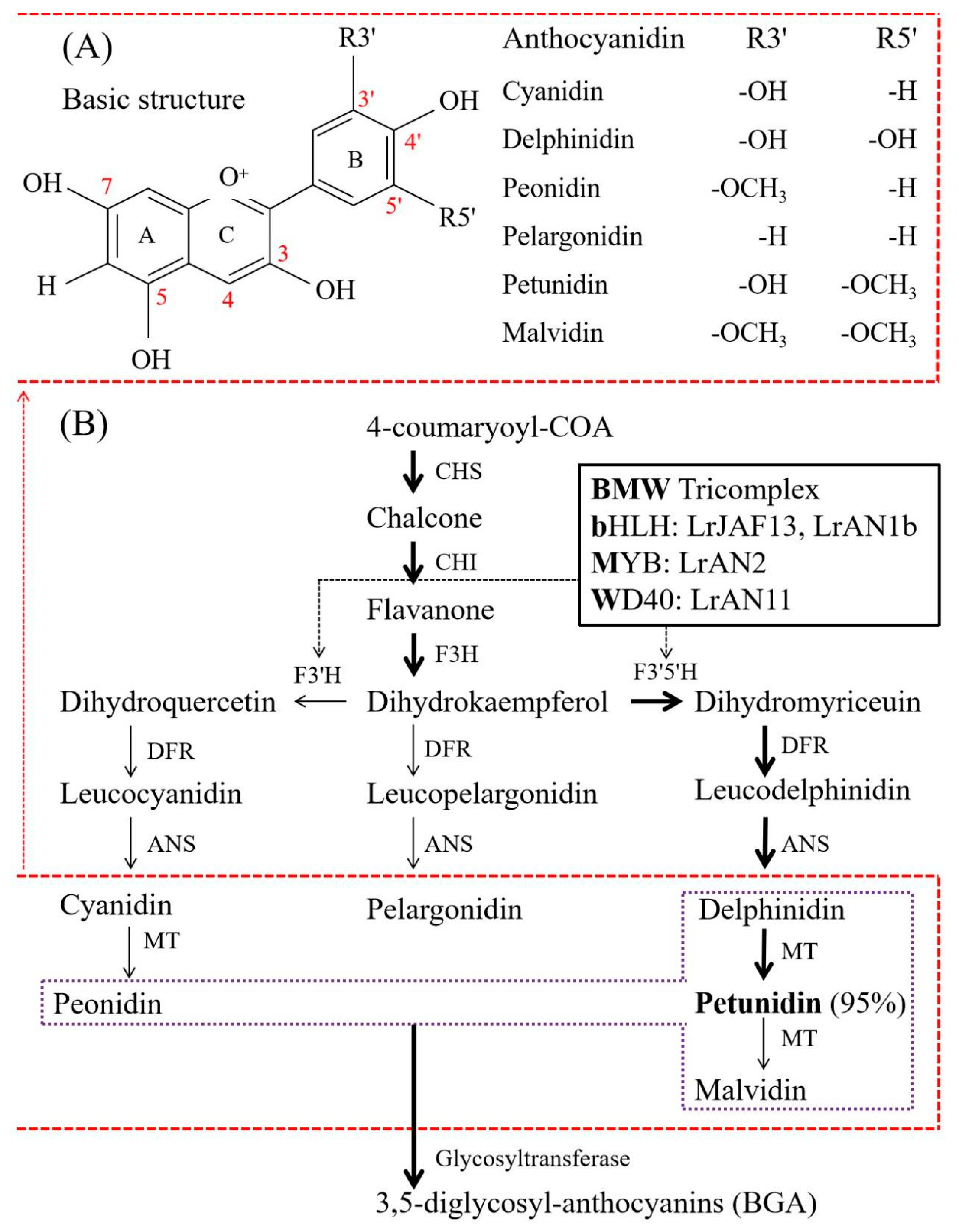
Figure 1. (A) Basic structure of anthocyanidins and six most common anthocyanidins in nature. (B) The biosynthetic pathway of black goji anthocyanins (BGAs). Abbreviations: ANS, anthocyanidin synthase; CHI, chalcone isomerase; CHS, chalcone synthase; DFR, dihydroflavonol 4-reductase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′hydroxylase; F3′5′H, flavonoid 3′5′hydroxylase; MT, anthocyanin methyltransferase. The black arrow weight indicates the size of metabolic flux. The black dashed arrows indicate that the BMW tricomplex possibly regulates the transcription of F3′H and F3′5′H genes. Adapted from Yang et al. [22] and Zeng et al. [23].
Table 1. The identified anthocyanins in fresh or freeze-dried black goji a.
