Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Naruchit Thanuthanakhun | -- | 2147 | 2022-11-22 09:57:07 | | | |
| 2 | Vivi Li | Meta information modification | 2147 | 2022-11-23 07:43:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thanuthanakhun, N.; Kim, M.; Kino-Oka, M. Cell Behavioral Dynamics in Pluripotent Stem Cells Bioprocessing. Encyclopedia. Available online: https://encyclopedia.pub/entry/35811 (accessed on 07 February 2026).
Thanuthanakhun N, Kim M, Kino-Oka M. Cell Behavioral Dynamics in Pluripotent Stem Cells Bioprocessing. Encyclopedia. Available at: https://encyclopedia.pub/entry/35811. Accessed February 07, 2026.
Thanuthanakhun, Naruchit, Mee-Hae Kim, Masahiro Kino-Oka. "Cell Behavioral Dynamics in Pluripotent Stem Cells Bioprocessing" Encyclopedia, https://encyclopedia.pub/entry/35811 (accessed February 07, 2026).
Thanuthanakhun, N., Kim, M., & Kino-Oka, M. (2022, November 22). Cell Behavioral Dynamics in Pluripotent Stem Cells Bioprocessing. In Encyclopedia. https://encyclopedia.pub/entry/35811
Thanuthanakhun, Naruchit, et al. "Cell Behavioral Dynamics in Pluripotent Stem Cells Bioprocessing." Encyclopedia. Web. 22 November, 2022.
Copy Citation
Pluripotent stem cells (PSCs) are important for future regenerative medicine therapies. However, in the production of PSCs and derivatives, the control of culture-induced fluctuations in the outcome of cell quality remains challenging. A detailed mechanistic understanding of how PSC behaviors are altered in response to biomechanical microenvironments within a culture is necessary for rational bioprocessing optimization.
pluripotent stem cells
cell behaviors
culture microenvironments
stem cell bioprocessing
Waddington’s epigenetic landscape
1. Introduction
Pluripotent stem cells (PSCs), which can be isolated from embryos as embryonic stem cells (ESCs) or reprogrammed from somatic cells as induced pluripotent stem cells (iPSCs), are remarkable for their unlimited self-renewal in vitro and ability to differentiate into all cell types of the three embryonic germ layers, including the ectoderm, mesoderm, and endoderm [1]. These cellular capacities make them highly attractive candidates for regenerative medicine. They have been investigated for various clinical complications associated with globally high morbidity rates, including age-related macular degeneration, neurological disorders, and type 1 diabetes [2][3][4]. Despite an extensive exploration of the therapeutic potential of PSCs, there remain challenges toward real-world implementation of these cells in terms of cell bioprocessing and manufacturability.
Cell-based therapies require a high cell density for downstream differentiation and transplantation. Therefore, the development and optimization of robust, scalable, and cost-effective bioprocessing systems are needed to provide an adequate quantity of cells with stable quality [5][6]. Different cell culture systems have emerged for propagating and differentiating PSCs to generate various target cell types. However, the cell variability and suboptimal quality observed within and between batches reflect the existence of fluctuation in the bioprocessing of PSCs (Figure 1) [7][8][9][10]. Typically, the variation in cell quality has been identified to be dependent on the cell origin (origin-induced) and the culture environment (culture-induced) [11]. During in vitro culture adaptation, PSCs accumulate epigenetic changes and potentially undergo phenotypic transition, influencing their clonal self-renewal and lineage differentiation propensity [9][12][13]. It has been shown that variable input factors applied to the culture process, including raw culture materials, culture conditions, and operational parameters, determine the actual microenvironments of the growing cells and how they behave in culture [14]. The dynamic alteration of the cell–microenvironment interactions can profoundly impact many intracellular biological events and cell pluripotency features [10][15][16]. Previously, cellular characteristics and functional variation were investigated among culture platforms. The growth-dependent self-organization within cell colonies under two-dimensional (2D) culture conditions is associated with inter- and intra-colony heterogeneity and spatial differences in the transcription of genes involved in cell cycle regulation, pluripotency, and epithelial function [17][18][19]. The distinct patterns of cell behaviors and growth phase transition during expansion have been further linked with the subsequent preferential differentiation [18][20]. Moreover, in a three-dimensional (3D) aggregate culture system, the growth and survival of PSCs, as well as the cell aggregate structural integrity, are variable, depending on their intercellular interactions and endogenous extracellular matrix (ECM) secretion capability [21][22][23]. After prolonged expansion, the increasing local heterogeneity in ECM accumulation and cytoskeletal alignment, in concert with the uneven distribution of nutrients and essential biochemicals, likely exacerbates growth limitation and spontaneous cell differentiation within 3D cell aggregates [22][24][25]. These prior studies demonstrated that multifactorial variables over courses of culture processes play a role in modulating cell behaviors and regulating the PSC properties and potential. From the perspective of bioprocess engineering, how to efficiently stabilize this fluctuation during PSC culture has become a key challenge in boosting process performance and final cell product quality.

