Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Naruchit Thanuthanakhun and Version 2 by Vivi Li.
Pluripotent stem cells (PSCs) are important for future regenerative medicine therapies. However, in the production of PSCs and derivatives, the control of culture-induced fluctuations in the outcome of cell quality remains challenging. A detailed mechanistic understanding of how PSC behaviors are altered in response to biomechanical microenvironments within a culture is necessary for rational bioprocessing optimization.
- pluripotent stem cells
- cell behaviors
- culture microenvironments
- stem cell bioprocessing
- Waddington’s epigenetic landscape
1. Introduction
Pluripotent stem cells (PSCs), which can be isolated from embryos as embryonic stem cells (ESCs) or reprogrammed from somatic cells as induced pluripotent stem cells (iPSCs), are remarkable for their unlimited self-renewal in vitro and ability to differentiate into all cell types of the three embryonic germ layers, including the ectoderm, mesoderm, and endoderm [1]. These cellular capacities make them highly attractive candidates for regenerative medicine. They have been investigated for various clinical complications associated with globally high morbidity rates, including age-related macular degeneration, neurological disorders, and type 1 diabetes [2][3][4][2,3,4]. Despite an extensive exploration of the therapeutic potential of PSCs, there remain challenges toward real-world implementation of these cells in terms of cell bioprocessing and manufacturability.
Cell-based therapies require a high cell density for downstream differentiation and transplantation. Therefore, the development and optimization of robust, scalable, and cost-effective bioprocessing systems are needed to provide an adequate quantity of cells with stable quality [5][6][5,6]. Different cell culture systems have emerged for propagating and differentiating PSCs to generate various target cell types. However, the cell variability and suboptimal quality observed within and between batches reflect the existence of fluctuation in the bioprocessing of PSCs (Figure 1) [7][8][9][10][7,8,9,10]. Typically, the variation in cell quality has been identified to be dependent on the cell origin (origin-induced) and the culture environment (culture-induced) [11]. During in vitro culture adaptation, PSCs accumulate epigenetic changes and potentially undergo phenotypic transition, influencing their clonal self-renewal and lineage differentiation propensity [9][12][13][9,12,13]. It has been shown that variable input factors applied to the culture process, including raw culture materials, culture conditions, and operational parameters, determine the actual microenvironments of the growing cells and how they behave in culture [14]. The dynamic alteration of the cell–microenvironment interactions can profoundly impact many intracellular biological events and cell pluripotency features [10][15][16][10,15,16]. Previously, cellular characteristics and functional variation were investigated among culture platforms. The growth-dependent self-organization within cell colonies under two-dimensional (2D) culture conditions is associated with inter- and intra-colony heterogeneity and spatial differences in the transcription of genes involved in cell cycle regulation, pluripotency, and epithelial function [17][18][19][17,18,19]. The distinct patterns of cell behaviors and growth phase transition during expansion have been further linked with the subsequent preferential differentiation [18][20][18,20]. Moreover, in a three-dimensional (3D) aggregate culture system, the growth and survival of PSCs, as well as the cell aggregate structural integrity, are variable, depending on their intercellular interactions and endogenous extracellular matrix (ECM) secretion capability [21][22][23][21,22,23]. After prolonged expansion, the increasing local heterogeneity in ECM accumulation and cytoskeletal alignment, in concert with the uneven distribution of nutrients and essential biochemicals, likely exacerbates growth limitation and spontaneous cell differentiation within 3D cell aggregates [22][24][25][22,24,25]. These prior studies demonstrated that multifactorial variables over courses of culture processes play a role in modulating cell behaviors and regulating the PSC properties and potential. From the perspective of bioprocess engineering, how to efficiently stabilize this fluctuation during PSC culture has become a key challenge in boosting process performance and final cell product quality.
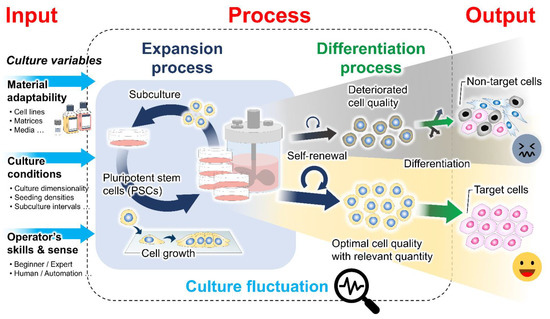
Figure 1. Culture-induced fluctuation in the cell preparation bioprocess. The expansion process contributes to producing an adequate quantity of undifferentiated cells with stable quality for further differentiation and clinical translation. The existence of cell quality variability has been recognized to be influenced by intrinsic cellular properties and extrinsic variables in the cell culture, including raw material adaptability, culture conditions, and the operator’s skills and sense. Differences in the culture inputs and spatiotemporal changes in the cell structural organization during culture determine the biomechanical properties of the cellular microenvironments. The interactions between cells and their microenvironments may result in fluctuations in the phenotypic features of the PSC products.