| No. | Anthocyanins | Molecular Formula | References |
|---|---|---|---|
| Petundin derivatives | |||
| 1 | Petundin 3-O-galactoside-5-O-glucoside | C28H33O16 + | [2][6][11][27][32] |
| 2 | Petundin 3,5-O-diglucosides | C28H33O17 + | [2][6][11][18][27] |
| 3 | Petunidin 3-O-rutinoside-5-O-glucoside | C34H43O21 + | [14][19][22][32] |
| 4 | Petunidin 3-O-glucoside (maloyl)-5-O-glucoside | C31H35O19 + | [2][6][11][39] |
| 5 | Petunidin 3-O-glucoside (feruloyl)-5-O-glucoside | C38H41O20 + | [2][6][11][39] |
| 6 | Petunidin 3-O-rutinoside (feruloyl)-5-O-glucoside | C44H51O24 + | [19][22][24] |
| 7 | Petunidin 3-O-rutinoside (caffeoyl)-5-O-glucoside | C43H37O24 + | [2][6][11][19][27] |
| 8 | Petunidin 3-O-rutinoside (cis-caffeoyl)-5-O-glucoside | C43H50O24 + | [5] |
| 9 | Petunidin 3-O-rutinoside (trans-caffeoyl)-5-O-glucoside | C43H50O24 + | [5][22] |
| 10 | Petunidin 3-O-rutinoside (p-coumaroyl)-5-O-glucoside | C43H37O23 + | [14][20][22] |
| 11 | Petunidin 3-O-rutinoside (cis-p-coumaroyl)-5-O-glucoside | C43H37O23 + | [2][5][6][11][19][22][27][32] |
| 12 | Petunidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | C43H37O23 + | [1][2][5][6][11][18][19][22][27][32][41] |
| 13 | Petunidin 3-O-rutinoside (glucosyl-cis-p-coumaroyl)-5-O-glucoside | C49H53O28 + | [19][22] |
| 14 | Petunidin 3-O-rutinoside (glucosyl-trans-p-coumaroyl)-5-O-glucoside | C49H53O28 + | [19][22] |
| 15 | Petunidin 3-O-[6-O-(4-O-(cis-p-coumaroyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C43H49O23 + | [28][33][39] |
| 16 | Petunidin 3-O-[6-O-(4-O-(trans-p-coumaroyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C43H49O23 + | [28][29][31][33][39] |
| 17 | Petunidin 3-O-[6-O-(4-O-p-caffeoyl-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C43H49O24 + | [14][39] |
| 18 | Petunidin 3-O-[6-O-(4-O-(trans-p-caffeoyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C43H49O24 + | [28][33] |
| 19 | Petunidin 3-O-[6-O-(4-O-(4-O-cis-(β-D-glucopyranoside)-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C49H59O28 + | [14][28][33][39] |
| 20 | Petunidin3-O-[6-O-(4-O-(4-O-trans-(β-D-glucopyranoside)-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C49H59O28 + | [14][28][33][39] |
| 21 | Petunidin 3-O-[6-O-(4-O-(4-O-(β-D-glucopyranosyl)-cis-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C49H59O28 + | [14][39] |
| 22 | Petunidin 3-O-[6-O-(4-O-(4-O-(β-D-glucopyranosyl)-trans-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C49H59O28 + | [14][39] |
| 23 | Petunidin 3-O-[6-O-α-L-rhamnopyranosyl-β-D-glucopyranoside]-5-O- [β-D-glucopyranoside] |
C34H43O21 + | [4] |
| Malvidin derivatives | |||
| 24 | Malvidin 3-O-rutinoside-5-O-glucoside | C35H45O21 + | [19] |
| 25 | Malvidin 3-O-rutinoside (feruloyl)-5-O-glucoside | C45H53O24 + | [19] |
| 26 | Malvidin 3-O-rutinoside (p-coumaroyl)-5-O-glucoside | C44H51O23 + | [34] |
| 27 | Malvidin 3-O-rutinoside (cis-p-coumaroyl)-5-O-glucoside | C44H51O23 + | [2][6][11][20][28][40] |
| 28 | Malvidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | C44H51O23 + | [20][22][23] |
| 29 | Malvidin 3-O-rutinoside (glucosyl-cis-p-coumaroyl)-5-O-glucoside | C50H61O28 + | [20] |
| 30 | Malvidin 3-O-rutinoside (glucosyl-trans-p-coumaroyl)-5-O-glucoside | C50H61O28 + | [20] |
| 31 | Malvidin 3-O-[6-O-(4-O-p-coumaroyl-α-L-rhamnosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C44H51O23 + | [15][40] |
| 32 | Malvidin 3-O-[6-O-(4-O-(4-O-trans-(β-D-glucopyranoside)-p- coumaroyl)-a-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O- [β-D-glucopyranoside] |
C44H51O23 + | [20] |
| Delphinidin derivatives | |||
| 33 | Delphinidin 3-O-rutinoside (cis-p-coumaroyl)-5-O-glucoside | C42H47O23 + | [2][6][11][20][28] |
| 34 | Delphinidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | C42H47O23 + | [2][6][11][22][28][33] |
| 35 | Delphinidin 3-O-rutinoside (glucosyl-trans-p-coumaroyl)-5-O-glucoside | C48H57O28 + | [20] |
| 36 | Delphinidin 3-O-[6-O-(4-O-p-coumaroyl-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C42H47O23 + | [15][40] |
| 37 | Delphinidin 3-O-[6-O-(4-O-(trans-p-coumaroyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] |
C42H47O23 + | [29][34] |
| Peonidin derivatives | |||
| 38 | Peonidin 3-O-[6-O-(4-O-E-p-coumaroyl-O-α-rhamnopyranosyl) -β-glucopyranoside]-5-O-[β-glucopyranoside] |
C43H48O22 + | [26] |
| 39 | Peonidin 3-O-[6-O-(4-O-E-p-coumaroyl-O-α-rhamnopyranosyl) -β-glucopyranoside]-5-O-[β-glucopyranoside] |
C43H48O22 + | [26] |
a The anthocyanins in bold are abundant in black goji, as demonstrated by Yang et al. [22].