Figure 1. Culture-induced fluctuation in the cell preparation bioprocess. The expansion process contributes to producing an adequate quantity of undifferentiated cells with stable quality for further differentiation and clinical translation. The existence of cell quality variability has been recognized to be influenced by intrinsic cellular properties and extrinsic variables in the cell culture, including raw material adaptability, culture conditions, and the operator’s skills and sense. Differences in the culture inputs and spatiotemporal changes in the cell structural organization during culture determine the biomechanical properties of the cellular microenvironments. The interactions between cells and their microenvironments may result in fluctuations in the phenotypic features of the PSC products.
2. Mechanistic Roles of Cell Behavioral Dynamics in Modulating Cell Fate Decision
Recent evidence has indicated that complex reciprocity between cells and culture microenvironments produces dynamic changes in cell behaviors and intracellular mechanics, contributing to the spatiotemporal variations in cell state and functional regulation within culture [26][27][28][29]. In addition to biochemical cues, cultured cells continually perceive extrinsic stimuli through direct interactions with their surroundings and relay them intracellularly to regulate the multitudes of molecular pathways and gene transcriptional networks. This process is called mechanotransduction (Figure 2) [28][30]. At the cell surface, the anchorage of cells to the local ECM components and the neighboring cells, mediated by specialized adhesion molecules, such as integrin and cadherin, conjointly exerts a mechanosensation role and coordinates cell migration [31][32][33]. The strength of the cell adhesion interactions depends on the magnitude of mechanical constraints sensed by the cells, eliciting a graded mechanosignaling output from the adhesion sites [34][35].
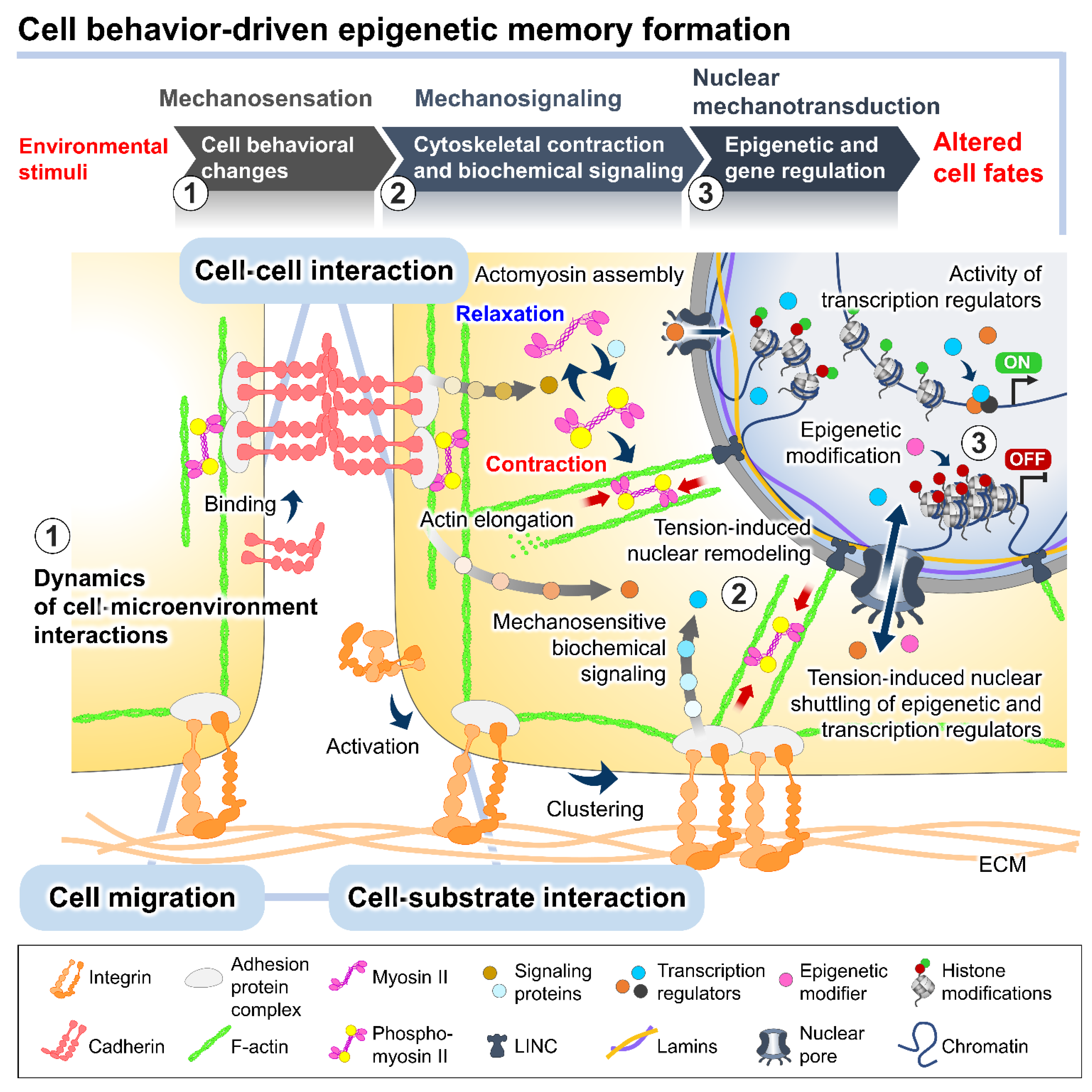
Figure 2. The plausible interplay between cell behavioral dynamics and intracellular mechanical regulation in cell fate modulation. In the culture microenvironments, cells sense and respond to extrinsic mechanical stimuli from the culture substrate, surrounding cells, and fluid dynamics exerted on cellular membranes and different biomechanosensors, such as integrins and E-cadherins. The alteration of the integrin-mediated cell–substrate and E-cadherin-mediated cell–cell adhesion behaviors triggered by the extrinsic forces can stimulate a wide range of intracellular biochemical signaling cascades and induce intrinsic force generation by regulating the actomyosin cytoskeletal contractility. The distribution of the intrinsic cytoskeletal tension mediates the nuclear structural remodeling and cytoplasmic-nuclear shuttling of several mechanosensitive transcription regulators, influencing the intranuclear events, such as epigenetic modifications and gene transcriptional activity, and consequently altering cell fates and functions.
