| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefania Croce | + 2114 word(s) | 2114 | 2020-12-08 09:22:25 |
Video Upload Options
This is an entry on the in vivo therapeutic applications of Mesenchymal stem/stromal cell derived Extracellular vesicles (MSC-EVs). The immunomodulatory and regenarative properties of this cell free product will be analyzed in preclinical aniomal models of immune-mediated diseases (graft versus host disease) and orga injury (lung, kidney, skin)
1. Introduction
Extracelluar Vesicles (EVs), mediators of multiple biological functions, are released by almost all cells under physiological and/or pathological conditions, and when cellular activation or stress is present [1][2]. EVs, characterized by a phospholipid bilayer membrane, consist of particles with different size, defined as medium/large-sized EVs (m/lEV enriched in microvesicles) if ranging from 100 to 1000 nm or small EVs (sEV, enriched in exosomes) if ranging from 20 to 100 nm [3]. MVs are budded from the parental cell membrane and can contain microRNAs, mRNAs, proteins and mitochondria. sEVs have endosomal biogenesis and can be composed of lipids, proteins, and nucleic acids[4]. We will focus on clinical applications of EV-derived from in vitro expanded Mesenchymal Stem/Stromal cells (MSCs), indicating that parental cells may have both cell-renewal (stem) and immunomodulatory capacities (stromal) [5][6]. Since the pioneer transplantations of MSCs have been documented[7][8], a huge amount of studies have been reporting the beneficial effects of these cells in different clinical applications, due to either their ability to modulate the immune response or their capacity to differentiate into several lineages, being so far a relevant source for the therapy of pathologies characterized by inflammation and degenerative processes [9][10][11][12][13]. Moreover, MSC capability to trans-differentiate in endothelial cells and produce pro-angiogenic factors has allowed to investigate their use in tissue regeneration (i.e. ischemic tissues) [14][15]. In the last years, the beneficial effects have been hampered by the possible, not to be excluded a priori, capacity that transplanted MSCs may unexpectedly differentiate or uncontrollably grow in the host [16][17], opening a scenario of still unknown complications for the receiver. These possible events lead the scientists to better investigate for in vivo cell free therapies. Initially, the therapeutic effects of MSCs were attributed to their capability to engraft in damaged tissues, however it has been shown that only a small proportion of infused MSCs reach the targets[18] and several recent studies well documented how they exert their beneficial effects through a paracrine action by the release of EVs [19]. The MSC-EVs may reach distant sites taking advantage of extracellular fluids and mediate immune responses or tissue regeneration by different mechanisms of action, including the interaction with membrane specific receptors and/or the direct fusion of EV membrane with target cell followed by the release of biological compounds in the cytosol [20][21]. Preclinical studies are showing that MSC-EVs have therapeutic effects quite comparable or even better than those obtained with transplanted MSCs [22][23]. The actual translation of MSC-EVs to the clinical stage still needs to be defined. In particular, several issues have to be investigated such as MSC-EV sources (adult or neonatal tissue), method of EV enrichment and characterization, dosage and route of administration [3]. Nevertheless, MSC-EVs are candidates to become an actual alternative for clinicians in the near future. This may lead to a number of advantages such as the prevention of possible immune reaction against heterologous MSCs, or the formation of ectopic tissue and/or tumor masses, therefore improving the safety in clinical setting. In addition, the number of curable pathologies would be increased by the fact that MSC-EVs are able to cross the blood brain barrier [24].