2.3. Biosynthesis of BGAs
The biosynthesis of phenolic compounds in plants is controlled by genes of the corresponding enzymes [52]. The anthocyanin biosynthetic pathway in black goji is presented in Figure 1B, in which the transcription factors, including bHLH, MYB and WD40, forming a BMW tricomplex [53], co-regulate the transcription of structural genes such as flavonoid 3′hydroxylase (F3′H), flavonoid 3′5′hydroxylase (F3′5′H), anthocyanidin synthase (ANS), dihydroflavonol 4-reductase (DFR) and glucosyltransferase to control BGA biosynthesis [17][54][55][56]. The enhanced anthocyanin gene transcripts (F3′5′H, ANS, DFR and glucosyltransferase) and the increased ratio of F3′5′H/F3′H transcripts account for the biosynthesis and accumulation of Pt derivatives in black goji [10][55], where BGAsN1b and BGAsN2, encoding MYB transcription factors, could be responsible for the Pt3R5G biosynthesis by activating the pathway and regulating the accumulation, respectively [1][54][55][56].
While a plant’s anthocyanin profile is dependent on its genetic information, geographical conditions also play an important role in the anthocyanin structures [52]. The special geography (high altitude) and harsh weather conditions (low temperature, less rain and strong sunlight) of northwestern China, especially Qinghai-Tibet Plateau, dramatically promote the expression of glycosyltransferase HG27071 [22] and ultimately influence the BGA composition by linking sugar moieties and acyl groups to the anthocyanidins, forming glycosylated and acylated anthocyanins [2][18]. The glycosylation and acylation substitution in turn stabilize the chemical structures of BGAs during biosynthesis and accumulation [11], as well as during extraction, industrial processing and gastrointestinal digestion [6][22]. It is important for black goji to survive in such an area with special geography and harsh weather conditions.
Considering the discussion above, black goji is an ideal plant for agriculture and ecology in northwest China, encompassing Qinghai and Gansu Provinces and Inner Mongolia, Xinjiang Uygur and Ningxia Hui Autonomous Regions. The provinces or regions have significant differences in geographical conditions, including altitude, annual mean temperature and rainfall [2]. The anthocyanin composition patterns of black goji from different provinces or regions are the same, but the contents of Pt3R5G and total BGAs in black goji from different provinces or regions exhibit significant differences [2][24]. Meanwhile, black goji from the same province or region does not exhibit significant differences in the content of Pt3R5G and total BGAs [2]. This phenomenon evidences the important impact of geographical conditions on the content of BGAs. The correlation between the Pt3R5G contents and altitude is positive (r = 0.487, p < 0.01), while the correlations between the Pt3R5G contents and temperature and humidity are negative (r = −0.509, p < 0.01 and −0.377, p > 0.05 respectively) [1]. As a result, black goji from Qinghai, which has the highest mean altitude (2800 to 3000 m), lowest annual mean temperature (−5 to 5 °C) and annual mean precipitation (17 to 49 mm), possesses higher contents of both Pt3R5G and total BGAs than those from other provinces or regions in the order of Qinghai > Gansu > Inner Mongolia > Xinjiang > Ningxia [2]. Specifically, the content of total BGAs is increased with higher altitude, due to changes in temperature and humidity conditions. The content of Pt3R5G in black goji is also positively affected (r = 0.026, p > 0.05) by the annual mean sunshine hours, being beneficial to the seedling and growth of black goji [1][57]. The findings support the deduction of Wang et al. [58] that black goji from Qinghai has a higher market value compared to that from other provinces.
Due to the important impact of geographical origin on the content of BGAs, BGAs in turn could be used as an indicator for determining the authenticity of the geographical origins of black goji, which is important in quality control [58]. Wang et al. [2] adopted the data of individual BGA concentrations together with multivariate statistical analysis techniques to differentiate black goji from different geographical areas. The results of the principal component analysis (PCA) and linear discriminant analysis (LDA) provide a 100% successful differentiation rate. Liu et al. [35] observed a similar result, showing that PCA and the hierarchical cluster analysis (HCA) carried out using the content of individual BGAs present a clear separation of black goji according to geographical origins. In addition, by adopting the data of BGA concentrations obtained by near-infrared (NIR) spectroscopy and chemometrics, synergy interval partial least squares (Si-PLS), LDA, K-nearest neighbors (KNN), back propagation artificial neural network (BPANN) and least-squares support vector machine (LS-SVM) regression were systematically evaluated and compared during the development of a determinant model of black goji geographical origin and variety characterization [59]. The recognition rate of LS-SVM was >98.18%, showing excellent generalization for identification results.
On the other hand, the geographical conditions of Qinghai Province suitable for the biosynthesis and accumulation of these anthocyanins are limited, resulting in commercial devaluation and nutritional depreciation of black goji. Anthocyanins are specialized metabolites, the accumulation of which requires elicitors, and ethylene is an enhancer of anthocyanin contents during the growth of berries and vegetables. Foliar-applied ethephon (an ethylene-generating compound) on black carrot increased the anthocyanin contents by 25% [60][61]. Ethephon treatment by immersion of developing fruit of strawberry and ‘Rubi’ table grape also resulted in ripe fruit having greater anthocyanin contents [62][63]. The improvements could be that ethylene caused an increased expression of genes and enzymes as well as transcription factors related to the biosynthesis and accumulation of anthocyanins [60][63]. Ethylene also enhanced the acylation of anthocyanins (i.e., more stable), which protects plants from UV-B damage and subsequent cell death [11][61]. Moreover, postharvest ethylene treatment increased the anthocyanin contents in Cesanese red wine grapes by 17% [64]. Therefore, black goji from other provinces could be regulated by ethylene both in field and postharvest stages to obtain a higher content of BGAs.
