Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cui, H.; Xie, W.; Hua, Z.; Cao, L.; Xiong, Z.; Tang, Y.; Yuan, Z. Phytochemicals Used for Hair Dyeing. Encyclopedia. Available online: https://encyclopedia.pub/entry/35849 (accessed on 08 March 2026).
Cui H, Xie W, Hua Z, Cao L, Xiong Z, Tang Y, et al. Phytochemicals Used for Hair Dyeing. Encyclopedia. Available at: https://encyclopedia.pub/entry/35849. Accessed March 08, 2026.
Cui, Hongyan, Wenjing Xie, Zhongjie Hua, Lihua Cao, Ziyi Xiong, Ying Tang, Zhiqin Yuan. "Phytochemicals Used for Hair Dyeing" Encyclopedia, https://encyclopedia.pub/entry/35849 (accessed March 08, 2026).
Cui, H., Xie, W., Hua, Z., Cao, L., Xiong, Z., Tang, Y., & Yuan, Z. (2022, November 22). Phytochemicals Used for Hair Dyeing. In Encyclopedia. https://encyclopedia.pub/entry/35849
Cui, Hongyan, et al. "Phytochemicals Used for Hair Dyeing." Encyclopedia. Web. 22 November, 2022.
Copy Citation
Natural dyes have been used since ancient times, when they were used not only for hair coloration, but also for medicinal, decoration and religious purposes. Many organic compounds have been identified as the principal coloring matters in hair dye plants and investigated for dyeing performance under experimental conditions. Natural colorants can be classified based on dye source, application method and chemical structure.
plant hair dye
natural colorant
coloration mechanism
1. Introduction
Nowadays, with the growing global awareness of the adverse effects of synthetic hair dyes, the demand for safer and more environmentally friendly hair dyes is increasing. Hair dye products can be grouped into three categories according to wash fastness: temporary, semi-permanent and permanent hair dyes [1]. Permanent hair dyes refer to synthetic oxidative hair dyes, by which colors are produced in the hair cortex from small primary intermediates (e.g., p-phenylenediamine and p-aminophenol) and couplers (e.g., m-aminophenol, m-hydroxyphenol and resorcinol) through oxidation reactions in the presence of hydrogen peroxide as the oxidizing agent [2]. Permanent hair dyes represent the most widely used coloring matter in commercial hair dye cosmetics due to their strong dyeing performance, predictable colors and rich range of tones [3]. However, several studies have reported allergenicity [4][5], mutagenicity [6][7], carcinogenicity [8], and environmental toxicity [9] associated with the use of synthetic hair dye ingredients and the potential health risks have attracted widespread attention. By contrast, natural dyes are temporary or semi-permanent non-oxidative hair dyes that can be adsorbed onto the cuticle and some parts of the cortex of the hair shaft to produce color. Natural dyes derived from various parts of plants (e.g., fruits, flowers, leaves, seeds and roots) are generally regarded as low-irritating, less allergenic, sustainable and eco-friendly green products with additional health benefits (e.g., antioxidant, anti-inflammatory and antimicrobial properties) [10].
Natural dyes have been used since ancient times, when they were used not only for hair coloration, but also for medicinal, decoration and religious purposes [11][12]. In the early days, hair dyes were obtained from metallic compounds, plant extracts, dried plants or their mixtures [13]. Before the invention of first synthetic aniline dye, mauve, in 1856, different plant extracts and herbal preparations such as mullein, birch bark, turmeric, and saffron have been used for hair dyeing. The early record of natural hair dyeing dates back to ancient Egyptian times when Rameses II reinforced his red hair color using henna [13]. The ancient Greeks used to bleach their hairs using a rinse of potassium lye solution followed by rubbing with a type of ointment made of yellow flower petals and pollen [14]. The Romans dyed their hair black by using walnut extracts [15]. Today, the renascence of natural botanical ingredients in cosmetics and health care products has led to research work into the phytochemistry and coloring potential of these traditionally used hair dye plants. Compounds including quinones, tannins, flavonoids, indigo, curcuminoids and carotenoids were identified as the dominant naturally-occurring hair coloring matters and some plants accumulating these phytochemicals, such as Lawsonia inermis (henna) [16][17][18], Juglans regia (walnut) [11][12][16], Curcuma longa (turmeric) [19][20], Haematoxylon campechianum (logwood) [16][19][21] were extensively investigated. Natural dyes used in commercial cosmetics are mainly extracted from plants by solvent extraction [19], ultrasonic assisted extraction [22], microwave assisted extraction [10], supercritical fluid extraction [23], and enzyme-assisted extraction [24] etc.
2. Phytochemicals Used for Hair Dyeing
2.1. Quinones
Quinones are colored compounds with a basic benzoquinone chromophore consisting of two carbonyl groups. The three main classes of quinones are naphthoquinones, anthraquinones and benzoquinones [25]. Among which, naphthoquinones, widely distributed in plants and microorganisms, are the most frequently encountered quinone hair dyes. In plants, these compounds usually exist in the free form with several isomers, among which, 1,4-naphthoquinones are the most stable [26]. The light absorbance of quinone dyes depends on their skeleton structure and is affected by the presence of various substituents. The introduction of substituents, especially free or methylated hydroxyl groups, may induce a red shift of the absorption maxima. Some substituents, such as amino or substituted amino groups, may have significant influences on the color properties of quinone dyestuffs [27]. Representative naphthoquinones for hair dyeing purposes are lawsone, juglone, and shikonin (Figure 1).
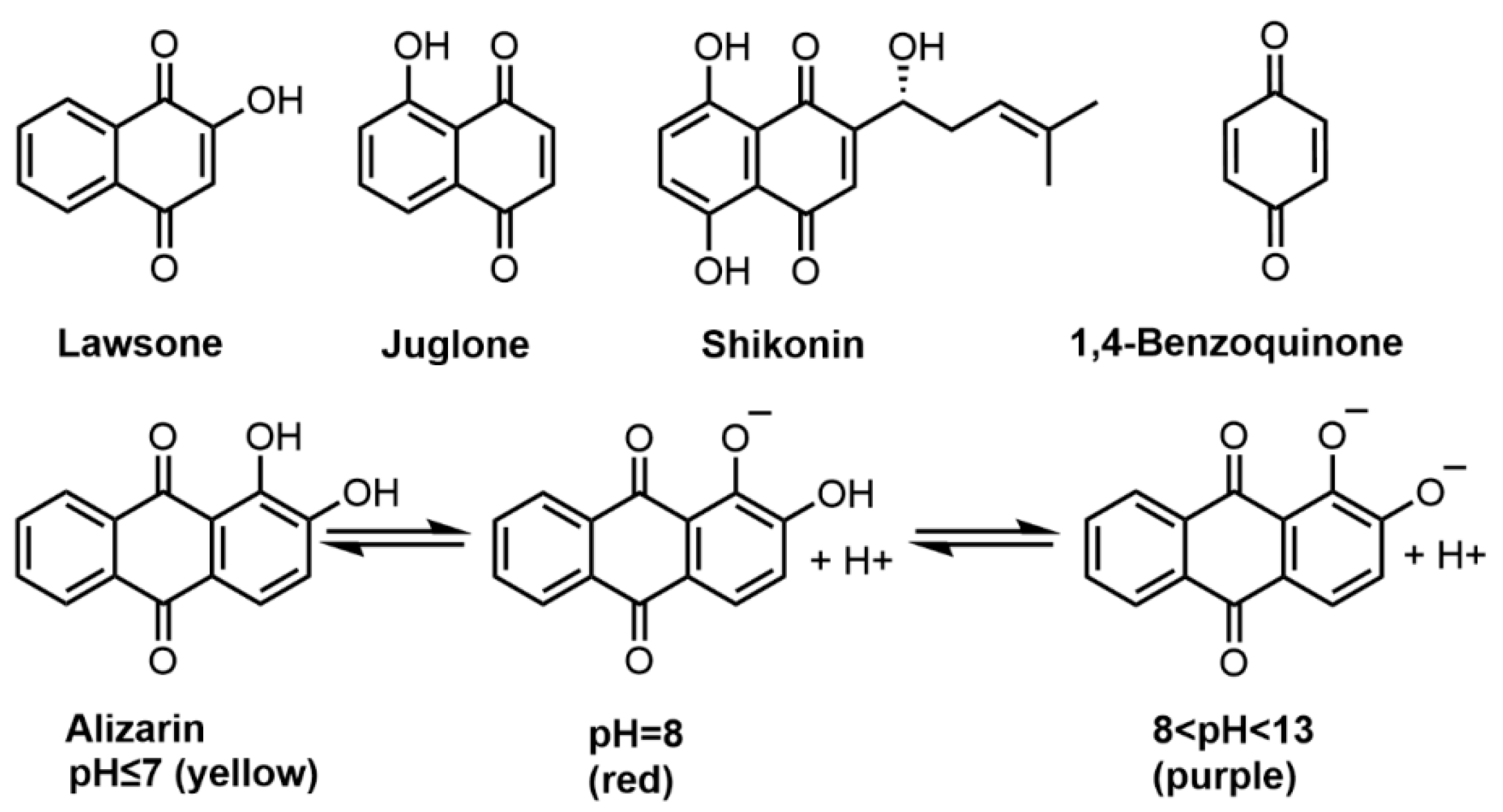
Figure 1. Molecular structures of lawsone, juglone, shikonin, 1,4-benzoquinone, alizarin and the color changes at acid–base conditions.