2. EV Applications in Prevention or Treatment of Acute and Chronic Graft Versus Host Disease
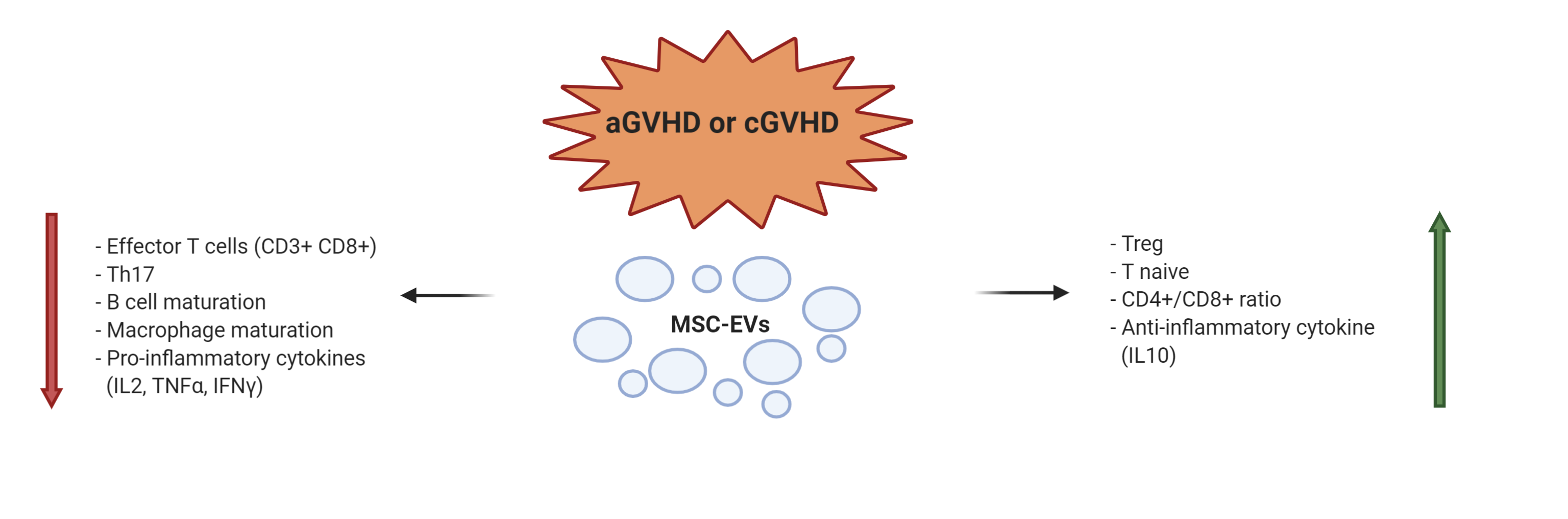
Graft versus host disease (GVHD) is a frequent and severe consequence of allogeneic hematopoietic stem cell transplantation. It is an immunologically mediated process due to activated donor T cell proliferation homing toward target tissues and causing host tissue damage. About 50% of patients, receiving immunosuppressant drugs, may result steroid-refractory [25]. EVs were used as an innovative approach in the prevention of aGVHD after allo-Hematopoietic Stem Cell Transplantation (HSCT) in a mouse model. In order to prevent life-threatening aGVHD, EVs were obtained from human umbilical cord-MSCs (UC-MSC-EVs) and administered intravenously. After infusions at day 0 and 7 after HSCT, the in vivo aGVHD development and the recipient survival were monitored. Recipients treated with human UC-MSC-EVs had significantly lower numbers of CD3+CD8+ cytotoxic T cells, and reduced serum levels of IL-2, TNF-α, and IFN-γ. At variance, a higher ratio of CD3+CD4+ and CD3+CD8+ T cells and higher serum levels of IL-10 were observed.This study indicated that the prophylactic effects of human UC-MSC-EVs for aGVHD was essentially due to proliferation suppression of allo-reactive T cells, altered imbalance of T cell subpopulations, inhibition of pro-inflammatory cytokine release, and induction of anti-inflammatory cytokines [26].The immunologic aspect of recipients presenting aGVHD following human BM-MSC derived EVs infusion was evaluated in a mouse model described by Fujii et al [27]. They reported decreased frequency and number of both CD4+ and CD8+ effector T lymphocytes and an increased level of naïve T cells and a higher number of Tregs. The beneficial therapeutic effect of MSC-EV infusions has been recently demonstrated in a xenogeneic model of GVHD, where an irradiated mouse was infused with human PBMCs. Immortalized human embryonic MSC-EVs were intraperitoneally injected beginning on day 1 after PBMC infusion. Subsequent doses were administered every 3 days until animal death or end of the study (day 34).The reduction of GVHD symptoms and the prolonged recipient survival were associated to the increased number of circulating Tregs [28]. The therapeutic properties of EVs have been investigated in a chronic GVHD (cGVHD) mouse model [29]. Human BM-MSC-EVs were infused once a week for 6 weeks. EV treated mice showed a prolonged survival with amelioration of skin, lung and liver fibrosis, compared to non treated group. In MSC-EV treated mice, activation of CD4+ T cells and their infiltration into the lung were reduced. The immunomodulatory effect of EVs was ascribed to the inhibition of Th17 and induction of Tregs. Summarizing the results of the considered preclinical applications in an immune mediated disease such as GVHD, we can underline that MSC-EVs, as their MSC counterpart, may act on almost all immune cells leading to a reduction of activated T lymphocytes and pro-inflammatory cytokines, together with the suppression of macrophage maturation and B cell response, and an induction of Treg cells and anti-inflammatory cytokines (Figure 1).