2.4. Factors Influencing the Composition of BGAs
2.4.1. Degree of Ripeness
Anthocyanin biosynthesis can be influenced by various factors that result in the accumulation of anthocyanins in the specific cells or organs at particular developmental stages, leading to different anthocyanin levels in fruits at different growth stages [17][65]. The dynamic change in BGAs at three different growth stages (juvenile, expanding and mature) has been studied [1]. It was observed that the biosynthesis and accumulation of Pt3R5G are slow at the juvenile stage but increase significantly during the fruit expanding stage, especially at maturity. Compared with the juvenile stage, the Pt3R5G concentration is increased nearly 3- and 11-fold at the next two development stages, respectively. The increased level of anthocyanins is attributed to the high transcript levels of anthocyanin-related genes and regulators during the ripening process of black goji [66]. Among them, HG27071 and BGAsN2 gene expressions share a similar trend to Pt3R5G concentration during the development of black goji [1][22]. Specifically, a negative correlation (−0.072) is observed between BGAsN2 expression level and Pt3R5G concentration in the early green fruit stage [1]. This may be possible because the structural genes associated with the anthocyanin biosynthetic pathway (Figure 1B) might have not been effectively activated at the juvenile stage, leading to down-accumulation of anthocyanins [67]. However, the correlations are positive at the later stages (expanding, r = 0.344 and mature, r = 0.544) [1]. It is speculated that the synergistic effect of BGA biosynthesis, regeneration and translocation occurs primarily at the mature stage. These findings are helpful in determining the right time for black goji harvesting, which is important to ensure the quality of black goji.
2.4.2. Variety
In recent years, wild black goji resources have decreased dramatically due to destructive picking and the deterioration of the ecological environment [1]. Therefore, cultivated black goji were developed to meet the increased market demands [1][6]. Cultivated black goji exhibits the same profile of anthocyanins as wild black goji [2][6]. The reason is that cultivated black goji is basically transplanted from wild black goji, leading to an identical genetic profile for the two forms [6]. Interestingly, cultivated black goji has a relatively greater fruit size as compared to wild black goji [68]. Positive correlations are observed between anthocyanin contents and the fruit size of black goji [1]. Thus, it is speculated that cultivated black goji may have more BGAs than wild black goji. However, it was reported that the contents of both Pt3R5G and total BGAs are significantly lower in cultivated black goji than in wild fruits [1][6]. The reason is that cultivation has negative effects on the biosynthesis and accumulation of BGAs. The negative effects are caused by routine irrigation, fertilization, weeding and other cultivation operations [69]. The findings suggest that cultivar is important for black goji in biosynthesizing and accumulating more BGAs. Gamage et al. [70] proposed that greenhouse technology could be a suitable approach to produce new varieties of black goji which can be grown in a diverse range of climatic and soil conditions while preserving the high anthocyanin content in their fruit. Precise molecular breeding techniques, based on the characterization of the structural genes involved in BGA biosynthesis (Figure 1B), are also an appropriate approach to produce a type of black goji with a high anthocyanin yield.
2.4.3. Processing Techniques
Dried black goji is the most common product of black goji on the market, and oven-dried black goji has become more prevalent as thousands of drying units have been built in recent years by local governments [24]. The effects of different drying techniques on BGAs have been studied [2][24][71]. Three methods, i.e., freeze-drying (−40 °C), air-drying (room temperature, 22 to 25 °C) and oven-drying (50 °C), were compared. Freeze-dried black goji exhibits the same anthocyanin profile as the fresh black goji, but has less Pt3R5G (64.08%) than the fresh black goji (80%), which may occur during the transportation from the remote producing areas to the specialized laboratories, which takes about 8 h [21]. However, air- and oven-dried black goji show significant differences in both profiles and amounts of anthocyanins as compared to fresh and freeze-dried black goji. The content of Pt3R5G in BGAs from air- and oven-dried black goji was 11.79% and 0.72%, respectively, significantly lower than that of fresh and freeze-dried black goji [24][71]. Additionally, all anthocyanins in fresh and freeze-dried black goji have the structure of 5-O-glucoside, while most in air- and oven-dried black goji have no 5-O-glucoside structure [24][71]. Meanwhile, Cy and Pg derivatives were only found in oven-dried black goji [24], whereas the mechanism remains unclear. It is suggested that freeze-drying maximizes the retention of BGAs, and the thermal processing, i.e., air-drying and especially oven-drying, can cause severe structural changes and degradation of BGAs, as documented by Lu et al. [72] and Wang et al. [73]. This is understandable because anthocyanins have poor stability under light and high temperature conditions [74].
However, a contrary conclusion was reported, showing that temperature has a limited effect on BGA contents [75]. The mechanism was explored by studying the effects of acylation on the thermostability as well as the photostability and pH stability of BGAs [18]. Two individual BGAs, Pt3R5G and Pt 3,5-O-diglucosides (non-acylated), were compared, showing no significant differences for Pt3R5G under various temperature and light conditions, while the degradation of Pt 3,5-O-diglucosides is remarkable. In addition, only 15% of Pt 3,5-O-diglucosides and almost 50% of Pt3R5G are retained at pH 5 and 7, while more than 90% of the two anthocyanins are retained at pH 1 and 3. It is suggested that acylation significantly boosts the stability of BGAs not only in an acidic environment but also at neutral and alkaline pH, and lower pH (1 to 3) conditions are more favorable for the extraction or processing of BGAs, as documented by Tang and Giusti [32] and Shen et al. [40].
