| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anxo Fernández-Ferreiro | + 3612 word(s) | 3612 | 2020-12-15 05:03:36 | | | |
| 2 | Anxo Fernández-Ferreiro | Meta information modification | 3612 | 2020-12-15 08:19:22 | | | | |
| 3 | Nicole Yin | Meta information modification | 3612 | 2020-12-16 04:07:35 | | |
Video Upload Options
Cystinosis is a rare autosomal recessive disease that affects about 1 in 100,000–200,000 people among the general population, characterized by high levels of cystine within the lysosomes in cells of certain types of tissues. The accumulation of this substance is caused by mutations in the CTNS gene which codes for cystinosin, the carrier that transports cystine out of the lysosome. The presence of cystine crystals in different tissues leads to the progressive impairment and dysfunction of multiple organs, such as kidneys, pancreas, brain, thyroid and eyes.
1. Introduction
Although renal damage prevails in premature forms of the disease, all forms of cystinosis affect ocular structures: cornea, conjunctiva, iris, ciliary body, choroid, retina and optic nerve. The most frequently described ocular manifestation is the deposition of cystine crystals in the cornea, but the exact mechanisms of crystal formation are not yet fully understood. Stromal crystals have a needle shape and are oriented parallel to the corneal stromal lamellae[1], which suggests that the structure of collagen in the stroma plays a very important role in the evolution of cystine crystals[2]. This deposition is one of the most troublesome complications affecting the quality of life of patients with cystinosis, especially as the prognosis improves and life expectancy increases, causing photophobia, visual impairment and, finally, blindness[3][4]. In addition, this accumulation with time can cause corneal scars, keratitis and cataracts[5].
2. Diagnosis and Management
Corneal crystals are visible on ophthalmological examination as of 16 months of age in most patients[6]. Recent exploration techniques with in vivo confocal microscopy and optical coherence tomography (OCT), anterior segment optical coherence tomography (AS-OCT) and in vivo confocal microscopy (IVCM) of the anterior segment, are useful methods to evaluate crystals and detect their morphological characteristics and corneal alterations[7][8].
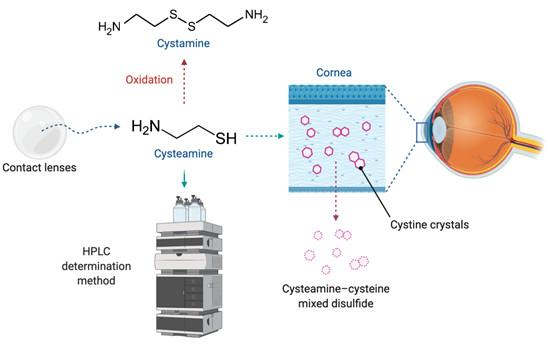
Early diagnosis and treatment of the ocular manifestations of childhood nephropathic cystinosis are essential. Therefore, early initiation and adherence to topical therapy have a significant impact on disease progression[3]. Nowadays, although new prodrug strategies based on N-acyl or glutaric acid derivatives of cystamine have been researched[9][10], the aminothiol cysteamine remains the only available treatment of cystinosis[11]. It lowers intracellular levels of cystine by forming a cysteamine–cysteine mixed disulfide, which can egress the lysosome using the undamaged excretion pathway for lysine[12] (Figure 1). The authorization of oral cysteamine (Cystagon®) by the Food and Drug Administration (FDA) in 1994, and by the European Medicine Agency (EMA) in 1997, has totally changed the management and prognosis of the patients with cystinosis[6][13][14]. However, it does not prevent corneal crystal accumulation because it cannot reach the cornea due to its lack of vascularization[15]. Consequently, it is necessary to instill cysteamine on the ocular surface in order to eliminate the cystine crystals at this location[6].
In 2012 Cystaran® (0.44% cysteamine ophthalmic solution) was approved by the FDA as an orphan drug indicated for the treatment of corneal cystine crystal deposits. The posology indicated in the prescribing information is one drop in each eye, every waking hour[16]. This posology, with its very frequent administrations, complicates patient’s adherence to the treatment. In order to improve this aspect, in 2017 Cystadrops® (0.55% cysteamine ophthalmic solution) was approved by the EMA and by the FDA in 2020[17]. It contains sodium carmellose which provides a high viscosity to the formulation, achieving a longer residence time on the ocular surface and allowing a dosage of just four times per day[18]. Therapeutic options marketed for ophthalmic treatment are scarce. Although ethical issues sometimes should outweigh economical and feasibility issues, the pharmaceutical industry does not allocate sufficient resources for the study of rare diseases and the development of orphan drugs. Because the commercialized cysteamine presentations are not available in most countries, hospital pharmacy departments are responsible for preparing homemade eyedrops as a therapeutic alternative [19]. The problem is that on many occasions, these formulations lack exhaustive stability controls under different storage conditions, cysteamine being a very easily oxidizable molecule[20].