The binding of cell adhesion molecules promotes the recruitment of multiple structural and signaling proteins at the cytoplasmic domains. The association of adhesion protein complexes primarily facilitates a direct force transmission from adhesion contacts towards internal cell compartments and nuclei via physical cytoskeletal connections [36][37]. Moreover, the exerted forces on cell adhesion can be converted into biochemical signals by altering the compositions and activities of regulatory proteins at the adhesion complexes, which generates a sequence of signaling pathways and changes in the cytoskeletal alignment and tensional dynamics [38][39]. The adhesion-mediated activation of Rho A, a member of the Rho GTPase family, and ROCK signaling promotes the phosphorylation of motor protein myosin II and inhibits the activity of myosin II phosphatase [40][41]. The kinetics of the assembly between the phosphorylated myosin II and filamentous actin termed actomyosin complex produces alternating cycles of actin cyto-skeletal contraction and relaxation [42]. Active Rho protein at cell–cell adherens junctions produces signals through its effectors to establish apical actomyosin networks [17][38]. Whereas the localization of p120-catenin elicits the recruitment and activation of other Rho GTPases, Rac and Cdc42, inducing actin polymerization and suppressing the Rho/ROCK-dependent actomyosin contractility [43]. The antagonism of the Rho GTPase members in controlling contraction or elongation of actin bundles serves as a molecular switch to manipulate the balance between cell adhesive and migratory behaviors and direct the intra- and intercellular tensional homeostasis.
The actomyosin-induced contraction propagates mechanical cues to the nucleus, which are directly transmitted to the chromatin domains via a linker of nucleoskeleton and cytoskeleton (LINC) complexes and further modulates the cytoplasmic-to-nuclear translocation of epigenetic modifiers and transcription regulators [27][44][45]. The direct tethering of actomyosin to the nucleoskeleton and associated chromatin influences the dynamics of nuclear deformation and nuclear lamina–genome interactions based on increased or decreased actomyosin activities [46]. Studies demonstrated that actomyosin-induced nuclear flattening and chromatin condensation in the mechanical stress-applied cells involve the alteration of histone methylation and the transcriptional upregulation of mechanoresponsive and quiescent genes [27][47][48].