The effects of purification on the thermostability of BGAs have also been studied [36]. The thermostability of purified BGAs, unpurified BGAs and purified BGA–whey protein isolate (WPI) synthesis were compared, showing that they follow the order of purified BGA–WPI synthesis > purified BGAs > unpurified BGAs. It is suggested that the purification as well as combination with other substrates can improve the thermostability of BGAs, as highlighted by Qin et al. [76]. Inspired by this, chitosan (CS, 0.2–0.3 mg/mL) and casein phosphopeptide (CPP, 0.5%) composite gel system are used to prepare BGAs-loaded CS-CPP nanoparticles [77], and arabic gum (1%) and β-CD (50%) or gelatin (7%) and β-CD (40%) are used as the coating wall to microencapsulate BGAs [78][79]. The BGA nanoparticles and microencapsulates exhibit higher stability than the native BGAs when exposed to light, air and temperature. The co-pigmentation by glycosylation with glucose or polyphenol extracts is also likely to enhance the stability of BGAs [80]. It was reported that the addition of glucose in the freeze-dried black goji and the addition of polyphenol extracts from raspberry, Lonicera edulis and blackcurrant in the BGA solutions extend the half-lives of BGAs [73][81].
References
- Liu, Z.; Dong, B.; Liu, C.; Zong, Y.; Shao, Y.; Liu, B.; Yue, H. Variation of anthocyanin content in fruits of wild and cultivated Lycium ruthenicum. Ind. Crop. Prod. 2020, 146, 112208.
- Wang, Z.; Yan, Y.; Nisar, T.; Zou, L.; Yang, X.; Niu, P.; Sun, L.; Guo, Y. Comparison and multivariate statistical analysis of anthocyanin composition in Lycium ruthenicum Murray from different regions to trace geographical origins: The case of China. Food Chem. 2018, 246, 233–241.
- Zhang, J.; Sun, L.; Dong, Y.; Fang, Z.; Nisar, T.; Zhao, T.; Wang, Z.-C.; Guo, Y. Chemical compositions and alpha-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chem. 2019, 299, 125102.
- Jin, H.; Zhao, J.; Zhou, W.; Shen, A.; Yang, F.; Liu, Y.; Guo, Z.; Zhang, X.; Tao, Y.; Peng, X.; et al. Preparative separation of a challenging anthocyanin from Lycium ruthenicum Murr. by two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography. RSC Adv. 2015, 5, 62134–62141.
- Yossa Nzeuwa, I.B.; Xia, Y.; Qiao, Z.; Feng, F.; Bian, J.; Liu, W.; Qu, W. Comparison of the origin and phenolic contents of Lycium ruthenicum Murr. by high-performance liquid chromatography fingerprinting combined with quadrupole time-of-flight mass spectrometry and chemometrics. J. Sep. Sci. 2017, 40, 1234–1243.
- Wang, Z.-C.; Nisar, T.; Sun, L.; Fang, Z.; Yan, Y.; Li, D.; Xie, H.; Wang, H.; Guo, Y. Effect of in vitro gastrointestinal digestion on the composition and bioactivity of anthocyanins in the fruits of cultivated Lycium ruthenicum Murray. CYTA—J. Food 2019, 17, 552–562.
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766.
- Peng, Q.; Liu, H.; Shi, S.; Li, M. Lycium ruthenicum polysaccharide attenuates inflammation through inhibiting TLR4/NF-kappaB signaling pathway. Int. J. Biol. Macromol. 2014, 67, 330–335.
- Peng, Q.; Liu, H.; Lei, H.; Wang, X. Relationship between structure and im-munological activity of an arabinogalactan from Lycium ruthenicum. Food Chem. 2016, 194, 595–600.
- Qi, Y.; Zhu, C.; Chen, J.; Liu, G.; Yang, Z.; Chen, W. Comparative analysis of the quality and health-promoting compounds of two-shaped fruits of wild Lycium ruthenicum Murr. from the Qinghai-Tibet Plateau. Acta Physiol. Plant. 2019, 41, 101.
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865.
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical profiles, nutritional constituents and antioxidant activity of black wolfberry (Lycium ruthenicum Murr.). Ind. Crop. Prod. 2020, 154, 112692.
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8.
- Chen, S.; Zhou, H.; Zhang, G.; Meng, J.; Deng, K.; Zhou, W.; Wang, H.; Wang, Z.; Hu, N.; Suo, Y. Anthocyanins from Lycium ruthenicum Murr. ameliorated d-galactose-Induced memory impairment, oxidative stress, and neuroinflammation in adult rats. J. Agr. Food Chem. 2019, 67, 3140–3149.
- Luo, Y.; Fang, J.-L.; Yuan, K.; Jin, S.-H.; Guo, Y. Ameliorative effect of purified anthocyanin from Lycium ruthenicum on atherosclerosis in rats through synergistic modulation of the gut microbiota and NF-κB/SREBP-2 pathways. J. Funct. Foods 2019, 59, 223–233.