Concerning topical treatments for ocular cystinosis, the patients’ therapeutic compliance is a major factor. In this regard, a new topical treatment, which only needs administrations every several hours or even days, would improve it[21]. The problem of the necessary frequent administration of topical cysteamine stems from the fact that eye drops are rapidly cleared from the ocular surface due to reflex tearing, constant blinking and nasolacrimal drainage resulting in a short contact time on the eye[22]. In addition, this organ has the tendency to maintain its residence volume at approximately 10 µL and, consequently, the bioavailability of a topically applied drug is typically <5%[23].
Figure 1. Cysteamine released from a drug delivery system to the ocular surface, where it forms the complex cysteamine-cysteine, facilitating the removal of cystine crystals from the cornea. Cysteamine is easily oxidized to cystamine, and can be detected from several determination methods, such as high-performance liquid chromatography (HPLC).
3. Drug Delivery Systems (DDSs)
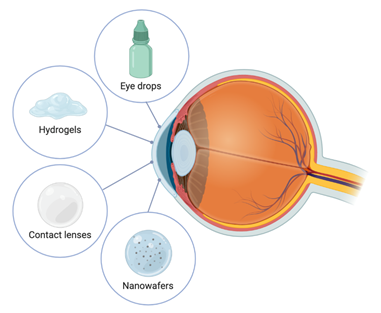
Ocular drug delivery has always been a challenge due to the limitations of conventional eye drops. The high tear turnover rate (1 µL/mL), loss of drug due to rapid blinking, reflex tear production, the tear-film barrier and high nasolacrimal drainage are factors that limit the absorption of topically applied ophthalmic formulations. To increase the drug bioavailability, a higher residence time on the ocular surface needs to be achieved. During the last decades, research has been done on the development of DDSs offering longer retention and a sustained release of the drug molecule to pass through these barriers[24]. The variability of DDSs intended for ophthalmic administration has experienced a large increase in the last decade. However, the difficulties associated with the stability of cysteamine, combined with the fact that cystinosis is a rare disease, means the development of DDSs intended to treat this disease has been more limited than in other ophthalmic diseases (Figure 2).
3.1. Hydrogels
Hydrogels are networks of polymer chains extensively swollen with water, which they retain within their structure. Their porosity and high-water content make hydrogels suitable for encapsulation of water-soluble drugs, since they are processed at room temperature and organic solvents are rarely needed[25][26].
Hydrogels can be designed from natural or synthetic polymers. Natural polymers present the advantage of minimal toxicity due to their high biocompatibility. However, their main drawback is their considerably shorter drug release compared to synthetic hydrogels, which limits their use as long-term sustained DDS[26][27].
By formulating cysteamine as a bioadhesive ophthalmic gel with controlled drug release, the administration frequency could be reduced and, consequently, increase therapeutic compliance. Particularly, hydrogels which are transparent and bioadhesive are highly desirable for topical ophthalmic application[5]. Concerning the treatment of ocular cystinosis, several types of polymers have been used for the development of topical hydrogels.
3.1.1. Synthetic Hydrogels
Synthetic hydrogels based on poly(acrylic acid) (PAA), commercially available as Carbopol®, can be obtained crosslinked with allylsucrose or allylpentaerythrol (carbomer) for pharmaceutical application. PAA is highly coiled and tightly packed but once dispersed in water the polymer swells to form a colloidal dispersion that behaves as an anionic electrolyte. In addition, the nonNewtonian pseudoplastic rheology of PAA hydrogels enhances the process of blinking because it causes an important reduction in apparent viscosity as a function of the high external shear-stresses applied by the eyelid[21]. Considering these properties, Buchan et al. designed a hydrogel composed of carbomer 934 for the topical administration of cysteamine on the ocular surface. The formed hydrogel was bioadhesive, transparent and offered significantly less resistance to blinking than Newtonian liquids of equivalent consistency, resulting in longer contact times on the surface of the eye. Furthermore, dissolution studies showed a first-order release of the active drug from the sample matrix with no destruction of the gel properties due to the addition of cysteamine. Accordingly, the authors affirm that this kind of hydrogels based on pseudoplastic fluids form weak networks with desirable properties to increase the residence time of cysteamine on the ocular surface[21].
For their part, McKenzie et al. carried out rheology, bioadhesion, dissolution and stability studies with several synthetic polymers in order to test their suitability for ophthalmic delivery. In this sense, they agree with Buchan et al. regarding the fact that carbomer 934 is suitable for ophthalmic delivery of cysteamine. However, their studies showed problems related to gel opacity[5].
Although both previous formulations have been characterized, they lack in vivo studies which are indispensable to show the permanence of the gels on the ocular surface, especially considering that the aim of the studies was to extend the retention time of the cysteamine.
Figure 2. Drug delivery systems developed for cysteamine ophthalmic administration.