3. Emerging Methods for Enhancing PSC Expansion through the Regulation of Cell Behaviors
Spatiotemporal differences in cellular microenvironments and structural self-organization along the culture of PSCs potentially contribute to cell-to-cell phenotypic variability [14][16]. Fine-tuning three key components of cell mechanical transducers at the cell-microenvironment interface (cell–substrate interaction, cell–cell interaction, and cell migration) by applying alternative culture substrates and biochemical molecules has been recently introduced by several groups [35][49][50][51][52]. The interaction of PSCs with their surrounding ECM plays a role in coordinating the balances of force generation at the cell-ECM contacts and the overall strength of the intracellular and intercellular contraction [27]. Recent advancements in the field of biomaterials provide a wide range of surface coating agents, including recombinant ECM proteins and synthetic biomimetic matrices [35][53]. Differences in the biochemical composition, molecular structure, and mechanical characteristics of culture matrices strongly influence cell behaviors and pluripotent capacity [54]. The distinct structural isoforms of the ECM adhesion molecules, such as laminin-511, -521, -332, -211, and -111, have been shown to affect the efficiency of proliferation and differentiation of PSCs [54][55][56]. Previous research demonstrated that E8 fragments of laminin-511 and -332, which are the minimal forms retaining the integrin-binding specificity, successfully maintained iPSCs in an undifferentiated state with a normal karyotype and pluripotency for more than 30 passages [55]. However, differences in the binding affinity to E8 fragments of laminin-511, -332, and -211 determine the degree of cell colony compaction and actomyosin contractility, consequently switching the differentiation propensity of cultured iPSCs towards distinct ocular lineages involving Wnt and YAP signaling modulation [54].
The availability of synthetic polymer- and peptide-based matrices with tunable mechanical properties allows users to regulate an optimal strength of cell–substrate adhesion in a target cell-specific manner, thereby promoting an efficient generation of desired cells [35][50][57][58][59][60]. Stiffness represents a key mechanical property of coating material, critically dictating the subcellular allocation and activity of the integrin-mediated adhesion molecules, and further modifying the cell interactions with neighboring cells [61]. Cultivating ESCs on tunable decellularized fibroblast-derived matrices indicated that the extent of substrate stiffness modulates their cell–substrate adhesive potential and cell motility, mediating either induction or inhibition of the epithelial to mesenchymal transition program and controlling the activity of pluripotent gene expression [28]. The ranges of optimal stiffness should be considered when developing culture matrices to facilitate pluripotency maintenance and long-term cell expansion [62]. Recent research on synthetic hydrogel systems has introduced a concept of cell behavioral control through the in situ modifying of structural and adhesive microenvironments [63]. Combined hydrogel matrices have been optimized to switch between pluripotency-permissive and differentiation-permissive states via ionic de-cross-linking [63]. Interestingly, controlling the timing of matrix switching can regulate the ESCs to differentiate into ectoderm or mesendoderm lineages [63]. These culture matrices have been used successfully to generate an integrated platform for growing undifferentiated ESCs and subsequently differentiating them into terminally specialized cells [63]. Additionally, hydrogel-based matrices have been applied to fabricate labile substrates with patterned islands, which restrict the cell–substrate adhesion to designated areas and induce the self-assembly cell aggregation for producing size- and shape-controlled 3D cell aggregates [64]. Regulating the aggregation kinetics by adjusting the labile substrate ligand density allows for the controlling of the porous structure of cell aggregates and indirectly determining stem cell fate [64].
The exogenous regulation of integrin- or E-cadherin-mediated adhesion can attune the properties and functions of cultured PSCs [27][31][65]. Applying a uniform mode of integrin- or E-cadherin-based adhesion regulation attenuates the spatial cell heterogeneity in culture [31]. A non-colony culture system based on recombinant E-cadherin-immobilized surfaces has been proposed to grow undifferentiated PSCs in a more homogeneous microenvironments with moderation of cell–cell contacts [66]. The cultures of ESCs and iPSCs on an E-cadherin-coated substrate, which retain their E-cadherin-based interaction, have been found to increase cell proliferation and maintain cell viability and pluripotency during subculture [66][67]. Furthermore, to modulate the cell–cell interaction, the E-cadherin function-blocking agent botulinum hemagglutinin (HA) has been used as a culture tool to selectively remove cells that deviate from an undifferentiated state during the expansion of iPSCs [49]. Due to the weakened E-cadherin-mediated cell–cell adhesion in deviated cells, HA-induced E-cadherin disruption causes the detachment of deviated cells from cell colonies; however, the undifferentiated cells can restore the E-cadherin-mediated cell–cell interaction and retain their pluripotency following HA removal [49]. Moreover, routine HA treatment in serial passages has been shown to facilitate the long-term maintenance of the iPSC population in an undifferentiated state [52]. It has been suggested that the temporal relaxation of cell–cell junctions by HA can stimulate cell migratory behaviors and cytoskeletal rearrangement, resulting in a relatively uniform dispersion of cells in colonies [52]. In addition, the HA-mediated temporal cell–cell adhesion disruption has been adopted to establish an in situ cell aggregate break-up method for high-density suspension expansion [68]. In cell aggregate growth in conventional culture, large-size aggregates enhance collagen type I accumulation on the aggregate periphery, restricting the homogeneous microenvironments and consequently resulting in undesirable cell proliferation and cell necrosis within the aggregate. The HA-mediated dissociation of cell–cell adhesion facilitates the break-up of aggregates into small sizes, allowing a significant increase in the expansion fold of cells with no adverse effect on maintaining pluripotency [68]. These studies represent current progress in tailoring cell behaviors in PSC cultures. The use of emerging culture strategies that integrate the precise control of culture microenvironments and cell behavioral dynamics may ultimately contribute to regulating the maintenance of undifferentiated state and pluripotent ability of cultured PSCs along the expansion process.