- Yan, Y.; Peng, Y.; Tang, J.; Mi, J.; Lu, L.; Li, X.; Ran, L.; Zeng, X.; Cao, Y. Effects of anthocyanins from the fruit of Lycium ruthenicum Murray on intestinal microbiota. J. Funct. Foods 2018, 48, 533–541.
- Zeng, S.; Wu, M.; Zou, C.; Liu, X.; Shen, X.; Hayward, A.; Liu, C.; Wang, Y. Comparative analysis of anthocyanin biosynthesis during fruit development in two Lycium species. Physiol. Plantarum. 2014, 150, 505–516.
- Hu, N.; Zheng, J.; Li, W.; Suo, Y. Isolation, stability, and antioxidant activity of anthocyanins from Lycium ruthenicum Murray and Nitraria Tangutorum Bobr of Qinghai-Tibetan Plateau. Sep. Sci. Technol. 2014, 49, 2897–2906.
- Jin, H.; Liu, Y.; Yang, F.; Wang, J.; Fu, D.; Zhang, X.; Peng, X.; Liang, X. Characterization of anthocyanins in wild Lycium ruthenicum Murray by HPLC-DAD/QTOF-MS/MS. Anal. Methods 2015, 7, 4947–4956.
- Pan, Z.; Cui, M.; Dai, G.; Yuan, T.; Li, Y.; Ji, T.; Pan, Y. Protective effect of anthocyanin on neurovascular unit in cerebral ischemia/reperfusion injury in rats. Front. Neurosci. 2018, 12, 947.
- Wang, Z.; Sun, L.; Fang, Z.; Nisar, T.; Zou, L.; Li, D.; Guo, Y. Lycium ruthenicum Murray anthocyanins effectively inhibit α-glucosidase activity and alleviate insulin resistance. Food Biosci. 2021, 41, 100949.
- Yang, X.; Lin, S.; Jia, Y.; Rehman, F.; Zeng, S.; Wang, Y. Anthocyanin and spermidine derivative hexoses coordinately increase in the ripening fruit of Lycium ruthenicum. Food Chem. 2020, 311, 125874.
- Zeng, S.; Liu, Y.; Wu, M.; Liu, X.; Shen, X.; Liu, C.; Wang, Y. Identification and validation of reference genes for quantitative real-time PCR normalization and its applications in Lycium. PLoS ONE 2014, 9, e97039.
- Tian, Z.; Aierken, A.; Pang, H.; Du, S.; Feng, M.; Ma, K.; Gao, S.; Bai, G.; Ma, C. Constituent analysis and quality control of anthocyanin constituents of dried Lycium ruthenicum Murray fruits by HPLC-MS and HPLC-DAD. J. Liq. Chromatogr. R. T. 2016, 39, 453–458.
- Zhao, J.; Xu, F.; Ji, T.; Li, J. A new spermidine from the fruits of Lycium ruthenicum. Chem. Nat. Compd. 2014, 50, 880–883.
- Samec, D.; Valek-Zulj, L.; Martinez, S.; Grúzc, J.; Piljac, A.; Piljac-Zegarac, J. Phenolic acids significantly contribute to antioxidant potency of Gynostemma pentaphyllum aqueous and methanol extracts. Ind. Crop. Prod. 2016, 84, 104–107.
- Chen, C.; Shao, Y.; Tao, Y.; Mei, L.; Shu, Q.; Wang, L. Main anthocyanins compositions and corresponding H-ORAC assay for wild Lycium ruthenicum Murr. fruits from the Qaidam Basin. J. Pharm. Technol. Drug Res. 2013, 2, 1.
- Jin, H.; Liu, Y.; Guo, Z.; Yang, F.; Wang, J.; Li, X.; Peng, X.; Liang, X. High-performance liquid chromatography separation of cis-trans anthocyanin isomers from wild Lycium ruthenicum Murr. employing a mixed-mode reversed-phase/strong anion-exchange stationary phase. J. Agric. Food Chem. 2015, 63, 500–508.
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radical Bio. Med. 2019, 136, 96–108.
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90.
- Tang, J.; Yan, Y.; Ran, L.; Mi, J.; Sun, Y.; Lu, L.; Gao, Y.; Zeng, X.; Cao, Y. Isolation, antioxidant property and protective effect on PC12 cell of the main anthocyanin in fruit of Lycium ruthenicum Murray. J. Funct. Foods 2017, 30, 97–107.
- Tang, P.; Giusti, M.M. Black goji as a potential source of natural color in a wide pH range. Food Chem. 2018, 269, 419–426.
- Wu, T.; Lv, H.; Wang, F.; Wang, Y. Characterization of polyphenols from Lycium ruthenicum fruit by UPLC-Q-TOF/MS(E) and their antioxidant activity in Caco-2 Cells. J. Agr. Food Chem. 2016, 64, 2280–2288.
- Celli, G.B.; Ghanem, A.; Brooks, M.S. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using response surface methodology. Ultrason. Sonochem. 2015, 27, 449–455.
- Liu, Z.; Tang, X.; Liu, C.; Dong, B.; Shao, Y.; Liu, B.; Yue, H. Ultrasonic extraction of anthocyanins from Lycium ruthenicum Murr. and its antioxidant activity. Food Sci. Nutr. 2020, 8, 2642–2651.
- Liu, P.; Li, W.; Hu, Z.; Qin, X.; Liu, G. Isolation, purification, identification, and stability of anthocyanins from Lycium ruthenicum Murr. LWT—Food Sci. Technol. 2020, 126, 109334.