3. EV applications in Acute and Chronic Kidney Disease
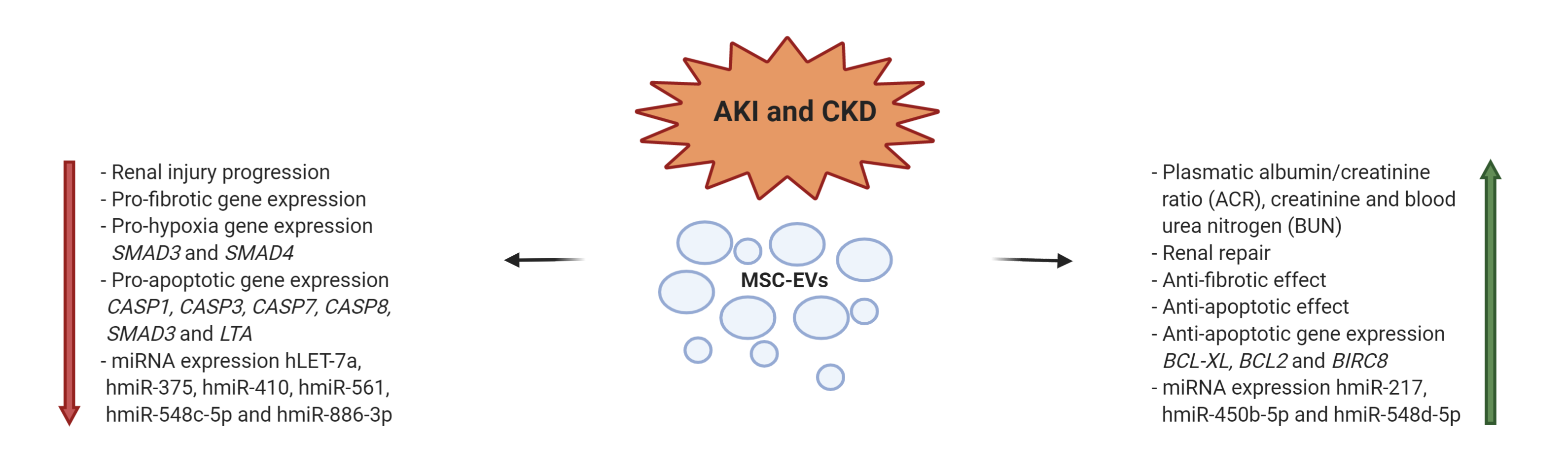
A large percentage of patients with Acute Kidney Injury (AKI) or Chronic Kidney Disease (CKD) undergoes to renal failure, requiring hemodialysis or even kidney transplantation [30][31]. Several preclinical studies are available regarding the use of EVs as therapeutic approach [32]. In a number of studies, in vivo models describe the positive effect of EVs, ascribed to their regenerative tissue and immunoregulation capacities. These results have been obtained with heterogeneous experimental settings. EVs were derived from MSCs of different tissues, such as BM [33][34][35][36], cord blood [37], Warton’s Jelly [38][39], renal [40][41], liver [42][43], or urine [44]. Different doses and schedules were also applied; the routes of administration included the tail infusion, the organ perfusion, or the direct administration in the kidney [33][34][35][36][38][40][41][42][43][44]. Different approaches in the induction of AKI and CKD animal models were also adopted [33][34][35][36][38][40][41][42][43][44]. We will focus on preclinical approaches where human BM-MSCs were the source of EVs. Gatti et al. reported the beneficial effect of BM-MSC-EVs in favoring the recovery of AKI and CKD induced by ischemia–reperfusion injury. The EVs content was represented by mRNAs and miRNAs. Even in this study, the organ tissue repair was derived from inhibition of cellular apoptosis and stimulation of tubular epithelial cell proliferation. The same group, investigated the effects of BM-MSC-EVs administered in single or multiple doses in AKI SCID mouse model. Renal function was improved by single administration, but was normalized only after multiple injections. The protection was mainly ascribed to an anti-apoptotic effect of EVs. Many other preclinical studies are reported in literature supporting that the therapeutic effect of MSC-EVs in kidney diseases is due to the transfer of miRNAs, with activation of cell proliferation and survive of kidney tubular cells.[45][46][47][48] (Figure 2).