Lawsone (2-hydroxy-1,4-naphthoquinone) is the main coloring component of henna leaves, also known as CI Natural Orange 6, which acts as a direct or mordant dye for hair fiber to give a red-orange color [17]. In Asia and North Africa, the leaves of henna are widely used for hair and skin coloration. In a traditional manner, henna paste (powdered henna leaves mixed with warm water) is directly applied onto the hair where lawsone can gradually diffuse from the paste into the hair shaft to bind with keratin. There are many natural dye formulations based on henna on the market, usually in the powder form. For example, a gel formula consisting of powdered henna, tea, and hibiscus leaves can dye bleached hair brown [18]. Besides direct dyeing, henna can also be used with various mordants. Ali et al. [20] reported that the plant hair dyes prepared from henna, curcumin, and Tagetes erecta extracts can be used as both direct dye to color gray hair brown and as mordant dye to obtain black color when combined with iron (II) sulfate. Additionally, lawsone has a very low allergic potential. In most cases, allergic reactions are not caused by the henna itself, but by the synthetic coloring additives that are added to henna mixtures [28]. It is worth noting that, when pure henna (usually considered as a weak sensitizer) is used in combination with p-phenylenediamine, the risks for inducing sensitization and broad immune responses (including hypersensitivity) increase significantly [29]. The European Union Scientific Committee on Consumer Safety (SCCS) concluded that henna was slightly and transiently irritating to the eye but it can be safely used as a hair dye when the content of lawsone is less than 1.4% [30]. Besides lawsone, there are flavonoids, tannins, phenolic compounds, alkaloids and other active components in henna. Researchers have reported that henna extract has anti-inflammatory, anticonvulsant [31], antioxidant, immunomodulatory [32], wound healing [33][34], and other pharmacological activities.
Juglone (5-hydroxy-1,4-naphthoquinone) is an isomer of lawsone and can be obtained from the leaves, roots, shells, and barks of walnut plants. Juglone and juglone-containing walnut green husk extracts were used in skin coloring preparations [35], hair dyes [11][12][16] and antimicrobial agents [36]. As a hair dye, juglone gives hair a brownish color when mordanted with iron (II) ions. Beiki et al. reported the use of walnut husk extract to dye bleached hair with good washability and antibacterial activity [12]. A variety of in vitro test methods were used to evaluate the safety of plant hair dyes based on walnut husk extract, categorizing it as non-irritant to the skin but slightly irritating to the eye [16]. Additionally, some studies reported dose-dependent cytotoxicity of juglone in human fibroblasts and keratinocytes [37][38]. These findings indicate that cosmetic preparations containing juglone should be used with care.
Likewise, shikonin has also been used as a direct hair dye for brown color since ancient times. Shikonin ((R)-5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-en-1-yl)naphthalene-1,4-dione) is a dominant component in the roots of Lithospermum erythrorhizon (gromwell root), a perennial herbaceous plant native to China, Japan, and Korea [39][40]. Wang et al. reported that the dyeing performance of gromwell root extracts on bleached hairs with the most pronounced color change observed under acidic conditions [21]. Besides, shikonin is believed to endow fibers with antibacterial and anti-ultraviolet properties [41].
Anthraquinones are compounds with a central 1,4-diketo-cyclohexa-2,5-diene (quinone) structure connected to peripheral phenyl rings [42]. Anthraquinone dyes are typical donor/acceptor types and the substituent effects of which can provide a wide range of colors. A notable structural feature is the hydrogen bonding between α-substituents and the carbonyl group, which enhances light fastness [43]. Anthraquinones are found in various parts of plants (flowers, leaves, fruits, roots, and rhizomes). Rheum officinale (rhubarb), Hamamelis mollis (witch hazel), Aloe vera (aloe), and Rhamnus davurica (buckthorn) are known genera rich in these compounds [44]. In plants, anthraquinones usually exist in the form of glycosides and rarely in the free form. Rubia cordifolia (madder) is a common source of the anthraquinone dye alizarin for dyeing purposes [25]. The structure and color of alizarin are pH-dependent (Figure 1). When the pH value is less than 7, alizarin is in its unionized form with both phenolic hydroxyl groups closed and appears yellow with the absorbance maximum at 430 nm [45][46]. At weak alkaline pH 8, alizarin is partially deprotonated only with its β-OH and appears red with two absorption peaks at 430 nm and 530 nm [46]. When pH increases (8 < pH < 13), both phenolic hydroxyl groups lost hydrogen, and the absorption peak of alizarin moves rapidly to 530 nm, showing a purple color [47]. Boga et al. investigated hair dyeing with alizarin at pH 8 (using yak hair as a model) and found it can rapidly turn white yak hair into reddish color [11].
Benzoquinone is the basic subunit of quinone compounds. 1,4-benzoquinone is a low molecular weight benzoquinone dye extracted from young shoots of the Pyrus lindleyi (pear) and can be used for direct dyeing [25]. It was reported that 1,4-benzoquinone can give yak hairs a brown color at pH 2.7 or pH 8 in a concentration dependent manner, and the color intensity does not seem to be strongly affected by pH [11].
Overall, quinones are a class of naturally abundant colorants with great potential to obtain a broad spectrum of colors on hair, ranging from deep purple to orange and yellow. Currently, the application of quinone colorants in commercial hair cosmetics is limited by poor solubility, strong odor, and photodegradation susceptibility.
2.2. Tannins
Tannins are a large group of polyphenolic molecules having several phenolic hydroxyl groups and other groups such as carbonyls to form strong complexes with various macromolecules [48] (Figure 2). Tannins are broadly distributed in the plant kingdom and are the most abundant secondary metabolites [49]. They are generally classified into two types: hydrolysable tannins and condensed tannins [50]. Hydrolysable tannins contain glucose or polyhydric alcohols esterified with gallic acid (e.g., gallotannins) or hexahydroxydiphenic acid (e.g., ellagitannins) while condensed tannins consist of flavolans or polymeric parts of flavan-3-ols (catechins) and/or flavan 3:4-diols (leucoanthocyanidins) [48].