3.1.2. Natural Hydrogels
Regarding ophthalmic delivery of cysteamine using natural hydrogels, Bozda et al. synthetized viscous solutions of cysteamine hydrochloride by using hydroxypropylmethyl-cellulose (HPMC) and evaluated in vitro characteristics and stability. All the viscous solutions tested showed nonNewtonian flow behavior. Concerning in vitro release tests, they revealed that more than 80% of cysteamine hydrochloride was released from the HPMC solutions in 8 h. In addition, the formulations produced no irritation when they were tested on rabbit eyes[28].
On the other hand, McKenzie et al. showed that sodium hyaluronate and hydroxyethyl cellulose were both suitable for ophthalmic delivery of cysteamine. Among the obtained results, it is necessary to highlight the fact that sodium hyaluronate displayed optimum performance in the preformulation tests, being pseudoplastic and bioadhesive, as well as releasing cysteamine over 40 min[5].
In these sense, Luaces-Rodríguez et al.[4][19] selected two different polysaccharide hydrogels to formulate cysteamine: an ion sensitive hydrogel with the polymers gellan gum and kappa-carrageenan, and another composed of hyaluronic acid[5][29]. In this regard, the authors performed in vitro (characterization of the hydrogels, drug release and cell toxicity) and ex vivo (transcorneal permeation and Hen´s Egg Chorioallantoic membrane (HET-CAM)) assays. On the one hand, in vitro release studies determined that both hydrogels can control the release of cysteamine over time, showing zero-order kinetics for 4 h. At the same time, they affirmed that these hydrogels could act as corneal absorption promoters, as they allow a higher permeation of cysteamine through bovine cornea compared to a solution. On the other hand, ex vivo assays showed no irritation on the ocular surface. Finally, the authors also accomplished in vivo studies based on direct measures of biopermanence time by positron emission tomography (PET), demonstrating that both formulations presented a high retention time on the ocular surface of rats[4]. Hydrogels previously described, as well as their main characteristics, are listed in Table 1.
In spite of the fact that important advances have been made regarding the development of new hydrogels, further studies should be carried out. Specifically regarding the development of smart hydrogels which can be very advantageous because they dramatically change their volume and other properties in response to environmental stimuli such as temperature or pH[30]. In addition, the synthesis of hydrogels based on nanoparticles could be a very promising strategy, which has provided interesting results in other pathologies[31].
Table 1. Hydrogels developed as cysteamine delivery systems.
|
Polymer Name |
Polymer Type |
% Released Cysteamine |
Time |
Reference |
|
Carbomer 934 |
Synthetic |
80 |
210 min 1 |
[21] |
|
Hyaluronic acid |
Natural |
60.7 |
24 h |
[4] |
|
88% Deacylated gellan gum and 12% kappa carrageenan |
Natural |
36.3 |
24 h |
[4] |
|
Carbomer 934 |
Synthetic |
80 |
20 min |
[5] |
|
Hydroxyethyl cellulose |
Natural |
80 |
14 min |
[5] |
|
Hyaluronic acid |
Natural |
80 |
14 min |
[5] |
|
Hydroxypropylmethyl-cellulose |
Natural |
81.2 |
8 h |
[28] |
1 Cystamine–phenylalanine conjugate.
3.2. Nanowafers
Nanowafers are tiny transparent circular discs that can be applied on the ocular surface with a fingertip[32]. Marcano et al. synthetised cysteamine-nanowafers via a hydrogel template strategy. In this study, they fabricated poly(vinyl alcohol) (PVA) nanowafers loaded with cysteamine. They contained arrays of drug-loaded nanoreservoirs from which the drug was released in a tightly controlled way for an extended period of time. At the end of this period, the nanowafer dissolved and faded away. Cysteamine-nanowafers are highly transparent; in fact, the refractive index of a cysteamine-nanowafer is very close to that of a soft contact lens. Hence, nanowafer application on the cornea does not affect the normal vision. These authors found that cysteamine was stable in the nanowafer and in a therapeutically effective form for up four months when stored at room temperature[22].
In addition, they carried out in vivo studies to determine the efficacy of the cysteamine-nanowafer in comparison to topical cysteamine eye drop formulation (0.44%). Two groups of CTNS−/− mice (three per group) were treated with the cysteamine-nanowafer (10 μg of cysteamine, once a day) and cysteamine eye drops (5 μL, 22 μg) twice a day for 30 days. These studies revealed that compared to the baseline corneal cystine crystal volume, cysteamine eye drops reduced the crystal volume by 55%, while cysteamine-nanowafer reduced the crystal volume by 90%, confirming that the cysteamine nanowafer treatment was significantly more efficacious. The authors stated that this higher efficacy was due to the longer residence time of the drug molecules on the eye achieved with the nanowafer, which enabled their diffusion into the ocular surface epithelium[22].
The development of this nanowafer means a very innovative and interesting alternative to properly control cysteamine release on the ocular surface. In this sense, other studies are needed to explore the possibilities that nanosystems, which have demonstrated successful results for other pathologies, could offer for ocular cystinosis treatment[31][33].