4. EV applications in lung injury
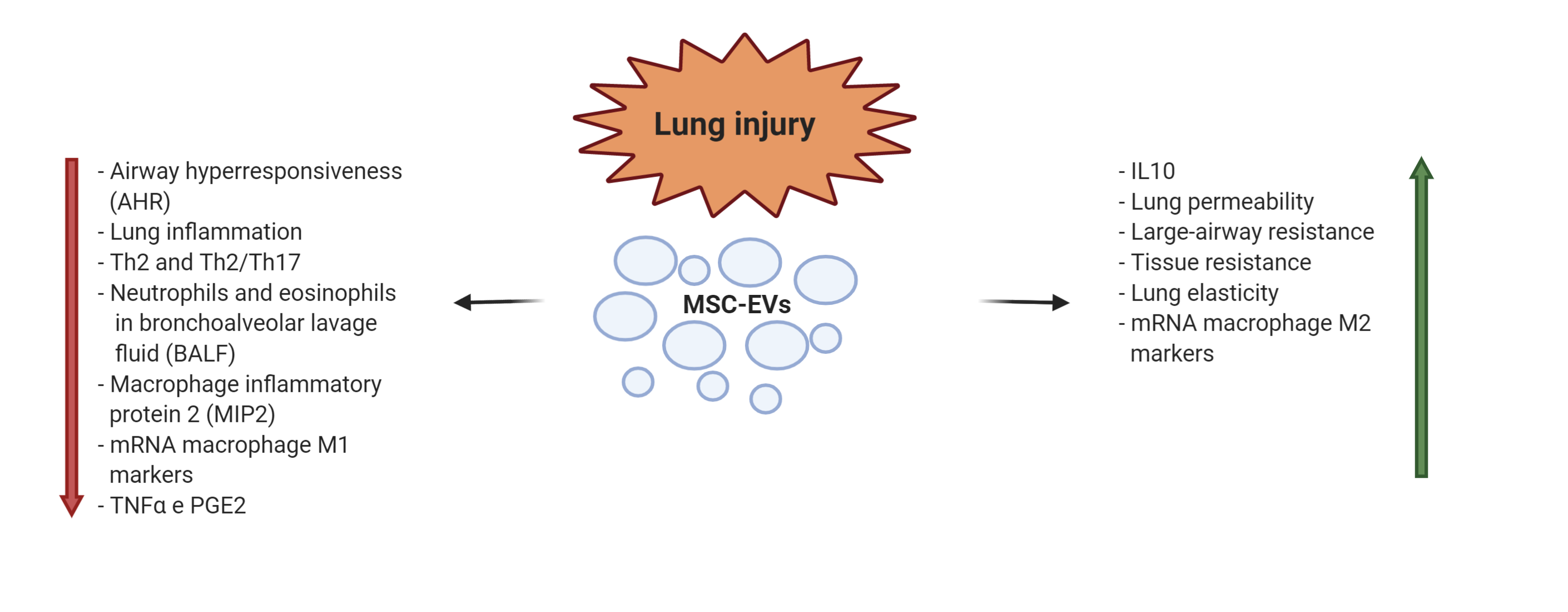
Lung injury is characterized by severe airway inflammation with activation of alveolar epithelial cells, macrophages, pulmonary microvascular endothelial cells, and neutrophils bringing to tissue damages. The inflammatory response can be caused by inhalation of toxic particles or by infection. The consequences are acute or chronic pulmonary diseases, often with few clinical treatments available, characterized by difficulty in breathing and reduced pulmonary functions [49]. Due to their already mentioned capacities to modulate the immune response and to attenuate the tissue damages, observed in several preclinical studies [50][51], EVs have been proposed for clinical application in this context. In an endotoxin-induced mouse model of acute lung injury (ALI), the intratracheal administration of BM-MSC-EVs induced a decrease in inflammatory cytokines produced by neutrophils and macrophages. The result was a reduction in pulmonary edema and lung protein permeability [52]. Several data are consistent with therapeutic properties of MSC-EV early administration in experimental models of bronchopulmonary dysplasia (BPD) [53]. However, Willis et al. [54] demonstrated that even late infusion of EVs derived from Wharton's Jelly-MSCs restored lung architecture, decreased pulmonary fibrosis and blood vessel loss in an experimental model of neonatal BPD. The interesting conclusion of the study was that EV infusions were effective not only to prevent the development of BPD but also to provide beneficial effects in established BPD. MSC-EVs were also tested in an animal model of allergic airway inflammation, where EVs resulted as effective as the parental MSCs in mitigating Th2/Th17-mediated allergic airway inflammation, with the reduction of pro-inflammatory cytokines in bronchoalveolar fluid [55]. Mechanical ventilation is the main supportive treatment of acute respiratory distress syndrome (ARDS), but it may lead to ventilator-induced lung injury (VILI). Recently, AT-MSC-EVs were administered by a small mechanical ventilator in a VILI mouse model. The study reported the protective effect on VILI of EV miR-146a in reducing the expression of pro-inflammatory cytokines by inhibiting Toll-Like Receptor 4 pathway, leading to the inhibition of calcium channel TRPV4 and extracellular calcium influx [56]. (Figure 3).