- Qin, B.; Liu, X.; Cui, H.; Ma, Y.; Wang, Z.; Han, J. Aqueous two phase assisted by ultrasound for the extraction of anthocyanins from Lycium ruthenicum Murr. Prep. Biochem. Biotech. 2017, 47, 881–888.
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Xue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76.
- Wang, Y.; Luan, G.; Zhou, W.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Subcritical water extraction, UPLC-Triple-TOF/MS analysis and antioxidant activity of anthocyanins from Lycium ruthenicum Murr. Food Chem. 2018, 249, 119–126.
- Shen, M.; Liu, K.; Liang, Y.; Liu, G.; Sang, J.; Li, C. Extraction optimization and purification of anthocyanins from Lycium ruthenicum Murr. and evaluation of tyrosinase inhibitory activity of the anthocyanins. J. Food Sci. 2020, 85, 696–706.
- Zhang, Y.; Chen, F.; Sang, J. Green approach for sample preparation and determination of anthocyanins from Lycium ruthenicum Murr. using a β-cyclodextrin-based extraction method coupled with UPLC-DAD analysis. Food Anal. Method. 2018, 11, 2141–2148.
- Wang, H.; Xia, X.; Yu, H.; Zhao, X.; Zhong, X.; Li, Q.; Tang, J.; Zhao, Y. Effect of liquid fermentation on bread fortified with Lycium ruthenicum: A quality attribute and in vitro digestibility study. Food Chem. 2019, 299, 125131.
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, isolation, and purification of anthocyanin. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.1.1–F1.1.11.
- Pacheco-Palencia, L.A.; Talcott, S.T. Chemical stability of acai fruit (Euterpe oleracea Mart.) anthocyanins as influenced by naturally occurring and externally added polyphenolic cofactors in model systems. Food Chem. 2010, 18, 17–25.
- Liobikas, J.; Skemiene, K.; Trumbeckaite, S.; Borutaite, V. Anthocyanins in cardioprotection: A path through mitochondria. Pharmacol. Res. 2016, 113, 808–815.
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radical Res. 2006, 40, 1014–1028.
- Fraige, K.; Pereira-Filho, E.R.; Carrilho, E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC-DAD-MS and exploratory analysis by principal component analysis. Food Chem. 2014, 145, 395–403.
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39.
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26.
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.D.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075.
- Ichiyanagi, T.; Kashiwada, Y.; Shida, Y.; Ikeshiro, Y.; Kaneyuki, T.; Konishi, T. Nasunin from eggplant consists of cis-trans isomers of delphinidin 3--5-glucopyranoside. J. Agric. Food Chem. 2005, 53, 9472–9477.
- Guo, J.; Yue, T.; Yuan, Y.; Wang, Y. Chemometric classification of apple juices according to variety and geographical origin based on polyphenolic profile. J. Agric. Food Chem. 2013, 61, 6949–6963.
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827.
- Li, T.; Fan, Y.; Qin, H.; Dai, G.; Li, G.; Li, Y. Transcriptome and flavonoids metabolomic analysis identifies regulatory networks and hub genes in black and white fruits of Lycium ruthenicum Murray. Front. Plant Sci. 2020, 11, 1256.
- Qi, Y.; Wei, H.; Gu, W.; Shi, W.; Jiang, L.; Deng, L. Transcriptome profiling provides insights into the fruit color development of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Protoplasma 2021, 258, 33–43.
- Zong, Y.; Zhu, X.; Liu, Z.; Xi, X.; Li, G.; Cao, D.; Wei, L.; Li, J.; Liu, B. Functional MYB transcription factor encoding gene AN2 is associated with anthocyanin biosynthesis in Lycium ruthenicum Murray. BMC Plant Biol. 2019, 19, 169.
- Liu, Z.; Shu, Q.; Wang, L.; Yu, M.; Hu, Y.; Zhang, H.; Tao, Y.; Shao, Y. Genetic diversity of the endangered and medically important Lycium ruthenicum Murr. revealed by sequence-related amplified polymorphism (SRAP) markers. Biochem. Syst. Ecol. 2012, 45, 86–97.
- Wang, Z.; Yan, Y.; Nisar, T.; Sun, L.; Zeng, Y.; Guo, Y.; Wang, H.; Fang, Z. Multivariate statistical analysis combined with e-nose and e-tongue assays simplifies the tracing of geographical origins of Lycium ruthenicum Murray grown in China. Food Control 2019, 98, 457–464.
- Li, Y.; Zou, X.; Shen, T.; Shi, J.; Zhao, J.; Mel, H. Determination of geographical origin and anthocyanin content of black goji berry (Lycium ruthenicum Murr.) using near-infrared spectroscopy and chemometrics. Food Anal. Method. 2017, 10, 1034–1044.
- Barba-Espín, G.; Glied, S.; Crocoll, C.; Dzhanfezova, T.; Joernsgaard, B.; Okkels, F.; Lütken, H.; Müller, R. Foliar-applied ethephon enhances the content of anthocyanin of black carrot roots (Daucus carota ssp. sativus var. atrorubens Alef.). BMC Plant Biol. 2017, 17, 70.
- Barba-Espín, G.; Lütken, H.; Glied, S.; Crocoll, C.; Joernsgaard, B.; Müller, R. Anthocyanin elicitation for bio-sustainable colourant production in carrot. Acta Hortic. 2019, 1242, 87–92.