3.3. Contact Lenses
3.3.1. Contact Lenses as Drug Delivery Systems
Contact lenses (CLs) are medical devices widely used by over 125 million individuals in the world to correct vision problems[34], and were proposed as ocular DDSs since the first prototypes were synthesized nearly 50 years ago. CLs are polymeric structures formed after a polymerization process of different monomers in the presence of a crosslinking agent. This system absorbs a large amount of water (30%–80%) to form hydrogels having an aqueous phase permeable to oxygen. Depending on the nature and proportion of the different monomers that make up the polymeric structure of the CLs, conventional hydrogels or silicone hydrogels can be obtained. Modern materials currently used are an evolution of the well-known lens materials based on poly-2-hydroxyethylmetacrilate (p-HEMA) and silicone hydrogels[35][36].
Over the last two decades, the use of CLs as DDSs has been studied for the treatment of numerous ophthalmic pathologies[37]. Once a CL is placed over the eye, a thin layer of fluid is formed between the lens and the cornea, which takes about 30 min to dilute. When a drug is included in this layer, the time of contact between the drug and the cornea would be increased and, therefore, bioavailability would increase to approximately 50% when compared to the administration of ocular drops (between 1%–5%). Further, conjunctival absorption would diminish and, consequently, a smaller amount of drug would enter the systemic circulation, avoiding the appearance of adverse effects[34]. This increase in bioavailability would allow reduction of the dosage, enabling high therapeutic compliance for patients, and correct vision problems at the same time. The main limitation they present as DDSs is that polymers that make up the lenses and drugs have low affinity, leading to insufficient drug loading and too rapid delivery[38]. The main objective of many investigations in recent years has been to achieve a controlled and sustained drug release from the CL to the tear film located between the CL and the cornea so that corneal absorption can take place from there.
3.3.2. Modified Contact Lenses
The simplest method for incorporating drugs into CLs is by immersing them in concentrated solutions of the active ingredient although, as previously mentioned, this technique tends to lead to insufficient charges and excessively rapid releases. To achieve controlled sustained release, a series of modifications have been studied, such as the incorporation of polymeric nanoparticles, microemulsions, micelles, liposomes, diffusion barriers (e.g., vitamin E) and sophisticated loading techniques such as molecular imprinting, ion ligand polymeric systems, drug-loaded films or supercritical fluid technology. With all that, it is important to note that the final modified lenses must maintain the conditions of oxygen permeability, transparency, comfort, water content, mechanical properties, ionic permeability, relatively neutral pH, tonicity and stability, preferably at room temperature[35][38][39].
Cysteamine is a hydrophilic small molecule (77.15 g/mol) and easily oxidizable, that shows little affinity for unmodified commercial CLs, giving rise to short releases and risking toxicity effects. Vitamin E can be incorporated into commercial silicone hydrogel CLs as a diffusion barrier, a strategy initially proposed by Peng et al.[40][41][42][43][44], achieving a higher bioavailability than eye drops. Vitamin E is a biocompatible hydrophobic molecule that exhibits low solubility in water, and which creates a tortuous pathway prolonging the drug diffusion time through the CL. Hydrophilic drugs like cysteamine must overcome the said obstacle to reach the ocular surface, increasing diffusion time. A vitamin E barrier is included through immersion of CLs in a solution of vitamin E in ethanol. The composition of the solution may differ according to the desired amount to be administered. Subsequently, the CL has to be placed in water to remove any remaining of ethanol, while the vitamin gets trapped within the CL. The antioxidant properties of vitamin E provide a certain degree of protection for a molecule as sensitive to oxidation as cysteamine. In addition, Vitamin E incorporation can block UV radiation, an additional benefit to cystinosis patients. On the other hand, even though the transparency of the CL is not affected, it can affect its size, oxygen diffusion and ionic permeability, which depend on the thickness of the barrier[34][45].
Hsu et al. studied the use vitamin-E-modified silicone-hydrogel CLs to extend the delivery of cysteamine. ACUVUE OASYS® (Senofilcon A) with 19.14% vitamin and 1-DAY ACUVUE® TruEye™ (Narafilcon B) with 10.22% vitamin E prolonged release durations about 3 h and 25 min, respectively. The mass of cysteamine released from the CLs decreased when vitamin E was incorporated. ACUVUE® OASYS® delivered about 583.4 μg of drug, but with the incorporation of 19.14% vitamin E this decreased to 408.8 μg. In the same way, the 1-DAY ACUVUE® TruEye™ released 654.9 μg, but the mass released was reduced to 600.1 and 527.7 μg for 10.22% and 22.24% vitamin E loadings, respectively. Authors predicted that either a single 19.14% vitamin E loaded ACUVUE® OASYS® or two 10.22% vitamin E loaded 1-DAY ACUVUE® TruEye™ per day were the safest options to deliver therapeutic doses of cysteamine to cornea. Results were based on an in vivo mathematical model used as an initial predictor of dose-related toxicity in animal studies. In this work it was also concluded that it was not clear whether a two hour release duration may be enough, since the current therapy uses 8–10 eye drops administered throughout the day[45].