5. EV applications in skin wound repair
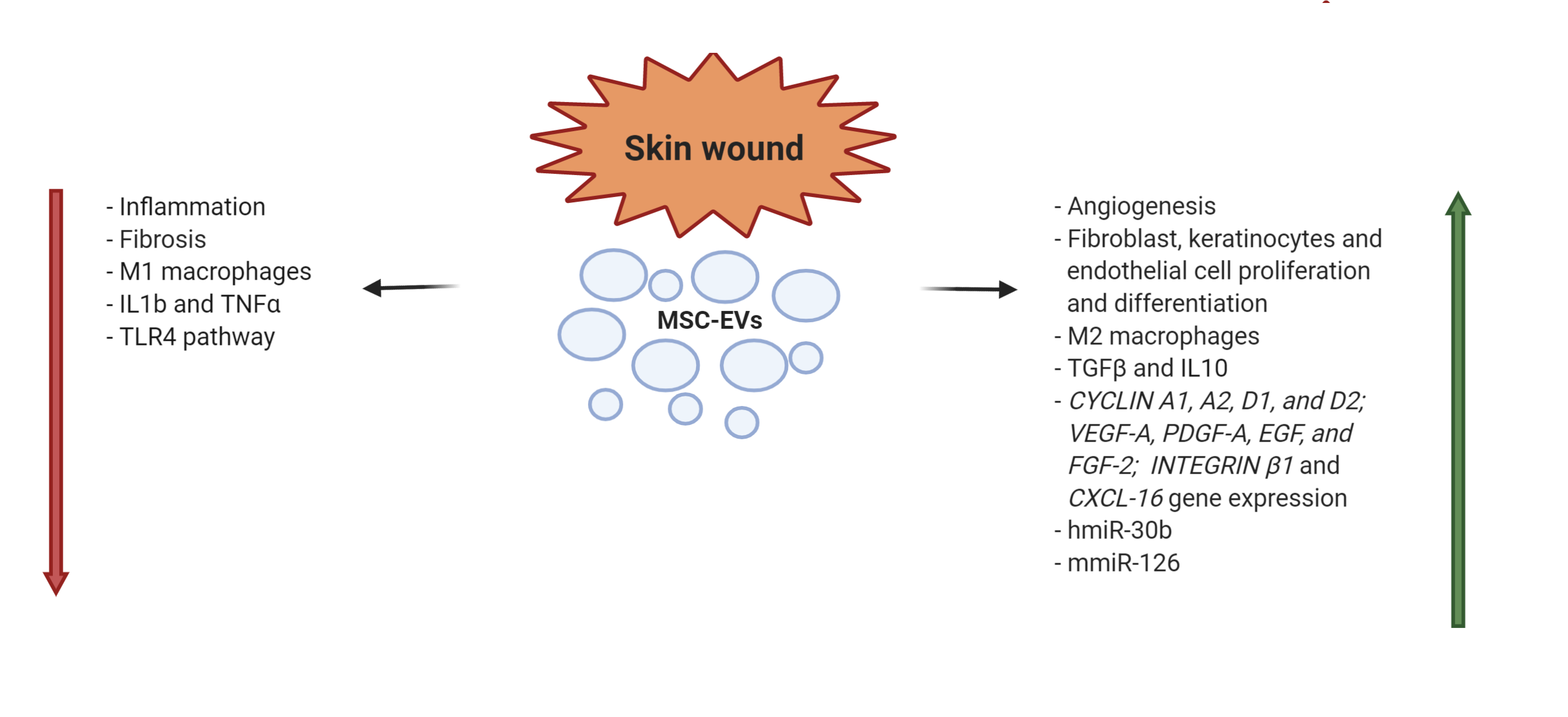
Skin wound healing is characterized by four stages: (i) hemostasis; (ii) inflammation; (iii) proliferation; and (iv) maturation/remodeling [57][58]. For their capacity to control inflammation, activate angiogenesis, stimulate cell migration and proliferation, as well as to modulate the cellular differentiation, EVs can act in each of these phases [59]. In a severe burned skin rat model it has been demonstrated that human UC-MSC-EVs attenuated burn-induced inflammation. The infusion of EVs reduced the macrophage production of TNF-α and IL-1β and increased IL-10 levels. These observations were described as the result of the miR-181c transfer from EVs to macrophages, leading to the suppression of the Toll like receptor 4 signaling pathway and of the inflammatory response [60]. Ferreira et al. [61]showed in in vitro experiments that AT-MSC-EVs co-cultured with fibroblasts and keratinocytes enhanced cell proliferation, while the increase of cell migration was demonstrated by scratch wound healing assays. These key processes in skin wound healing are due to the activation of AKT pathway, one of the major biochemical processes regulating migration of epithelial cells. Moreover the EV effect on wound healing was evaluated in a excisional rat wound model, where topical application of MSC-EVs determined an acceleration of the process. All these data were confirmed in a subsequent study, where AT-MSC-EVs differentially expressed 292 out of 333 miRNAs (199 up-regulated, 92 down-regulated) able to inhibit genes NPM1, PDCD4, CCL5, and NUP62, and contributing to the regeneration of skin fibroblasts [62]. In an experimental rabbit model of skin wound, locally injected EVs obtained from BM- and AT-MSCs, induced tissue restoration even better than MSC treatment. Moreover, in this model, the reparative action of AT-EVs resulted significantly better than that of BM-EVs [63]. (Figure4).