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337.
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.W.; Warnock, G.L.; Levings, M.K.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061.e5.
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438.
- Wang, Y.; Chou, B.K.; Dowey, S.; He, C.; Gerecht, S.; Cheng, L. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res. 2013, 11, 1103–1116.
- Chen, V.C.; Ye, J.; Shukla, P.; Hua, G.; Chen, D.; Lin, Z.; Liu, J.C.; Chai, J.; Gold, J.; Wu, J.; et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015, 15, 365–375.
- Koyanagi-Aoi, M.; Ohnuki, M.; Takahashi, K.; Okita, K.; Noma, H.; Sawamura, Y.; Teramoto, I.; Narita, M.; Sato, Y.; Ichisaka, T.; et al. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20569–20574.
- Cahan, P.; Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 2013, 14, 357–368.
- Gokhale, P.J.; Au-Young, J.K.; Dadi, S.V.; Keys, D.N.; Harrison, N.J.; Jones, M.; Soneji, S.; Enver, T.; Sherlock, J.K.; Andrews, P.W. Culture adaptation alters transcriptional hierarchies among single human embryonic stem cells reflecting altered patterns of differentiation. PLoS ONE 2015, 10, e0123467.
- Phadnis, S.M.; Loewke, N.O.; Dimov, I.K.; Pai, S.; Amwake, C.E.; Solgaard, O.; Baer, T.M.; Chen, B.; Pera, R.A.R. Dynamic and social behaviors of human pluripotent stem cells. Sci. Rep. 2015, 5, 14209.
- Paniza, T.; Deshpande, M.; Wang, N.; Neil, O.R.; Zuccaro, M.V.; Smith, M.E.; Madireddy, A.; James, D.; Ecker, J.; Rosenwaks, Z.; et al. Pluripotent stem cells with low differentiation potential contain incompletely reprogrammed DNA replication. J. Cell Biol. 2020, 219, e201909163.
- Poetsch, M.S.; Strano, A.; Guan, K. Human induced pluripotent stem cells: From cell origin, genomic stability, and epigenetic memory to translational medicine. Stem Cells 2022, 40, 546–555.
- Chowdhury, F.; Na, S.; Li, D.; Poh, Y.C.; Tanaka, T.S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2009, 9, 82–88.
- Kim, M.H.; Kino-oka, M. Bioengineering considerations for a nurturing way to enhance scalable expansion of human pluripotent stem cells. Biotechnol. J. 2020, 15, 1900314.
- Du, J.; Fan, Y.; Guo, Z.; Wang, Y.; Zheng, X.; Huang, C.; Liang, B.; Gao, L.; Cao, Y.; Chen, Y.; et al. Compression generated by a 3D supracellular actomyosin cortex promotes embryonic stem cell colony growth and expression of Nanog and Oct4. Cell Syst. 2019, 9, 214–220.e5.
- Yu, L.; Li, J.; Hong, J.; Takashima, Y.; Fujimoto, N.; Nakajima, M.; Yamamoto, A.; Dong, X.; Dang, Y.; Hou, Y.; et al. Low Cell-matrix adhesion reveals two subtypes of human pluripotent stem cells. Stem Cell Rep. 2018, 11, 142–156.