2. Mechanistic Roles of Cell Behavioral Dynamics in Modulating Cell Fate Decision
Recent evidence has indicated that complex reciprocity between cells and culture microenvironments produces dynamic changes in cell behaviors and intracellular mechanics, contributing to the spatiotemporal variations in cell state and functional regulation within culture [26][27][28][29][26,27,28,29]. In addition to biochemical cues, cultured cells continually perceive extrinsic stimuli through direct interactions with their surroundings and relay them intracellularly to regulate the multitudes of molecular pathways and gene transcriptional networks. This process is called mechanotransduction (Figure 2) [28][30][28,30]. At the cell surface, the anchorage of cells to the local ECM components and the neighboring cells, mediated by specialized adhesion molecules, such as integrin and cadherin, conjointly exerts a mechanosensation role and coordinates cell migration [31][32][33][31,32,33]. The strength of the cell adhesion interactions depends on the magnitude of mechanical constraints sensed by the cells, eliciting a graded mechanosignaling output from the adhesion sites [34][35][34,35].
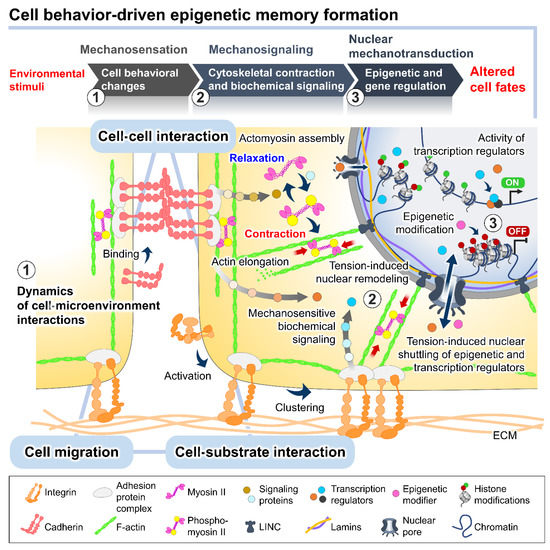
Figure 2. The plausible interplay between cell behavioral dynamics and intracellular mechanical regulation in cell fate modulation. In the culture microenvironments, cells sense and respond to extrinsic mechanical stimuli from the culture substrate, surrounding cells, and fluid dynamics exerted on cellular membranes and different biomechanosensors, such as integrins and E-cadherins. The alteration of the integrin-mediated cell–substrate and E-cadherin-mediated cell–cell adhesion behaviors triggered by the extrinsic forces can stimulate a wide range of intracellular biochemical signaling cascades and induce intrinsic force generation by regulating the actomyosin cytoskeletal contractility. The distribution of the intrinsic cytoskeletal tension mediates the nuclear structural remodeling and cytoplasmic-nuclear shuttling of several mechanosensitive transcription regulators, influencing the intranuclear events, such as epigenetic modifications and gene transcriptional activity, and consequently altering cell fates and functions.
The binding of cell adhesion molecules promotes the recruitment of multiple structural and signaling proteins at the cytoplasmic domains. The association of adhesion protein complexes primarily facilitates a direct force transmission from adhesion contacts towards internal cell compartments and nuclei via physical cytoskeletal connections [36][37][36,37]. Moreover, the exerted forces on cell adhesion can be converted into biochemical signals by altering the compositions and activities of regulatory proteins at the adhesion complexes, which generates a sequence of signaling pathways and changes in the cytoskeletal alignment and tensional dynamics [38][39][38,39]. The adhesion-mediated activation of Rho A, a member of the Rho GTPase family, and ROCK signaling promotes the phosphorylation of motor protein myosin II and inhibits the activity of myosin II phosphatase [40][41][40,41]. The kinetics of the assembly between the phosphorylated myosin II and filamentous actin termed actomyosin complex produces alternating cycles of actin cyto-skeletal contraction and relaxation [42]. Active Rho protein at cell–cell adherens junctions produces signals through its effectors to establish apical actomyosin networks [17][38][17,38]. Whereas the localization of p120-catenin elicits the recruitment and activation of other Rho GTPases, Rac and Cdc42, inducing actin polymerization and suppressing the Rho/ROCK-dependent actomyosin contractility [43]. The antagonism of the Rho GTPase members in controlling contraction or elongation of actin bundles serves as a molecular switch to manipulate the balance between cell adhesive and migratory behaviors and direct the intra- and intercellular tensional homeostasis.
The actomyosin-induced contraction propagates mechanical cues to the nucleus, which are directly transmitted to the chromatin domains via a linker of nucleoskeleton and cytoskeleton (LINC) complexes and further modulates the cytoplasmic-to-nuclear translocation of epigenetic modifiers and transcription regulators [27][44][45][27,44,45]. The direct tethering of actomyosin to the nucleoskeleton and associated chromatin influences the dynamics of nuclear deformation and nuclear lamina–genome interactions based on increased or decreased actomyosin activities [46]. Studies demonstrated that actomyosin-induced nuclear flattening and chromatin condensation in the mechanical stress-applied cells involve the alteration of histone methylation and the transcriptional upregulation of mechanoresponsive and quiescent genes [27][47][48][27,47,48].