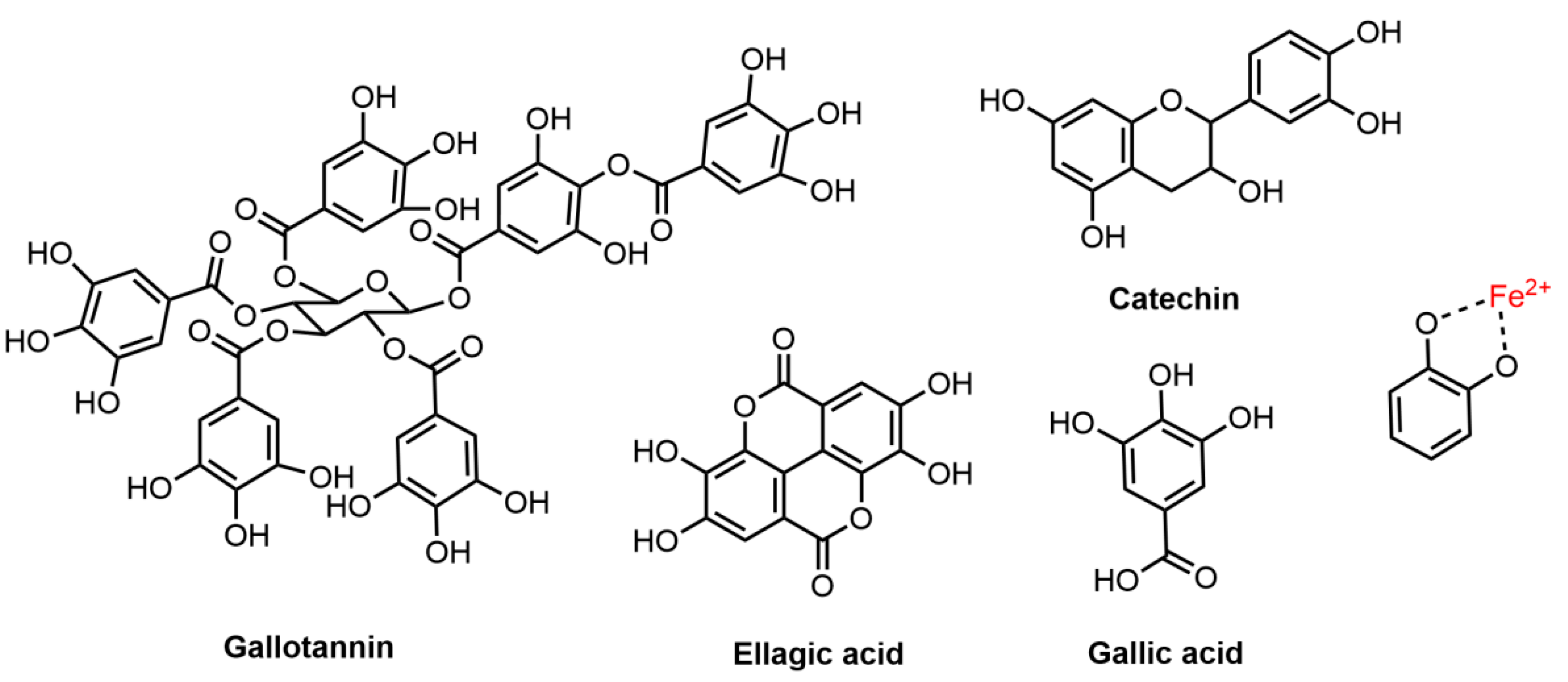
Figure 2. Chemical structures of tannins (gallotannin, ellagic acid, gallic acid and catechin) with a proposed mordanting mechanism when complexed with iron (II) ions.
Tannins are typical mordant dyes. One famous example used in history is the iron-gallink (tannin-iron coordination compounds) [51]. When hydrolysable tannins (pale yellow color) are complexed with iron (II) ions, a strong bluish-black color is produced and the coordination complex formed between tannin, mordant and hair fiber can enhance the color fastness [16]. Both gallotannins and gallic acids occur abundantly (tannin content over 65%) in Galla Chinensis (Chinese gallnut) and the gallnut extract was widely used as a black hair dye with iron (II) sulfate in eastern Asia [52]. Sargsyan et al. [53] studied the hair dyeing mechanism of matcha, which contains another type of hydrolysable tannin, catechin, and found a similar iron-gall complex formed between the mordanting iron (II) ions and the hydroxy groups of flavanol and keratinous hair fiber [54]. Additionally, the selection of metal ions and mordanting methods has shown to have significant effects on the color intensity and fastness of tannin dyeing [55].
On the other hand, tannins can also act as a bio-mordant to enhance the dyeing fastness of many plant dyes. Jahangiri et al. [56] investigated the effects of tannin-based bio-mordants and metallic mordant (alum) on wool dyed with madder root extract. The results showed that fibers pretreated with tannin produced very similar color and washing fastness with those pre-mordanted with alum (ΔE < 1). This was suggested to result from the formation of ionic complexes among protein fibers and organic molecules with ionizable groups at appropriate pHs [57]. In addition, due to the existence of abundant −OH groups, the application of tannins as mordants may also enhance color fastness by forming additional hydrogen bonds with both colorants and protein fibers [58].
2.3. Flavonoids
Flavonoids are formed in plants from the aromatic amino acids, i.e., phenylalanine and tyrosine, and generally occur as glycosylated derivatives. The core structure of flavonoid is flavan nucleus, which consists of 15 carbon atoms arranged in three rings [59]. Flavonoids known for hair dye applications include anthocyanins, hematoxylin, quercetin, acacetin, etc. (Figure 3). Anthocyanins are the largest group of polyphenols in the plant kingdom. They are responsible for the pink, red, purple, violet and blue colors of many fruits, vegetables and flowers [19]. Several studies have demonstrated the successful use of plants containing anthocyanins for hair coloring, including Ribes nigrum (blackcurrant) [60], Morus nigra (mulberry) [11], Phaseolus mungo (bleak bean) [61] and Cleistocalyx nervosum var. paniala [62]. Their colors are determined by the number of hydroxyl groups and degree of methylation as well as the number and position of sugar moieties (glycosides) and attached aliphatic or aromatic acids [60]. Six common derivatives of anthocyanins are presented in Figure 3. Their colors are highly influenced by the environmental acidity/alkalinity. At pH < 3, the flavan nucleus exists mainly as flavylium cation (AH+) showing a red color [60]. When pH increases, AH+ undergoes a rapid deprotonation to form a purple-colored quinonoidal base (A); and when pH > 7.5, an anionic quinonoidal base (A−) is formed with a blue color [63]. Besides, opening of the anthocyanin ring may result in the formation of a yellow-colored E-chalcone [64]. Cyanidin-3-glucoside was successfully applied to hair coloring as a source of red colorants and can change into blue color when complexed with iron (II) oxalate [60][62]. Therefore, anthocyanin dyes can be used for both direct dyeing and mordant dyeing. However, anthocyanins are unstable in aqueous formula whereas the addition of vitamin E acetate at 0.04% can enhance this natural colorant’s stability [60]. Anthocyanins-based preparations have shown to be efficient semi-permanent dyestuffs for hair with the dyed colors durable up to 5 wash cycles [61].