Figure 4. MSC-EV mechanisms of action in skin wound healing (h = human; m = murine).
6. Conclusions
MSC-EVs are considered by the scientific community an acellular biological product potentially interesting for therapies, with a number of advantages with respect to MSCs. MSC-EVs have a low immunogenicity, long half life, in vivo stability and a high efficacy of delivery because they are small and circulate readily, whereas the most of MSCs are trapped in the capillary bed of the lungs. In addition, their use avoids the transfer of cells which may have mutated or damaged DNA. However, there are still a number of critical parameters that need to be standardized, including production, purification, characterization and storage of EVs. Additional important issues are the sources of EV originating cells, the total amount to be delivered and the route of EV administration
References
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. Extracell Vesicles 2015, 4, 27066, doi: 10.3402/jev.v4.27066.
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60,89-98. doi: 10.1016/j.copbio.2019.01.005.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750, doi: 10.1080/20013078.2018.1535750.
- Théry, C.; Zitvogel, L.; Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569-79, doi: 10.1038/nri855.
- de Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.E.; Biagi, E.; Brini, A.T.; D'Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal stem/stromal cells: a new ''cells as drugs'' paradigm. Efficacy and critical aspects in cell therapy. Curr Pharm Des. 2013, 19, 2459-73. doi: 10.2174/1381612811319130015.
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019, 21, 1019-24. doi: 10.1016/j.jcyt.2019.08.002.
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439-41. doi: 10.1016/S0140-6736(04)16104-7.
- Le Blanc, K.; Götherström, C.; Ringdén, O.; Hassan, M.; McMahon, R.; Horwitz, E.; Anneren, G.; Axelsson, O.; Nunn, J.; Ewald, U. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005, 79, 1607-14. doi: 10.1097/01.tp.0000159029.48678.93
- Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011, 60, 788-98. doi: 10.1136/gut.2010.214841.
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017, 151, 971-981. doi: 10.1016/j.chest.2016.10.061.
- Lindsay, J.O.; Allez, M.; Clark, M.; Labopin, M.; Ricart, E.; Rogler, G.; Rovira, M.; Satsangi, J.; Farge, D.; Hawkey, C.J. ASTIC Trial Group. European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. European Crohn’s and Colitis Organisation Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: An analysis of pooled data from the ASTIC trial. Lancet Gastroenterol. Hepatol. 2017, 2, 399–406. doi: 10.1016/S2468-1253(17)30056-0.
- Meier, R.P.; Müller, Y. D.; More, P.; Gonelle-Gispert, C.; Bühler, L. H. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013, 11, 1348-64, doi: 10.1016/j.scr.2013.08.011.
- Bernardo, M.E.; Ball, L.M.; Cometa, A.M.; Roelofs, H.; Zecca, M.; Avanzini, M.A.; Bertaina, A.; Vinti, L.; Lankester, A.; Maccario, R.; et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011, 46, 200-7, doi: 10.1038/bmt.2010.87.
- Gnecchi, M.; Danieli, P.; Malpassa, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016, 1416, 123-146. doi: 10.1007/978-1-4939-3584-0_7.
- Mathew, S.A.; Naik, C.; Cahill, P.A.; Bhonde, R.R. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol Life Sci. 2020, 77, 253-265, doi: 10.1007/s00018-019-03268-1.
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E.; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. doi: 10.1056/NEJMoa1609583.