- Reis, L.; Forney, C.F.; Jordan, M.; Munro Pennell, K.; Fillmore, S.; Schemberger, M.O.; Ayub, R.A. Metabolic profile of strawberry fruit ripened on the plant following treatment with an ethylene elicitor or inhibitor. Front. Plant Sci. 2020, 11, 995.
- Scavroni, J.; Ferreira, L.C.; Ferrarese, M.d.L.L.; Ono, E.O.; Rodrigues, J.D. Ethephon and calcium chloride, a combination that improves skin color of ‘Rubi’ table grape. Rev. Bras. Frutic. 2018, 40, e777.
- De Santis, D.; Bellincontro, A.; Forniti, R.; Botondi, R. Time of postharvest ethylene treatments affects phenols, anthocyanins, and volatile compounds of Cesanese red wine grape. Foods 2021, 10, 322.
- Awad, M.A.; de Jager, A.; van der Plas, L.H.; van der Krol, A.R. Flavonoid and chlorogenic acid changes in skin of ’Elstar’ and ’Jonagold’ apples during development and ripening. Sci. Hortic. 2001, 90, 69–83.
- Ma, Y.J.; Duan, H.R.; Zhang, F.; Li, Y.; Yang, H.S.; Tian, F.P.; Zhou, X.H.; Wang, C.M.; Ma, R. Transcriptomic analysis of Lycium ruthenicum Murr. during fruit ripening provides insight into structural and regulatory genes in the anthocyanin biosynthetic pathway. PLoS ONE 2018, 13, e0208627.
- Zong, Y.; Li, S.; Xi, X.; Cao, D.; Wang, Z.; Wang, R.; Liu, B. Comprehensive influences of overexpression of a MYB transcriptor regulating anthocyanin biosynthesis on transcriptome and metabolome of tobacco leaves. Int. J. Mol. Sci. 2019, 20, 5123–5135.
- Fang, T.; Zhen, Q.L.; Liao, L.; Owiti, A.; Zhao, L.; Korban, S.S.; Han, Y. Variation of ascorbic acid concentration in fruits of cultivated and wild apples. Food Chem. 2017, 225, 132–137.
- Kovačević, D.B.; Putnik, P.; Dragović-Uzelac, V.; Vahčić, N.; Babojelić, M.S.; Levaj, B. Influences of organically and conventionally grown strawberry cultivars on anthocyanins content and color in purees and low-sugar jams. Food Chem. 2015, 181, 94–100.
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Black goji berry anthocyanins: Extraction, stability, health benefits, and applications. ACS Food Sci. Technol. 2021, 1, 1360–1370.
- Tan, L.; Dong, Q.; Cao, J.; Gen, D.; Hu, F. Extraction and identification of anthocyanins in Lycium ruthenicum Murr. Nat. Prod. Res. Dev. 2014, 26, 1797–1802.
- Lu, Y.; Kong, X.; Zhang, J.; Guo, C.; Qu, Z.; Jin, L.; Wang, H. Composition changes in Lycium ruthenicum fruit dried by different methods. Front. Nutr. 2021, 8, 737521.
- Wang, Y.; Fu, J.; Yang, D. In situ stability of anthocyanins in Lycium ruthenicum Murray. Molecules 2021, 26, 7073.
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agr. Food Chem. 2002, 50, 7461–7466.
- Deng, K.; Ouyang, J.; Hu, N.; Meng, J.; Su, C.; Wang, J.; Wang, H. Improved colorimetric analysis for subtle changes of powdered anthocyanins extracted from Lycium ruthenicum Murr. Food Chem. 2022, 371, 131080.
- Qin, X.; Yuan, D.; Wang, Q.; Hu, Z.; Wu, Y.; Cai, J.; Huang, Q.; Li, S.; Liu, G. Maillard-reacted whey protein isolates enhance thermal stability of anthocyanins over a wide pH range. J. Agric. Food Chem. 2018, 66, 9556–9564.
- Ran, L.; Mi, J.; Lu, L.; Chen, F.; Luo, Q.; Li, X.; Yan, Y.; Cao, Y.; Huang, Q. Preparation of anthocyanin-loaded nanoparticles from Lycium ruthenium Murr. and its protective effect on oxidative damage of EAhy926 cells induced by oxidized low-density lipoprotein. Food Sci. 2019, 40, 162–168.
- Han, A.; Jiang, H.; Jia, Q.; Ma, L.; Bai, H. Optimization of microencapsulation of anthocyanins from Lycium ruthenicum Murr. by response surface methodology and stability of the microcapsules. Food Sci. 2016, 37, 82–87.
- Han, F.; Wang, M.; Yan, H.; Wang, H.; Li, M. Study on microencapsulation of Lycium ruthenicum anthocyanins. J. Anhui Agric. Sci. 2017, 45, 74–77.
- Zhang, C.; Ma, Y.; Zhao, X.Y.; Mu, J. Influence of copigmentation on stability of anthocyanins from purple potato peel in both liquid state and solid state. J. Agric. Food Chem. 2009, 57, 9503–9508.
- Luan, G.; Wang, Y.; Ouyang, J.; He, Y.; Zhou, W.; Dong, Q.; Wang, H.; Hu, N. Stabilization of Lycium ruthenicum Murr. anthocyanins by natural polyphenol extracts. J. Food Sci. 2021, 86, 4365–4375.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
972
Revisions:
2 times
(View History)
Update Date:
23 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