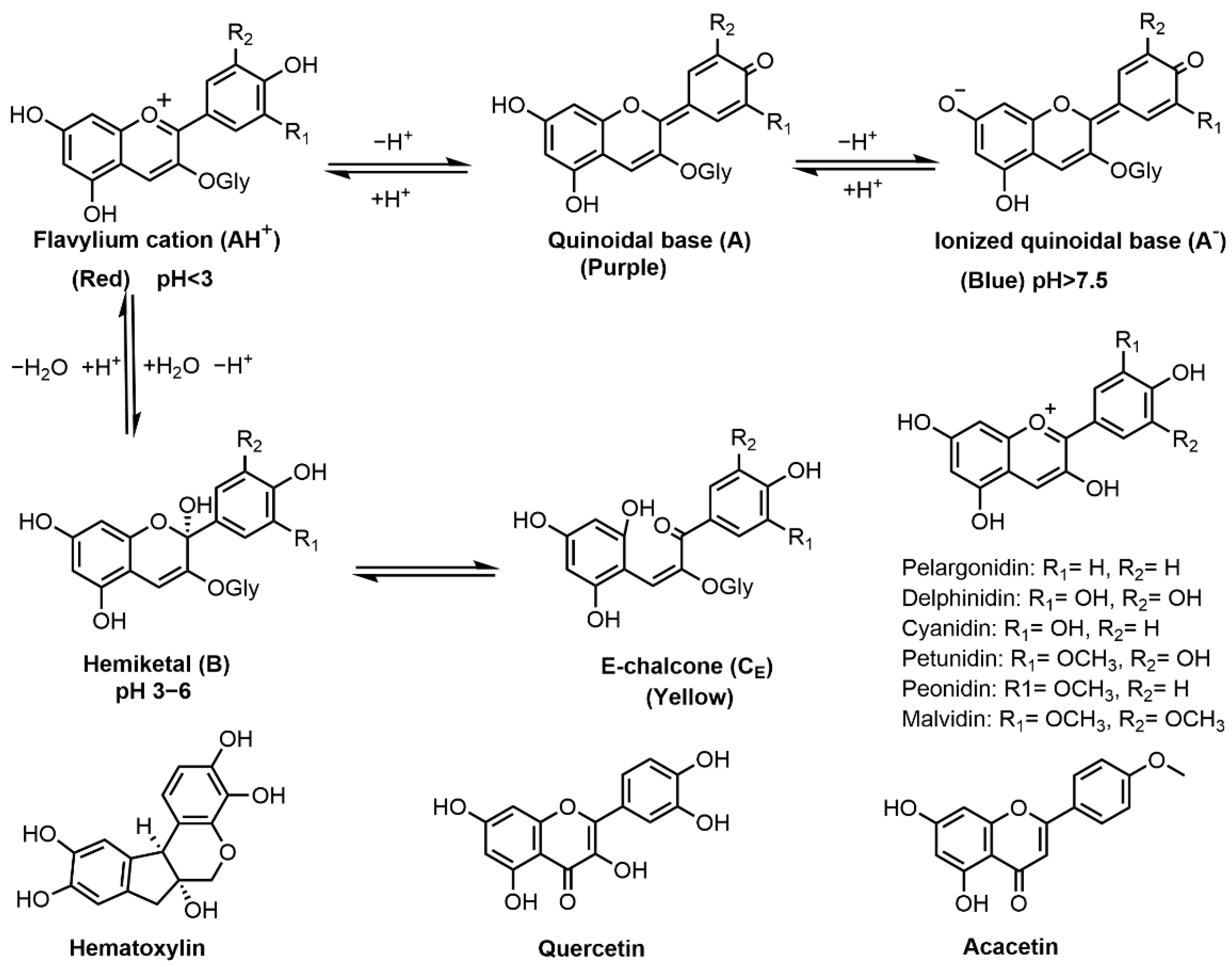
Figure 3. Chemical structures of flavonoids (anthocyanins, hematoxylin, quercetin and acacetin) and the effect of pH on anthocyanin structure and resultant color.
Hematoxylin, (6aR,11bS)-7,11b-dihydroindeno[2,1-c]chromene-3,4,6a,9,10(6H)-pentaol, derived from Haematoxylon campechianum (logwood) [65], can act directly or as mordant dye for hair fiber to give a reddish-brown color [16]. Wang et al. reported the use of logwood extract to dye bleached hair and the dyeing effect was optimal at pH 7 [21]. Thermodynamic and kinetic studies have shown that the adsorption of hematoxylin on hair is a spontaneous and exothermic process [65]. In vitro toxicological tests demonstrated that logwood extract is a safe non-irritant hair dye ingredient [16].
Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4-Hchromen-4-one)) is a polyphenolic flavonoid found in tea, onions and berries [66] and can act as a direct dye for hair coloring. Tibkawin et al. reported the use of teak leaf extract (quercetin is the main colorant) as a plant hair dye for bleached human hairs wherein the color obtained is dependent on the harvest states of leaves (young leaf extract produces reddish brown while mature leaf extract produces brown color) [67]. Quercetin is also known for its medicinal bioactivities, such as anticancer and antiviral activities [68]
Acacetin (5,7-dihydroxy-4-methoxyflavone) is a naturally-occurring flavonoid in the bark of Acacia farnesiana (huizache) [69]. Ali et al. studied the use of huizache extract to dye gray hair, and the color obtained by direct dyeing was less intense than that dyed with henna extract [20]. Besides, acacetin has a variety of pharmacological properties including neuroprotective, cardioprotective, anticancer, anti-inflammatory, antidiabetic, and antimicrobial activities [70].
Collectively, flavonoid colorants may come from a wide range of sources, but their colors are easily affected by environmental pH, metal ions, light, temperature, and oxygen [71][72].
2.4. Indigo
Indigo, also known as indigotin (CI Vat Blue 1), has been used as a vat dye and traditional medicine for thousands of years. In ancient times, freshly picked stems and leaves of “daqingye” indigoid plants [73], i.e., Indigo Naturalis, Baphicacanthus cusia (Nees) Bremek., Polygonum tinctorium Ait. and Isatis indigotica Fort. were soaked in vat water for several days to ferment and become dark blue [74]. Thereafter, lime was added and the residues were stirred and precipitated to obtain indigo colorant, which is a glycosylated form of indole precursor (e.g. isatan A and B) [75]. During fermentation, indole precursors, i.e., hydrolyzed and released indoles, combine spontaneously to form indigo in the presence of oxygen [76]. Intra- and inter-molecular hydrogen bonding are responsible for indigo’s insolubility in water and dilute acid [77].
In the dyeing process (Figure 4), indigo is first chemically reduced in alkaline medium to obtain its soluble reduced form. Sodium hydrosulfite is usually used as the reducing agent for textiles dyed with vat dyes [78][79]. Indigo carmine (indigo-5,5′-disulfonic acid di-sodium salt, C.I. Natural Blue 2) was considered as sulfonated indigo which can give hair a blue hue when used as an acid dye [80]. In the case of hair dyeing, indigo is usually mixed with a certain proportion of henna powders and water to make a paste which can color the hair dark brown [81]. Komboonchoo et al. [78] investigated the dyeing characteristics of indigo, lawsone and lawsone-indigo mixture under reducing and/or oxidizing conditions and found that the dyeing performance of indigo dyes strongly depends on the pH of the solution. Indigo, when dyed in a strong alkaline dyeing bath, shows enhanced dye uptake with a dark blue color due to the presence of soluble monophenol and bisphenol ions of its reduced form. Besides, some studies have reported that indigo has anti-inflammatory, antioxidant, antibacterial and immune regulatory activities [82][83]. Overall, indigo is a sustainable and environmentally friendly natural colorant and is widely used in commercial hair dye cosmetics with henna for dark colors.