- Gazdic, M.; Volarevic V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev. Rep. 2015, 11, 280-287. doi: 10.1007/s12015-014-9583-3.
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001, 169, 12-20, doi: 10.1159/000047856.
- Borgovan, T.; Crawford, L.; Nwizu, C.; Quesenberry, P. Stem cells and extracellular vesicles: biological regulators of physiology and disease. Am. J. Physiol. Cell. Physiol. 2019, 317, C155-C166, doi: 10.1152/ajpcell.00017.2019.
- Maguire, G. Stem cell therapy without the cells. Commun. Integr. Biol. 2013, 6, e26631, doi: 10.4161/cib.26631
- Katare, R.; Stroemer, P.; Hicks, C.; Stevanato, L.; Patel, S.; Corteling, R.; Miljan, E.; Vishnubhatla, I.; Sinden, J.; Madeddu, P. Clinical-grade human neural stem cells promote reparative neovascularization in mouse models of hindlimb ischemia. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 408-418, doi: 10.1161/ATVBAHA.113.302592.
- Silva, J.D.; de Castro, L.L.; Braga, C.L.; Oliveira, G.P.; Trivelin, S.A.; Barbosa-Junior, C.M.; Morales, M.M.; Dos Santos, C.C.; Weiss, D.J.; Lopes-Pacheco, M. Mesenchymal Stromal Cells Are More Effective Than Their Extracellular Vesicles at Reducing Lung Injury Regardless of Acute Respiratory Distress Syndrome Etiology. Stem Cells Int. 2019, 8262849, doi: 10.1155/2019/8262849.
- Mahmoudi, M.; Taghavi-Farahabadi, M.; Rezaei, N.; Hashemi, S. M. Comparison of the effects of adipose tissue mesenchymal stromal cell-derived exosomes with conditioned media on neutrophil function and apoptosis. Int. Immunopharmacol. 2019, 74, 105689, doi: 10.1016/j.intimp.2019.105689.
- Dabrowska, S.; Andrzejewska, A.; Lukomska, B.; Janowski, M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflammation 2019, 16, 178, doi: 10.1186/s12974-019-1571-8.
- Ghimire, S.; Weber, D.; Mavin, E.; Wang, X.N.; Dickinson, A.M.; Holler, E. Pathophysiology of GvHD and Other HSCT-Related Major Complications. Front. Immunol. 2017, 8, 79, doi: 10.3389/fimmu.2017.00079.
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo. L.; Wei, H.; Guan, L.; et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016, 25, 1874-1833, doi: 10.1089/scd.2016.0107.
- Fujii, S.; Miura, Y.; Fujishiro, A.; Shindo, T.; Shimazu, Y.; Hirai, H.; Tahara, H.; Takaori-Kondo, A.; Ichinohe, T.; Maekawa, T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells 2018, 36, 434-445, doi: 10.1002/stem.2759.
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, SK. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 2018, 20, 687-696, doi: 10.1016/j.jcyt.2018.02.372.
- Lai, P.; Chen, X.; Guo, L.; Wang, Y.; Liu, X.; Liu Y.; Zhou, T, Huang, T.; Geng, S.; Luo, C.; et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol. Oncol. 2018, 11, 135, doi: 10.1186/s13045-018-0680-7.
- Pozzoli, S.; Simonini, M.; Manunta, P. Predicting acute kidney injury: current status and future challenges. J. Nephrol. 2018, 31, 209-223, doi: 10.1007/s40620-017-0416-8.
- Rewa, O.; Bagshaw, S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193-207, doi: 10.1038/nrneph.2013.282.
- Biancone, L.; Bruno, S.; Deregibus, M.C.; Tetta, C.; Camussi, G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant. 2012, 27, 3037-3042, doi: 10.1093/ndt/gfs168.
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019, 9, 4468, doi: 10.1038/s41598-019-41100-9.
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 2016, 6, 34842, doi: 10.1038/srep34842.
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 2012, 7, e33115, doi:10.1371/journal.pone.0033115.
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant 2011, 26, 1474-1483. doi: 10.1093/ndt/gfr015.
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34, doi: 10.1186/scrt194
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton's Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40, doi: 10.1186/scrt428.
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through miR-30. Stem Cells Int. 2016, 2093940, doi: 10.1155/2016/2093940
- Choi, H.Y.; Moon, S.J.; Ratliff, B.B.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, S.; Lim, B.J.; Kim, B.S.; Plotkin, M.D.; et al. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One 2014, 9, e87853, doi: 10.1371/journal.pone.0087853.
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; Dimuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res. Ther. 2017, 8, 24, doi: 10.1186/s13287-017-0478-5.