- Kim, Y.; Jang, H.; Seo, K.; Kim, J.H.; Lee, B.; Cho, H.M.; Kim, H.J.; Yang, E.; Kim, H.; Gim, J.A.; et al. Cell position within human pluripotent stem cell colonies determines apical specialization via an actin cytoskeleton-based mechanism. Stem Cell Rep. 2022, 17, 68–81.
- Rosowski, K.A.; Mertz, A.F.; Norcross, S.; Dufresne, E.R.; Horsley, V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci. Rep. 2015, 5, 14218.
- Gorman, B.R.; Lu, J.; Baccei, A.; Lowry, N.C.; Purvis, J.E.; Mangoubi, R.S.; Lerou, P.H. Multi-scale imaging and informatics pipeline for in situ pluripotent stem cell analysis. PLoS ONE 2014, 9, e116037.
- Kim, M.-H.; Thanuthanakhun, N.; Fujimoto, S.; Kino-oka, M. Effect of initial seeding density on cell behavior-driven epigenetic memory and preferential lineage differentiation of human iPSCs. Stem Cell Res. 2021, 56, 102534.
- Kim, M.H.; Takeuchi, K.; Kino-oka, M. Role of cell-secreted extracellular matrix formation in aggregate formation and stability of human induced pluripotent stem cells in suspension culture. J. Biosci. Bioeng. 2019, 127, 372–380.
- Kato, Y.; Kim, M.H.; Kino-oka, M. Comparison of growth kinetics between static and dynamic cultures of human induced pluripotent stem cells. J. Biosci. Bioeng. 2018, 125, 736–740.
- Hashida, A.; Uemura, T.; Kino-oka, M. Kinetics on aggregate behaviors of human induced pluripotent stem cells in static suspension and rotating flow cultures. J. Biosci. Bioeng. 2020, 129, 494–501.
- Thanuthanakhun, N.; Kino-oka, M.; Borwornpinyo, S.; Ito, Y.; Kim, M.H. The impact of culture dimensionality on behavioral epigenetic memory contributing to pluripotent state of iPS cells. J. Cell. Physiol. 2021, 236, 4985–4996.
- Keong Kwok, C.; Sébastien, I.; Hariharan, K.; Meiser, I.; Wihan, J.; Altmaier, S.; Karnatz, I.; Feile, A.; Cabrera-Socorro, A.; Rasmussen, M.; et al. Scalable expansion of iPSC and their derivatives across multiple lineages. Reprod. Toxicol. 2022, 112, 23–35.
- Weissbein, U.; Plotnik, O.; Vershkov, D.; Benvenisty, N. Culture-induced recurrent epigenetic aberrations in human pluripotent stem cells. PLoS Genet. 2017, 13, e1006979.
- David, B.G.; Fujita, H.; Yasuda, K.; Okamoto, K.; Panina, Y.; Ichinose, J.; Sato, O.; Horie, M.; Ichimura, T.; Okada, Y.; et al. Linking substrate and nucleus via actin cytoskeleton in pluripotency maintenance of mouse embryonic stem cells. Stem Cell Res. 2019, 41, 101614.
- Kim, I.G.; Gil, C.H.; Seo, J.; Park, S.J.; Subbiah, R.; Jung, T.H.; Kim, J.S.; Jeong, Y.H.; Chung, H.M.; Lee, J.H.; et al. Mechanotransduction of human pluripotent stem cells cultivated on tunable cell-derived extracellular matrix. Biomaterials 2018, 150, 100–111.
- Bauwens, C.L.; Peerani, R.; Niebruegge, S.; Woodhouse, K.A.; Kumacheva, E.; Husain, M.; Zandstra, P.W. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 2008, 26, 2300–2310.
- Naqvi, S.M.; McNamara, L.M. Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661.
- Toh, Y.C.; Xing, J.; Yu, H. Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation. Biomaterials 2015, 50, 87–97.
- Martinez-Rico, C.; Pincet, F.; Thiery, J.P.; Dufour, S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J. Cell Sci. 2010, 123, 712–722.
- Cai, D.; Chen, S.C.; Prasad, M.; He, L.; Wang, X.; Choesmel-Cadamuro, V.; Sawyer, J.K.; Danuser, G.; Montell, D.J. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 2014, 157, 1146–1159.
- Roca-Cusachs, P.; Gauthier, N.C.; Del Rio, A.; Sheetz, M.P. Clustering of A5β1 integrins determines adhesion strength whereas Avβ3 and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA 2009, 106, 16245–16250.
- Labouesse, C.; Tan, B.X.; Agley, C.C.; Hofer, M.; Winkel, A.K.; Stirparo, G.G.; Stuart, H.T.; Verstreken, C.M.; Mulas, C.; Mansfield, W.; et al. StemBond hydrogels control the mechanical microenvironment for pluripotent stem cells. Nat. Commun. 2021, 12, 6132.