Years later, Dixon et al. showed that vitamin E incorporation into CLs increases the duration of cysteamine release in silicone-hydrogels ACUVUE® OASYS® (Senofilcon A) and ACUVUE® TruEye™ (Narafilcon A), the effect being more pronounced in TruEye™, possibly due to the low solubility of vitamin E in the lens matrix and higher aspect ratio of the barriers. The change observed in the composition of ACUVUE® TruEye™ in regard to Hsu’s work, was due to the fact that since 2010, the brand gradually replaced Narafilcon B to Narafilcon A[46]. They also explored the effect of vitamin E incorporation in p-HEMA hydrogel CLs, ACUVUE® Moist® (Etafilcon A). Release durations were significantly increased in ACUVUE® TruEye™ to 0.90, 2.0, and 4.0 h for vitamin E loadings of 10%, 20%, and 30%, respectively. For ACUVUE®OASYS® lenses, release durations increased to 0.89, 2.15, and 4.25 h for 10, 20 and 30% loadings, respectively. Vitamin E incorporation did not significantly increase the release duration for hydrogel ACUVUE® Moist® lenses, suggesting that vitamin E did not form barriers in hydrogel lenses. This data, along with the high aspect ratio in silicone hydrogels suggests that barriers could be forming at the interface of the silicone and hydrogel phases. Both cysteamine mass loaded and partition coefficients decreased for increasing vitamin E loadings, as seen in Table 2. CLs would need to be worn for about 4–5 h each day, less time than the typical duration of daily-wear. Finally, they also showed that daily use of cysteamine-loaded CLs did not cause any adverse response in the eyes of rabbits over a seven-day use[47].
Recently, the same research group developed CLs with carbon black to obtain tinted lenses to mitigate photophobia reducing transmittance. The presence of cystine crystals in the cornea causes photophobia, or extreme light sensitivity, in patients with cystinosis[48] which, after years of squinting, can cause intractable blepharospasm[49][50]. This incorporation can be used in cysteamine-vitamin E-loaded lenses, maintaining controlled release and keeping the lens parameters when carbon black concentrations of 0.3% are used[51]. Despite the advances made in the development of CLs as cysteamine delivery systems, more studies are needed to allow for their optimization. For this, it is important to select those CLs that have a more suitable composition for correct loading and release, and to develop methods that allow better control of release without affecting any of the CL’s parameters and preserving the stability of the cysteamine. Composition, total amount of cysteamine released and release time of the previously described CLs are summarized in Table 2.
Table 2. Contact lenses developed as cysteamine delivery systems.
|
Commercial Name |
Material |
Diffusion Barrier |
Total Cysteamine Release Amount (μg) |
Release Duration |
Reference |
|
1-DAY ACUVUE® TruEye™ |
Narafilcon B (silicone hydrogel) |
10.22% VE 1 |
600.1 ± 27.2 |
25 min |
[45] |
|
1-DAY ACUVUE® TruEye™ |
Narafilcon B (silicone hydrogel) |
22.24% VE |
527.7 ± 21.7 |
90 min |
[45] |
|
ACUVUE® OASYS® |
Senofilcon A (silicone hydrogel) |
19.14% VE |
408.8 ± 33.9 |
3 h |
[45] |
|
1-DAY ACUVUE® TruEye™ |
Narafilcon A (silicone hydrogel) |
10% VE |
651 ± 31.2 |
0.90 h |
[47] |
|
1-DAY ACUVUE® TruEye™ |
Narafilcon A (silicone hydrogel) |
20% VE |
603.1 ± 21.2 |
2 h |
[47] |
|
1-DAY ACUVUE® TruEye™ |
Narafilcon A (silicone hydrogel) |
30% VE |
538.9 ± 17.3 |
4 h |
[47] |
|
ACUVUE® OASYS® |
Senofilcon A (silicone hydrogel) |
10% VE |
464.0 ± 8.6 |
0.89 h |
[47] |
|
ACUVUE® OASYS® |
Senofilcon A (silicone hydrogel) |
20% VE |
408.1 ± 36.7 |
2.15 |
[47] |
|
ACUVUE® OASYS® |
Senofilcon A (silicone hydrogel) |
30% VE |
345.0 ± 33.2 |
4.25 h |
[47] |
|
Noncommercial |
- |
0.3% CB 2 |
148 ± 10 |
10 min |
[51] |
|
Noncommercial |
- |
0.3% CB + 20% VE |
123 ± 7 |
40 min |
[51] |
1 VE: vitamin E; 2 CB: carbon black.
CLs are presented as DDSs easy to administer and comfortable to the patient with dosing schedules that lead to better adherence than those obtained under frequent instillation of ophthalmic drops. Despite this, CLs are not available on the market due to issues like drug stability during processing/manufacturing, achievement of zero-order release kinetics, avoidance of drug release during the post-manufacturing monomer extraction step, protein adherence, drug release during storage and cost-benefits. However, they have potential applicability in the field of ophthalmic compounding, as drugs and excipients can be easily loaded onto them under sterile conditions as part of a relatively simple low-cost formulation process[38].