- Herrera Sanchez, M.B.; Brubo, S.; Grange, C.; Tapparo, M.; Cantaluppi, V.; Tetta, C.; Camussi, G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res. Ther. 2014, 5, 124, doi: 10.1186/scrt514.
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, N.; Deregibus, M.C., Brizzi, M.F.; Tetta, C.; Camussi, G. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front Immunol. 2018, 9, 1639, doi: 10.3389/fimmu.2018.01639.
- Jiang, Z-Z.; Liu, Y-M.; Niu, X.; Yin, J.Y.; Hu, B.; Guo, S-C-; Fan, Y.; Wang, Y.; Wang, N-S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24, doi: 10.1186/s13287-016-0287-2.
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114-124. doi: 10.1016/j.kint.2016.12.023.
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney Blood Press Res. 2016, 41, 119-128. doi: 10.1159/000443413.
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010, 5, e11803. doi: 10.1371/journal.pone.0011803.
- Tsuji, K.; Kitamura, S.; Wada, J. Secretomes from Mesenchymal Stem Cells against Acute Kidney Injury: Possible Heterogeneity. Stem Cells Int. 2018, 2018:8693137. doi: 10.1155/2018/8693137.
- Avecillas, J.F.; Freire, A.X.; Arroliga, A.C. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: incidence, diagnosis, and outcomes. Clin Chest Med. 2006, 27, 549-57; abstract vii. doi: 10.1016/j.ccm.2006.06.001.
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N. S.; Dhillon, N. K. Extracellular vesicles: novel communicators in lung diseases. Respir. Res. 2020, 21, 175, doi: 10.1186/s12931-020-01423-y.
- Liu, A.; Zhang, X.; He, H.; Zhou, L.; Naito, Y.; Sugita, S.; Lee, J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020, 20, 125-140. doi: 10.1080/14712598.2020.1689954.
- Zhu, Y. G.; Feng, X. M.; Abbott, J.; Fang, X. H.; Hao, Q.; Monsel, A.; Qu, J. M.; Matthay, M. A.; Lee, J. W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116-25, doi: 10.1002/stem.1504.
- Worthington, E.N.; Hagood, J.S. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int. J. Mol. Sci. 2020, 21, 2318. doi: 10.3390/ijms21072318.
- Willis, G. R.; Fernandez-Gonzalez, A.; Reis, M.; Yeung, V.; Liu, X.; Ericsson, M.; Andrews, N. A.; Mitsialis, S. A.; Kourembanas, S. Mesenchymal stromal cell-derived small extracellular vesicles restore lung architecture and improve exercise capacity in a model of neonatal hyperoxia-induced lung injury. J. Extracell. Vesicles. 2020, 9, 1790874, doi: 10.1080/20013078.2020.1790874.
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl. Med. 2015, 4, 1302-1316, doi:10.5966/sctm.2014-0280.
- Yu, Q.; Wang, D.; Wen, X.; Tang, X.; Qi, D.; He, J.; Zhao, Y.; Deng, W.; Zhu, T. Adipose-derived exosomes protect the pulmonary endothelial barrier in ventilator-induced lung injury by inhibiting the TRPV4/Ca2+ signaling pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L723-L741. doi: 10.1152/ajplung.00255.2019.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature. 2008, 15, 314-321. doi: 10.1038/nature07039.
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003, 83, 835-870. doi: 10.1152/physrev.2003.83.3.835
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146, doi:10.3389/fbioe.2020.00146.
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82.
- Ferreira, A.D.F.; Cunha, P.D.S.; Carregal, V.M.; da Silva, P.C.; de Miranda, M.C.; Kunrath-Lima, M.; de Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L.; Frezard, F.; et al. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem/Stromal Cells Accelerate Migration and Activate AKT Pathway in Human Keratinocytes and Fibroblasts Independently of miR-205 Activity. Stem Cells Int. 2017, 9841035, doi:10.1155/2017/9841035.
- Choi, E.W.; Seo, M.K.; Woo, E.Y.; Kim, S.H.; Park, E.J.; Kim, S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp. Dermatol. 2018, 10, 1170–1172.
- Pelizzo, G.; Avanzini, M. A.; Icaro Cornaglia A.; De Silvestri, A.; Mantelli, M.; Travaglino, P.; Croce, S.; Romano, P.; Avolio, L.; Iacob, G.; et al. Extracellular vesicles derived from mesenchymal cells: perspective treatment for cutaneous wound healing in pediatrics. Regen. Med. 2018, 13, 385-394, doi: 10.2217/rme-2018-0001.