- Gupton, S.L.; Waterman-Storer, C.M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 2006, 125, 1361–1374.
- Ciobanasu, C.; Faivre, B.; Le Clainche, C. Actomyosin-dependent formation of the mechanosensitive talin–vinculin complex reinforces actin anchoring. Nat. Commun. 2014, 5, 3095.
- Xu, Y.; Zhu, X.; Hahm, H.S.; Wei, W.; Hao, E.; Hayek, A.; Ding, S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl. Acad. Sci. USA 2010, 107, 8129–8134.
- Närvä, E.; Stubb, A.; Guzmán, C.; Blomqvist, M.; Balboa, D.; Lerche, M.; Saari, M.; Otonkoski, T.; Ivaska, J. A strong contractile actin fence and large adhesions direct human pluripotent colony morphology and adhesion. Stem Cell Rep. 2017, 9, 67–76.
- Harb, N.; Archer, T.K.; Sato, N. The Rho-Rock-myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS ONE 2008, 3, e3001.
- Chen, G.; Hou, Z.; Gulbranson, D.R.; Thomson, J.A. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 2010, 7, 240–248.
- Sato, M.K.; Ishihara, T.; Tanaka, H.; Ishijima, A.; Inoue, Y. Velocity-dependent actomyosin ATPase cycle revealed by in vitro motility assay with kinetic analysis. Biophys. J. 2012, 103, 711–718.
- Noren, N.K.; Liu, B.P.; Burridge, K.; Kreft, B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 2000, 150, 567–579.
- Alam, S.G.; Zhang, Q.; Prasad, N.; Li, Y.; Chamala, S.; Kuchibhotla, R.; Kc, B.; Aggarwal, V.; Shrestha, S.; Jones, A.L.; et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 2016, 6, 38063.
- Alisafaei, F.; Jokhun, D.S.; Shivashankar, G.V.; Shenoy, V.B. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc. Natl. Acad. Sci. USA 2019, 116, 13200–13209.
- Grespan, E.; Giobbe, G.G.; Badique, F.; Anselme, K.; Rühe, J.; Elvassore, N. Effect of geometrical constraints on human pluripotent stem cell nuclei in pluripotency and differentiation. Integr. Biol. 2018, 10, 278–289.
- Chi, Y.H.; Wang, W.P.; Hung, M.C.; Liou, G.G.; Wang, J.Y.; Chao, P.H.G. Deformation of the nucleus by TGFβ1 via the remodeling of nuclear envelope and histone isoforms. Epigenetics Chromatin 2022, 15, 1.
- Damodaran, K.; Venkatachalapathy, S.; Alisafaei, F.; Radhakrishnan, A.V.; Jokhun, D.S.; Shenoy, V.B.; Shivashankar, G.V. Compressive force induces reversible chromatin condensation and cell geometry–dependent transcriptional response. Mol. Biol. Cell 2018, 29, 3039–3051.
- Kim, M.H.; Sugawara, Y.; Fujinaga, Y.; Kino-Oka, M. Botulinum hemagglutinin-mediated selective removal of cells deviating from the undifferentiated state in hiPSC Colonies. Sci. Rep. 2017, 7, 93.
- Paiva, S.; Joanne, P.; Migdal, C.; Soler, E.L.; Hovhannisyan, Y.; Nicolas, A.; Agbulut, O. Polyacrylamide hydrogels with rigidity-independent surface chemistry show limited long-term maintenance of pluripotency of human induced pluripotent stem cells on soft substrates. ACS Biomater. Sci. Eng. 2020, 6, 340–351.
- Panda, A.K.; Ravikumar, R.; Gebrekrstos, A.; Bose, S.; Markandeya, Y.S.; Mehta, B.; Basu, B. Tunable substrate functionalities direct stem cell fate toward electrophysiologically distinguishable neuron-like and glial-like cells. ACS Appl. Mater. Interfaces 2021, 13, 164–185.
- Shuzui, E.; Kim, M.H.; Azuma, K.; Fujinaga, Y.; Kino-oka, M. Maintenance of an undifferentiated state of human-induced pluripotent stem cells through botulinum hemagglutinin-mediated regulation of cell behavior. J. Biosci. Bioeng. 2019, 127, 744–751.
- Lee, S.; Stanton, A.E.; Tong, X.; Yang, F. Hydrogels with enhanced protein conjugation efficiency reveal stiffness-induced YAP localization in stem cells depends on biochemical cues. Biomaterials 2019, 202, 26–34.