References
- A H Alsuhaibani; Michael D. Wagoner; A O Khan; Confocal microscopy of the cornea in nephropathic cystinosis. British Journal of Ophthalmology 2005, 89, 1530-1531, 10.1136/bjo.2005.074468.
- Phillip Dixon; Keith Christopher; Anuj Chauhan; Potential role of stromal collagen in cystine crystallization in cystinosis patients. International Journal of Pharmaceutics 2018, 551, 232-240, 10.1016/j.ijpharm.2018.09.021.
- Nesterova, G.; Gahl, W.A. Cystinosis: The evolution of a treatable disease. Pediatr. Nephrol. Berl. Ger. 2013, 28, 51–59.
- Luaces-Rodríguez, A.; Díaz-Tomé, V.; González-Barcia, M.; Silva-Rodríguez, J.; Herranz, M.; Gil-Martínez, M.; Rodríguez-Ares, M.T.; García-Mazás, C.; Blanco-Mendez, J.; Lamas, M.J.; et al. Cysteamine polysaccharide hydrogels: Study of extended ocular delivery and biopermanence time by PET imaging. Int. J. Pharm. 2017, 528, 714–722
- Barbara McKenzie; Graeme Kay; Kerr H. Matthews; Rachel M. Knott; Donald Cairns; Preformulation of cysteamine gels for treatment of the ophthalmic complications in cystinosis. International Journal of Pharmaceutics 2016, 515, 575-582, 10.1016/j.ijpharm.2016.10.044.
- William A. Gahl; Ernest M. Kuehl; Fumino Iwata; Anne Lindblad; Muriel I. Kaiser-Kupfer; Corneal Crystals in Nephropathic Cystinosis: Natural History and Treatment with Cysteamine Eyedrops. Molecular Genetics and Metabolism 2000, 71, 100-120, 10.1006/mgme.2000.3062.
- Csorba, A.; Maka, E.; Maneschg, O.A.; Szabó, A.; Szentmáry, N.; Csidey, M.; Resch, M.; Imre, L.; Knézy, K.; Nagy, Z.Z. Examination of corneal deposits in nephropathic cystinosis using in vivo confocal microscopy and anterior segment optical coherence tomography: An age-dependent cross sectional study. BMC Ophthalmol. 2020, 20, 73.
- Kowalczyk, M.; Toro, M.D.; Rejdak, R.; Załuska, W.; Gagliano, C.; Sikora, P. Ophthalmic Evaluation of Diagnosed Cases of Eye Cystinosis: A Tertiary Care Center’s Experience. Diagnostics 2020, 10, 911.
- McCaughan, B.; Kay, G.; Knott, R.M.; Cairns, D. A potential new prodrug for the treatment of cystinosis: Design, synthesis and in-vitro evaluation. Bioorg. Med. Chem. Lett. 2008, 18, 1716–1719.
- Omran, Z.; Moloney, K.A.; Benylles, A.; Kay, G.; Knott, R.M.; Cairns, D. Synthesis and in vitro evaluation of novel pro-drugs for the treatment of nephropathic cystinosis. Bioorg. Med. Chem. 2011, 19, 3492–3496
- Sukhmandeep Kaur; Phulen Sarma; Hardeep Kaur; Manisha Prajapat; Nishant Shekhar; Jaimini Bhattacharyya; Harpinder Kaur; Subodh Kumar; Bikash Medhi; Jagat Ram; et al.Dipankar DasPramod AvtiAjay PrakashR.S. SinghAnusuya Bhattacharyya Efficacy and safety of topical cysteamine in corneal cystinosis: a systematic review and meta- analysis.. American Journal of Ophthalmology 2020, null, null, 10.1016/j.ajo.2020.07.052.
- R L Pisoni; J G Thoene; H N Christensen; Detection and characterization of carrier-mediated cationic amino acid transport in lysosomes of normal and cystinotic human fibroblasts. Role in therapeutic cystine removal?. Journal of Biological Chemistry 1985, 260, 4791–4798.
- European Medicines Agency. Cystagon®. Prescribing Information. 1997. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cystagon (accessed on 7 November 2020).
- Schneider, J.A. Approval of cysteamine for patients with cystinosis. Pediatr. Nephrol. Berl. Ger. 1995, 9, 254.