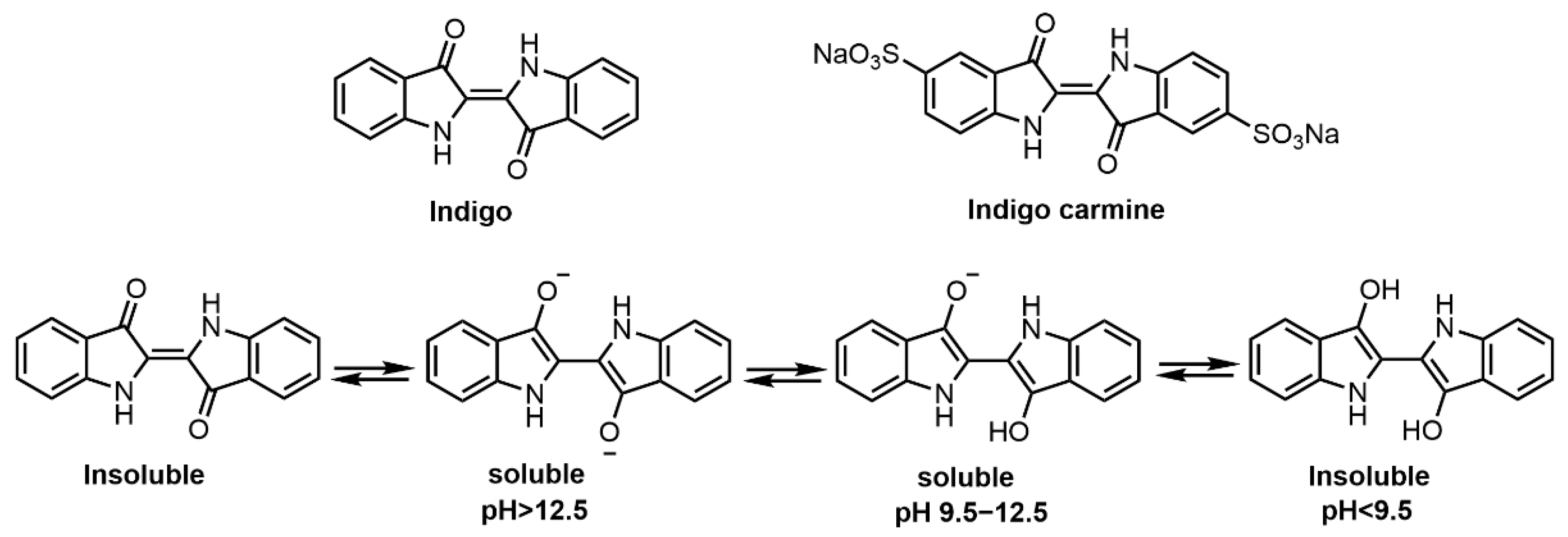
Figure 4. Molecular structures of indigo, indigo carmine and the pH effect on the soluble forms of indigos.
2.5. Curcuminoids
Curcuminoids are a bis-α, β-unsaturated diketone and in solution exist in equilibrium with the corresponding enol tautomer [11]. They are abundant in the rhizome of Curcuma longa (turmeric) and can be found in other Zingiberaceae and Araceae families. Under acidic and neutral conditions, the bis-keto form predominates, whereas at >pH 8 the enol tautomer is favored. Curcumin is practically water insoluble at acidic and neutral pHs while in alkali solution the formation of more soluble anionic species takes place [11]. Curcumin and other related curcuminoids (demethoxycurcumin and bisdemethoxycurcumin) are well known yellow curry colorants and textile dyes (Figure 5). It was reported that under acidic conditions, curcumin dispersed in a water/2-propanol/benzylalcohol solution can be used for direct hair dyeing, giving a distinct yellow color [11]. Curcumin can also be used for mordant dyeing. Under the action of iron (II) sulfate mordant, bleached hairs can be dyed into an orangish-brown color with resistance to 8 shampooing washes [19]. Besides, curcumin has been used in traditional medicine for treating diabetes, abdominal pains, menstrual disorders, wounds, eczema, jaundice, inflammations and for blood purification [84]. Curcumin extracted from turmeric was reported with potent antioxidant, anti-inflammatory, anticancer and hepatoprotective activities [85]. Nevertheless, poor water solubility and photostability limit the industrial use of curcuminoids in hair dye formations.
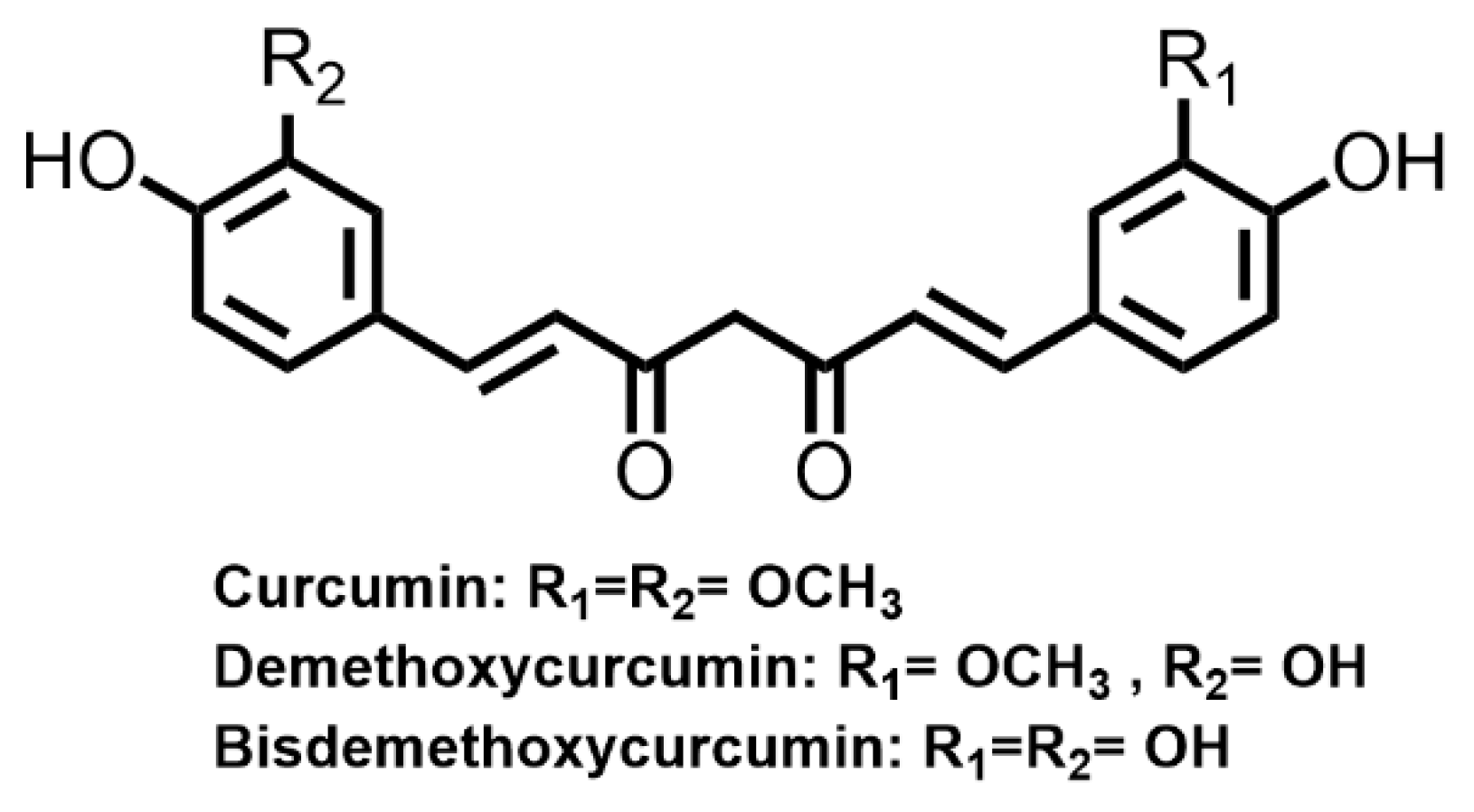
Figure 5. Molecular structures of the main types of curcuminoids.