- Shibata, S.; Hayashi, R.; Okubo, T.; Kudo, Y.; Katayama, T.; Ishikawa, Y.; Toga, J.; Yagi, E.; Honma, Y.; Quantock, A.J.; et al. Selective laminin-directed differentiation of human induced pluripotent stem cells into distinct ocular lineages. Cell Rep. 2018, 25, 1668–1679.e5.
- Miyazaki, T.; Futaki, S.; Suemori, H.; Taniguchi, Y.; Yamada, M.; Kawasaki, M.; Hayashi, M.; Kumagai, H.; Nakatsuji, N.; Sekiguchi, K.; et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012, 3, 1236.
- Laperle, A.; Hsiao, C.; Lampe, M.; Mortier, J.; Saha, K.; Palecek, S.P.; Masters, K.S. α-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Rep. 2015, 5, 195–206.
- Brafman, D.A.; Chang, C.W.; Fernandez, A.; Willert, K.; Varghese, S.; Chien, S. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 2010, 31, 9135–9144.
- Qian, X.; Villa-Diaz, L.G.; Kumar, R.; Lahann, J.; Krebsbach, P.H. Enhancement of the propagation of human embryonic stem cells by modifications in the gel architecture of PMEDSAH polymer coatings. Biomaterials 2014, 35, 9581–9590.
- Shimizu, E.; Iguchi, H.; Le, M.N.; Nakamura, Y.; Kobayashi, D.; Arai, Y.; Takakura, K.; Benno, S.; Yoshida, N.; Tsukahara, M.; et al. A chemically-defined plastic scaffold for the xeno-free production of human pluripotent stem cells. Sci. Rep. 2022, 12, 2516.
- Sung, T.C.; Li, H.F.; Higuchi, A.; Kumar, S.S.; Ling, Q.D.; Wu, Y.W.; Burnouf, T.; Nasu, M.; Umezawa, A.; Lee, K.F.; et al. Effect of cell culture biomaterials for completely xeno-free generation of human induced pluripotent stem cells. Biomaterials 2020, 230, 119638.
- Du, J.; Zu, Y.; Li, J.; Du, S.; Xu, Y.; Zhang, L.; Jiang, L.; Wang, Z.; Chien, S.; Yang, C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci. Rep. 2016, 6, 20395.
- Higuchi, A.; Kao, S.H.; Ling, Q.D.; Chen, Y.M.; Li, H.F.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Chang, S.C.; Lee, H.C.; et al. Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity. Sci. Rep. 2015, 5, 18136.
- Dixon, J.E.; Shah, D.A.; Rogers, C.; Hall, S.; Weston, N.; Parmenter, C.D.J.; McNally, D.; Denning, C.; Shakesheff, K.M. Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 5580–5585.
- Xie, A.W.; Binder, B.Y.K.; Khalil, A.S.; Schmitt, S.K.; Johnson, H.J.; Zacharias, N.A.; Murphy, W.L. Controlled self-assembly of stem cell aggregates instructs pluripotency and lineage bias. Sci. Rep. 2017, 7, 14070.
- Aban, C.E.; Lombardi, A.; Neiman, G.; Biani, M.C.; La Greca, A.; Waisman, A.; Moro, L.N.; Sevlever, G.; Miriuka, S.; Luzzani, C. Downregulation of E-Cadherin in pluripotent stem cells triggers partial EMT. Sci. Rep. 2021, 11, 2048.
- Nagaoka, M.; Koshimizu, U.; Yuasa, S.; Hattori, F.; Chen, H.; Tanaka, T.; Okabe, M.; Fukuda, K.; Akaike, T. E-cadherin-coated plates maintain pluripotent es cells without colony formation. PLoS ONE 2006, 1, e15.
- Nagaoka, M.; Duncan, S.A. Laboratory-scale purification of a recombinant E-cadherin-IgG Fc fusion protein that provides a cell surface matrix for extended culture and efficient subculture of human pluripotent stem cells. In Human Embryonic and Induced Pluripotent Stem Cells; Ye, K., Jin, S., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 25–35. ISBN 978-1-61779-267-0.
- Nath, S.C.; Tokura, T.; Kim, M.H.; Kino-oka, M. Botulinum hemagglutinin-mediated in situ break-up of human induced pluripotent stem cell aggregates for high-density suspension culture. Biotechnol. Bioeng. 2018, 115, 910–920.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
672
Revisions:
2 times
(View History)
Update Date:
29 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