- Hong Liang; Antoine Labbé; Jeannie Le Mouhaër; Céline Plisson; Christophe Baudouin; A New Viscous Cysteamine Eye Drops Treatment for Ophthalmic Cystinosis: An Open-Label Randomized Comparative Phase III Pivotal Study. Investigative Opthalmology & Visual Science 2017, 58, 2275-2283, 10.1167/iovs.16-21080.
- Food and Drug Administration. Cystaran®. Prescribing Information. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/200740s000lbl.pdf
- Food and Drug Administration. Cystadrops®. Prescribing Information. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211302s000lbl.pdf
- European Medicines Agency. Cystadrops®. Prescribing Information. 2016. Available online: https://www.ema.europa.eu/en/documents/product-information/cystadrops-epar-product-information_es.pdf
- Anxo Fernández-Ferreiro; Andrea Luaces-Rodríguez; Victoria Díaz-Tomé; María Gil-Martínez; María Teresa Rodríguez Ares; Rosario Touriño Peralba; José Blanco-Méndez; Miguel González-Barcia; Francisco Otero-Espinar; María Jesús Lamas; et al. [Cysteamine ophthalmic hydrogel for the treatment of ocular cystinosis].. Farmacia hospitalaria : organo oficial de expresion cientifica de la Sociedad Espanola de Farmacia Hospitalaria 2017, 41, 678-687.
- Ahmed Reda; Ann Van Schepdael; Erwin Adams; Prasanta Paul; David Devolder; Mohamed A. Elmonem; Koenraad Veys; Ingele Casteels; Lambertus Van Den Heuvel; Elena Levtchenko; et al.Matthias BaumgartnerMarc PattersonShamima RahmanVerena PetersEva MoravaJohannes Zschocke Effect of Storage Conditions on Stability of Ophthalmological Compounded Cysteamine Eye Drops. JIMD Reports 2017, 42, 47-51, 10.1007/8904_2017_77.
- Barbara Buchan; Graeme Kay; Anne Heneghan; Kerr H. Matthews; Donald Cairns; Barbara McKenzie; Gel formulations for treatment of the ophthalmic complications in cystinosis. International Journal of Pharmaceutics 2010, 392, 192-197, 10.1016/j.ijpharm.2010.03.065.
- Daniela C. Marcano; Crystal S. Shin; Briana Lee; Lucas C. Isenhart; Xing Liu; Feng Li; James V. Jester; Stephen C. Pflugfelder; Jennifer Simpson; Ghanashyam Acharya; et al. Synergistic Cysteamine Delivery Nanowafer as an Efficacious Treatment Modality for Corneal Cystinosis. Molecular Pharmaceutics 2016, 13, 3468-3477, 10.1021/acs.molpharmaceut.6b00488.
- Eliisa Mannermaa; Kati-Sisko Vellonen; Arto Urtti; Drug transport in corneal epithelium and blood–retina barrier: Emerging role of transporters in ocular pharmacokinetics. Advanced Drug Delivery Reviews 2006, 58, 1136-1163, 10.1016/j.addr.2006.07.024.
- Vrinda Gote; Sadia Sikder; Jeff Sicotte; Dhananjay Pal; Ocular Drug Delivery: Present Innovations and Future Challenges. Journal of Pharmacology and Experimental Therapeutics 2019, 370, 602-624, 10.1124/jpet.119.256933.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Luaces-Rodríguez, A.; Mondelo-García, C.; Zarra-Ferro, I.; González-Barcia, M.; Aguiar, P.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Intravitreal anti-VEGF drug delivery systems for age-related macular degeneration. Int. J. Pharm. 2020, 573, 118767.
- Chao-Chien Hu; Jen-Ray Chaw; Chin-Fu Chen; Hsia-Wei Liu; Controlled release bevacizumab in thermoresponsive hydrogel found to inhibit angiogenesis. Bio-Medical Materials and Engineering 2013, 24, 1941-1950, 10.3233/bme-141003.
- S Bozdag; K Gumus; O Gumus; Nurşen Ünlü; Formulation and in vitro evaluation of cysteamine hydrochloride viscous solutions for the treatment of corneal cystinosis. European Journal of Pharmaceutics and Biopharmaceutics 2008, 70, 260-269, 10.1016/j.ejpb.2008.04.010.
- Anxo Fernández-Ferreiro; Miguel González Barcia; Maria Gil-Martinez; Alba Vieites-Prado; I Lema; Bárbara Argibay; José Blanco-Méndez; María Jesús Lamas; Francisco J. Otero-Espinar; In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. European Journal of Pharmaceutics and Biopharmaceutics 2015, 94, 342-351, 10.1016/j.ejpb.2015.06.003.
- Lie-Wen Xia; Rui Xie; Xiao-Jie Ju; Wei Wang; Qianming Chen; Liang-Yin Chu; Nano-structured smart hydrogels with rapid response and high elasticity. Nature Communications 2013, 4, 2226, 10.1038/ncomms3226.