2.6. Carotenoids
Carotenoids are linear conjugated polyene-terranes and the general structure usually consists of a polyene chain with nine conjugated double bonds and two groups at both ends. Highly conjugated electronic systems of carotenoids contribute to their yellow, orange, red and purple colors [86]. Three types of carotenoids, i.e., zeaxanthin, peridinin, and lutein were successfully applied for hair dyeing (Figure 6). Carotenoid dyes can provide bright hues with good color fastness properties when associated with metallic mordants. Boonsong et al. reported the use of Eclipta alba (false daisy) extract (its main colorant is zeaxanthin) and Terminalia belerica (beleric myrobalan) extract (its main colorant is peridinin) as hair dye for bleached hair with ascorbic acid as a natural color developer and iron (II) sulfate as the mordant, which produced good dyeing performance and dye fastness [19]. Likewise, lutein obtained from Tagetes erecta (marigold) was used in natural hair dyeing to cover grey after mixing with Cymphomandra betacea (tamarillo) extract and Aloe vera mordant [87]. Besides, several carotenoids, such as bixin and norbixin, have been used as a good remedy for cardiac, astringent and febrifuge gonorrhea [85]. Similar to curcumin, carotenoids are hydrophobic dyes extracted using organic solvents such as hexane, acetone and ethyl acetate. Poor photostability is a hindrance to their applications in commercial cosmetic products.
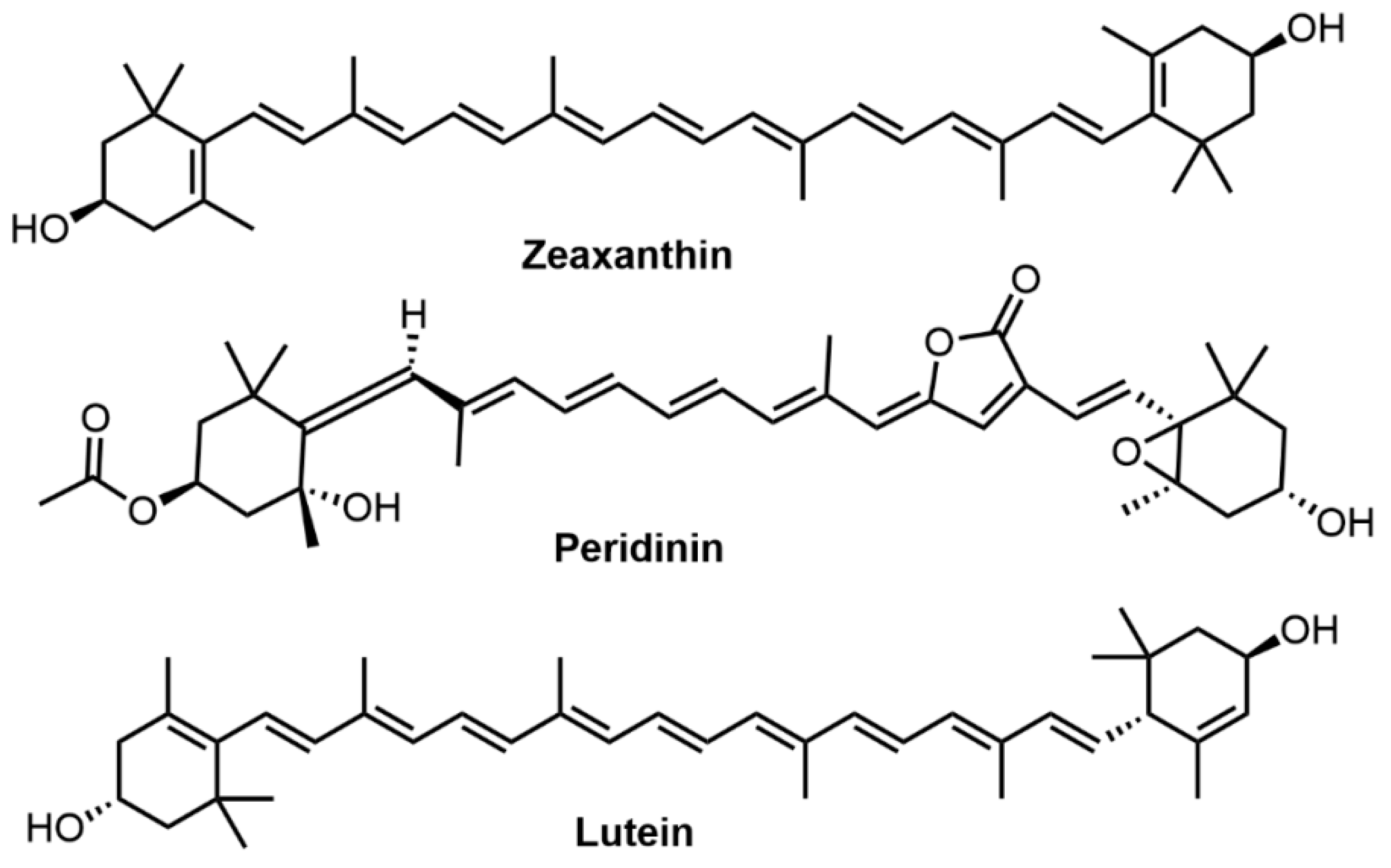
Figure 6. Molecular structures of zeaxanthin, peridinin, and lutein.
References
- Robbins, C.R. Dyeing Human Hair. In Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7, pp. 445–488.
- Ros, M.M.; Gago-Dominguez, M.; Aben, K.K.H.; Bueno-De-Mesquita, H.B.; Kampman, E.; Vermeulen, S.H.; Kiemeney, L.A. Personal hair dye use and the risk of bladder cancer: A case–control study from The Netherlands. Cancer Causes Control. 2012, 23, 1139–1148.
- Morel, O.J.X.; Christie, R.M. Current Trends in the Chemistry of Permanent Hair Dyeing. Chem. Rev. 2011, 111, 2537–2561.
- Hamann, D.; Yazar, K.; Hamann, C.R.; Thyssen, J.P.; Lidén, C. p-Phenylenediamine and other allergens in hair dye products in the United States: A consumer exposure study. Contact Dermat. 2014, 70, 213–218.
- Schuttelaar, M.-L.A.; Vogel, T.A. Contact Allergy to Hair Dyes. Cosmetics 2016, 3, 21.
- Nohynek, G.J.; Fautz, R.; Benech-Kieffer, F.; Toutain, H. Toxicity and human health risk of hair dyes. Food Chem. Toxicol. 2004, 42, 517–543.
- Burnett, C.M.; Loehr, R.F.; Corbett, J.F. Dominant lethal mutagenicity study on hair dyes. J. Toxicol. Environ. Health Part A 1977, 2, 657–662.
- Hp, C.; Reena, K.; Ng, K.Y.; Koh, R.Y.; Ch, N.; Chye, S.M. para-Phenylenediamine Containing Hair Dye: An Overview of Mutagenicity, Carcinogenicity and Toxicity. J. Environ. Anal. Toxicol. 2016, 6, 1000403.
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222.
- Ali, A.; Moinuddin; Allarakha, S.; Fatima, S.; Ali, S.A.; Habib, S. Risk of Carcinogenicity Associated with Synthetic Hair Dyeing Formulations: A Biochemical View on Action Mechanisms, Genetic Variation and Prevention. Indian J. Clin. Biochem. 2022, 39, 399–409.
- Boga, C.; Delpivo, C.; Ballarin, B.; Morigi, M.; Galli, S.; Micheletti, G.; Tozzi, S. Investigation on the dyeing power of some organic natural compounds for a green approach to hair dyeing. Dyes Pigment. 2013, 97, 9–18.