- Priyanka Bhatt; Priya Narvekar; Rohan Lalani; Mahavir Bhupal Chougule; Yashwant Pathak; Vijaykumar Sutariya; An in vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 281, 10.1208/s12249-019-1474-0.
- Xiaoyong Yuan; Daniela C. Marcano; Crystal S. Shin; Xia Hua; Lucas C. Isenhart; Stephen C. Pflugfelder; Ghanashyam Acharya; Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano 2015, 9, 1749-1758, 10.1021/nn506599f.
- Miral Shokry; Rania M. Hathout; Samar Mansour; Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: Augmented in-vivo efficacy and safe histological profile. International Journal of Pharmaceutics 2018, 545, 229-239, 10.1016/j.ijpharm.2018.04.059.
- M.A. Holgado; A. Anguiano-Domínguez; L. Martín-Banderas; Contact lenses as drug-delivery systems: a promising therapeutic tool. Archivos de la Sociedad Española de Oftalmología (English Edition) 2020, 95, 24-33, 10.1016/j.oftale.2019.07.007.
- García-Millán, E.; Castro-Balado, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Contact Lenses as Drug Delivery Systems. In Recent Progress in Eye Research; Eye and Vision Research Developments; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 91–154. ISBN 978-1-5361-2761-4.
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261.
- Gary D. Novack; Melissa Barnett; Ocular Drug Delivery Systems Using Contact Lenses. Journal of Ocular Pharmacology and Therapeutics 2020, 36, 595-601, 10.1089/jop.2020.0024.
- Ana Castro-Balado; Cristina Mondelo-García; Irene Zarra-Ferro; Anxo Fernández-Ferreiro; New ophthalmic drug delivery systems.. null 2020, 44, 149-157.
- Fernández-Ferreiro, A.; Castro-Balado, A.; García Quintanilla, L.; Lamas, M.; Otero-Espinar, F.; Mendez, J.; Gómez-Ulla, F.; Gil-Martínez, M.; Tomé, V.; Luaces-Rodríguez, A.; et al. Formulación Magistral Oftálmica Antiinfecciosa; SEFH. Sociedad Española de Farmacia Hospitalaria: Madrid, Spain, 2019; ISBN 978-84-09-10764-3.
- Peng, C.-C.; Kim, J.; Chauhan, A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials 2010, 31, 4032–4047.
- Peng, C.-C.; Chauhan, A. Extended cyclosporine delivery by silicone—Hydrogel contact lenses. J. Control. Release 2011, 154, 267–274.
- Peng, C.-C.; Burke, M.T.; Chauhan, A. Transport of Topical Anesthetics in Vitamin E Loaded Silicone Hydrogel Contact Lenses. Langmuir 2012, 28, 1478–1487.
- Peng, C.-C.; Ben-Shlomo, A.; Mackay, E.O.; Plummer, C.E.; Chauhan, A. Drug Delivery by Contact Lens in Spontaneously Glaucomatous Dogs. Curr. Eye Res. 2012, 37, 204–211.
- Peng, C.-C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 2012, 162, 152–158.
- Kuan-Hui Hsu; Richard C. Fentzke; Anuj Chauhan; Feasibility of corneal drug delivery of cysteamine using vitamin E modified silicone hydrogel contact lenses. European Journal of Pharmaceutics and Biopharmaceutics 2013, 85, 531-540, 10.1016/j.ejpb.2013.04.017.
- New 1-Day ACUVUE® TruEye® Brand Contact Lenses (narafilcon A) Now Available in U.S. Johnson & Johnson. Available online: https://www.jnj.com/media-center/press-releases/new-1-day-acuvue-trueye-brand-contact-lenses-narafilcon-a-now-available-in-us
- Phillip Dixon; Richard C. Fentzke; Arnab Bhattacharya; Aditya Konar; Sarbani Hazra; Anuj Chauhan; In vitro drug release and in vivo safety of vitamin E and cysteamine loaded contact lenses. International Journal of Pharmaceutics 2018, 544, 380-391, 10.1016/j.ijpharm.2017.11.059.
- Pascal Dureau; Michel Broyer; Jean-Louis Dufier; Evolution of Ocular Manifestations in Nephropathic Cystinosis: A Long-Term Study of a Population Treated With Cysteamine. Journal of Pediatric Ophthalmology and Strabismus 2003, 40, 142-146, 10.3928/0191-3913-20030501-07.
- Mancini, G.M.; Havelaar, A.C.; Verheijen, F.W. Lysosomal transport disorders. J. Inherit. Metab. Dis. 2000, 23, 278–292.
- Gahl, W.A. Cystinosis coming of age. Adv. Pediatr. 1986, 33, 95–126.
- Phillip Dixon; Anuj Chauhan; Carbon Black Tinted Contact Lenses for Reduction of Photophobia in Cystinosis Patients. Current Eye Research 2019, 44, 497-504, 10.1080/02713683.2018.1563701.