- Beiki, T.; Najafpour, G.D.; Hosseini, M. Evaluation of antimicrobial and dyeing properties of walnut (Juglans regia L.) green husk extract for cosmetics. Color. Technol. 2017, 134, 71–81.
- Dweck, A.C. Natural ingredients for colouring and styling. Int. J. Cosmet. Sci. 2002, 24, 287–302.
- Da França, S.A.; Dario, M.F.; Esteves, V.B.; Baby, A.R.; Velasco, M.V.R. Types of Hair Dye and Their Mechanisms of Action. Cosmetics 2015, 2, 110–126.
- Oumeish, O.Y. The cultural and philosophical concepts of cosmetics in beauty and art through the medical history of mankind. Clin. Dermatol. 2001, 19, 375–386.
- Tang, Y.; He, W.; Wu, Y.; Cai, R. Assessing the dyeing efficiency and irritation potentials of plant hair dyes: A multi-analytical in vitro approach. J. Cosmet. Dermatol. 2019, 18, 1564–1574.
- Tang, Y.; He, W.; Yang, S.; Liu, L. Stabilisation and detoxification of henna (Lawsonia inermis L.) extract for hair dye cosmetics by spray-drying encapsulation. Color. Technol. 2019, 135, 439–450.
- Himangshu, K.; Jyochhana Priya, M.; Gopal, P. Formulation and Evaluation of Natural Herbal Hair Dye Gel Using Lawsonia inermis (Henna Leaves) and Skin Irritation Studies in Albino Rats. J. Pharm. Pharmacol. 2022, 10, 18–24.
- Boonsong, P.; Laohakunjit, N.; Kerdchoechuen, O. Natural pigments from six species of Thai plants extracted by water for hair dyeing product application. J. Clean. Prod. 2012, 37, 93–106.
- Ali, S.; Maqbool, M.; Hussain, M.T. Efficacy of Some Plants Extracts for Natural Dyeing of Human Hair. J. Nat. Fibers 2020, 19, 2581–2595.
- Wang, H.; Chen, C. Formulation Studies and Properties Evaluation of Natural Semi-permanent Hair Dye Made from Gromwell Root and Sappan Wood. Sen’i Gakkaishi 2009, 65, 276–281.
- Sivakumar, V.; Vijaeeswarri, J.; Anna, J.L. Effective natural dye extraction from different plant materials using ultrasound. Ind. Crops Prod. 2011, 33, 116–122.
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847.
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315.
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural Quinone Dyes: A Review on Structure, Extraction Techniques, Analysis and Application Potential. Waste Biomass-Valorization 2021, 12, 6339–6374.
- Qiu, H.-Y.; Wang, P.; Lin, H.-Y.; Tang, C.-Y.; Zhu, H.-L.; Yang, Y.-H. Naphthoquinones: A continuing source for discovery of therapeutic antineoplastic agents. Chem. Biol. Drug Des. 2017, 91, 681–690.
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crops Prod. 2016, 94, 812–833.
- Tukenmez Demirci, G.; Kıvanç Altunay, İ.; Atış, G.; Kucukunal, A. Allergic contact dermatitis mimicking angioedema due to paraphenylendiamine hypersensitivity: A case report. Cutan. Ocul. Toxicol. 2012, 31, 250–252.
- de Ávila, R.I.; Veloso, D.F.M.C.; Teixeira, G.C.; Rodrigues, T.L.; Lindberg, T.; Lindstedt, M.; Fonseca, S.G.; Lima, E.M.; Valadares, M.C. Evaluation of in vitro testing strategies for hazard assessment of the skin sensitization potential of “real-life” mixtures: The case of henna-based hair-colouring products containing p-phenylenediamine. Contact Dermat. 2019, 81, 194–209.
- Scientific Committee on Consumer Safety (SCCS) Opinion on Lawsonia inermis (Henna) COLIPA n C169-SCCS/1511/13 Corrigendum, 12 November 2021. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_140.pdf (accessed on 14 November 2022).
- Syeda, N.F.; Hemalatha, G.; Smitha, T.; Rama, M. Assessment of Neuropharmacological Profile of Ethanolic Extract of Lawsonia Inermis Flowers. Mapana-J. Sci. 2020, 19, 37–48.
- Mikhaeil, B.R.; Badria, F.A.; Maatooq, G.T.; Amer, M.M.A. Antioxidant and Immunomodulatory Constituents of Henna Leaves. Z. Für Nat. C 2004, 59, 468–476.
- Nayak, B.S.; Isitor, G.; Davis, E.M.; Pillai, G.K. The evidence based wound healing activity of Lawsonia inermis Linn. Phytother. Res. 2007, 21, 827–831.
- Vakilian, S.; Norouzi, M.; Soufi-Zomorrod, M.; Shabani, I.; Hosseinzadeh, S.; Soleimani, M. L. inermis-loaded nanofibrous scaffolds for wound dressing applications. Tissue Cell 2018, 51, 32–38.
- Ramezani, N.; Raji, F.; Rezakazemi, M.; Younas, M. Juglone extraction from walnut (Juglans regia L.) green husk by supercritical CO2: Process optimization using Taguchi method. J. Environ. Chem. Eng. 2020, 8, 103776.
- Wang, J.; Liu, D.; Sun, X.; Bai, B.; Jiang, D.; Wu, Z. Label-free quantitative proteomic analysis of the inhibitory activities of juglone against translation and energy metabolism in Escherichia coli. Phytochem. Lett. 2016, 18, 55–58.
- Paulsen, M.T.; Ljungman, M. The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblasts. Toxicol. Appl. Pharmacol. 2005, 209, 1–9.
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic Action of Juglone and Plumbagin: A Mechanistic Study Using HaCaT Keratinocytes. Chem. Res. Toxicol. 2003, 17, 55–62.
- Valipour, M. Recent advances of antitumor shikonin/alkannin derivatives: A comprehensive overview focusing on structural classification, synthetic approaches, and mechanisms of action. Eur. J. Med. Chem. 2022, 235, 114314.
- Boulos, J.C.; Rahama, M.; Hegazy, M.-E.F.; Efferth, T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019, 459, 248–267.
- Dhandapani, R.; Sarkar, A.K. Antibacterial activity and UV property of shikonin on silk substrate. J. Text. Appar. Technol. Manag. 2007, 5, 1–7.
- Malik, E.M.; Müller, C.E. Anthraquinones As Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748.
- Rippon, D.M.L.J.A. The Coloration of Human Hair. The Coloration of Wool and Other Keratin Fibres; John Wiley & Sons: Bradford, UK, 2013; pp. 358–389.
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and its Antiproliferative Activities. Molecules 2016, 21, 1275.
- Acree, W.E., Jr.; Smart, K.; Abraham, M.H. Abraham model solute descriptors reveal strong intramolecular hydrogen bonding in 1,4-dihydroxyanthraquinone and 1,8-dihydroxyanthraquinone. Phys. Chem. Liq. 2017, 56, 416–420.
- Jiang, H.-Y.; Hu, X.-D.; Zhu, J.-J.; Wan, J.; Yao, J.-B. Studies on the photofading of alizarin, the main component of madder. Dye. Dyes Pigment. 2021, 185, 108940.
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. Quantum-chemical and correlation study of ionization of Alizarin. Russ. J. Gen. Chem. 2004, 74, 1558–1563.
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70.
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352.
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332.
- Han, S.Y.; Hong, S.-P.; Kang, E.K.; Kim, B.J.; Lee, H.; Kim, W.I.; Choi, I.S. Iron Gall Ink Revisited: Natural Formulation for Black Hair-Dyeing. Cosmetics 2019, 6, 23.
- Tang, Y.; Yang, S.; He, W.; Liu, L.; Zhang, Z. Stabilization of Chinese Gallnut (Galla Chinensis) Tannins by Spray-Drying Microencapsulation for Natural Hair Coloring. Fibers Polym. 2020, 21, 1283–1292.
- Sargsyan, L.; Hippe, T.; Manneck, H.; Vill, V. Tannin-Mordant Coloration with Matcha (camelia sinensis) and Iron(II)-Lactate on Human Hair Tresses. Molecules 2021, 26, 829.
- Sargsyan, L.; Vill, V.; Hippe, T. Investigations of vegetable tannins as hair dyes and their interactions with pre-bleached hair fibres. Int. J. Cosmet. Sci. 2020, 42, 320–327.
- Burkinshaw, S.M.; Kumar, N. The mordant dyeing of wool using tannic acid and FeSO4, Part 1: Initial findings. Dyes Pigment. 2009, 80, 53–60.
- Jahangiri, A.; Ghoreishian, S.M.; Akbari, A.; Norouzi, M.; Ghasemi, M.; Ghoreishian, M.; Shafiabadi, E. Natural Dyeing of Wool by Madder (Rubia tinctorum L.) Root Extract Using Tannin-based Biomordants: Colorimetric, Fastness and Tensile Assay. Fibers Polym. 2018, 19, 2139–2148.
- Yusuf, M.; Shahid, M.; Khan, M.I.; Khan, S.; Mohammad, F. Dyeing studies with henna and madder: A research on effect of tin (II) chloride mordant. J. Saudi Chem. Soc. 2011, 19, 64–72.
- Adeel, S.; Kiran, S.; Yousaf, M.S. Eco-friendly isolation of tannin based natural colorant from coconut coir (Cocos nucifera) for dyeing of bio-mordanted wool fabric. Glob. NEST J. 2020, 23, 65–72.
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042.
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of Anthocyanins from Blackcurrant ( Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes. J. Agric. Food Chem. 2018, 66, 6790–6798.
- Inman, C.; Lourith, N.; Kanlayavattanakul, M. Alternative application approach on black bean: Hair coloring product. Chem. Biol. Technol. Agric. 2020, 7, 1–7.
- Pipattanamomgkol, P.; Lourith, N.; Kanlayavattanakul, M. The natural approach to hair dyeing product with Cleistocalyx nervosum var. paniala. Sustain. Chem. Pharm. 2018, 8, 88–93.
- Wrolstad, R.E. Anthocyanin Pigments-Bioactivity and Coloring Properties. J. Food Sci. 2006, 69, C419–C425.
- Houbiers, C.; Lima, J.C.; Maçanita, A.L.; Santos, H. Color Stabilization of Malvidin 3-Glucoside: Self-Aggregation of the Flavylium Cation and Copigmentation with the Z-Chalcone Form. J. Phys. Chem. B 1998, 102, 3578–3585.
- Gao, L.; Gao, H. Haematoxylin sorption onto yak hair: Kinetic and thermodynamic studies. Color. Technol. 2013, 130, 21–26.
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89.
- Tibkawin, N.; Suphrom, N.; Nuengchamnong, N.; Khorana, N.; Charoensit, P. Utilisation of Tectona grandis (teak) leaf extracts as natural hair dyes. Color. Technol. 2021, 138, 355–367.
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374.
- Ali, A.; Akhtar, N.; Khan, B.A.; Khan, M.S.; Rasul, A.; Shahiq-uz-zaman; Khalid, N.; Waseem, K.; Mahmood, T.; Ali, L. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plants Res. 2012, 6, 1492–1496.
- Semwal, R.B.; Semwal, D.; Combrinck, S.; Trill, J.; Gibbons, S.; Viljoen, A. Acacetin—A simple flavone exhibiting diverse pharmacological activities. Phytochem. Lett. 2019, 32, 56–65.
- Belscak-Cvitanovic, A.; Levic, S.; Kalusevic, A.; Špoljarić, I.; Đorđević, V.; Komes, D.; Mršić, G.; Nedovic, V. Efficiency Assessment of Natural Biopolymers as Encapsulants of Green Tea (Camellia sinensis L.) Bioactive Compounds by Spray Drying. Food Bioprocess. Technol. 2015, 8, 2444–2460.
- Laleh, G.H.; Frydoonfar, H.; Heidary, R.; Jameei, R.; Zare, S. The Effect of Light, Temperature, pH and Species on Stability of Anthocyanin Pigments in Four Berberis Species. Pak. J. Nutr. 2006, 5, 90–92.
- Yingjiao, Z.; Zhu, Y.; Jian, C.; Xia, C.; Deng, J.; Li, H.; Yanling, L.; Juan, L.; Pei, L. Identification of three species commonly known as “daqingye” by internal leaf anatomy and high-performance liquid chromatography analyses. Acta Soc. Bot. Pol. 2018, 87, 1–10.
- Qi-Yue, Y.; Ting, Z.; Ya-Nan, H.; Sheng-Jie, H.; Xuan, D.; Li, H.; Chun-Guang, X. From natural dye to herbal medicine: A systematic review of chemical constituents, pharmacological effects and clinical applications of indigo naturalis. Chin. Med. 2020, 15, 1–13.
- Zhang, Q.; Xie, J.; Li, G.; Wang, F.; Lin, J.; Yang, M.; Du, A.; Zhang, D.; Han, L. Psoriasis treatment using Indigo Naturalis: Progress and strategy. J. Ethnopharmacol. 2022, 297, 115522.
- Blackburn, R.S.; Bechtold, T.; John, P. The development of indigo reduction methods and pre-reduced indigo products. Color. Technol. 2009, 125, 193–207.
- Božič, M.; Kokol, V. Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes. Dyes Pigment. 2008, 76, 299–309.
- Komboonchoo, S.; Bechtold, T. A study on the dyeing characteristics and electrochemical behaviour of lawsone–indigo mixtures. Color. Technol. 2011, 127, 153–158.
- Fabara, A.N.; Fraaije, M.W. An overview of microbial indigo-forming enzymes. Appl. Microbiol. Biotechnol. 2019, 104, 925–933.
- Komboonchoo, S.; Bechtold, T. Sorption Characteristics of Indigo Carmine as a Blue Colorant for Use in One-bath Natural Dyeing. Text. Res. J. 2009, 80, 734–743.
- Rao, Y.M.; Shayeda; Sujatha, P.S. Formulation and evaluation of commonly used natural hair colorants. Nat. Prod. Radiance 2008, 7, 45–48.
- Qin, S. Pharmacokinetics Research on Anti-Inflammatory Effect and Analgesic Effect of Indigo Naturalis. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 137–140.
- Lin, Y.-K.; Leu, Y.-L.; Huang, T.-H.; Wu, Y.-H.; Chung, P.-J.; Pang, J.-H.S.; Hwang, T.-L. Anti-inflammatory effects of the extract of indigo naturalis in human neutrophils. J. Ethnopharmacol. 2009, 125, 51–58.
- Kadu, S.S. Phytochemical and Physicochemical screening of Curcuma longa Linn. Rhizome by using different solvents. GP Glob. Res. J. Chem. 2020, 3, 55–59.
- Chengaiah, B.; Rao, K.M.; Kumar, K.M.; Alagusundaram, M.; Chetty, C.M. Medicinal importance of natural dyes-a review. Int. J. PharmTech Res. 2010, 2, 144–154.
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16.
- Packianathan, N.; Karumbayaram, S. Formulation and Evaluation of Herbal Hair Dye: An Ecofriendly Process. J. Pharm. Sci. Res. 2010, 2, 648–656.
More
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
2 times
(View History)
Update Date:
23 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





