
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeyong Yoon | + 701 word(s) | 701 | 2020-12-02 05:03:22 | | | |
| 2 | Vivi Li | -23 word(s) | 678 | 2020-12-15 06:53:47 | | |
Video Upload Options
Various lithium recovery technologies have been developed as securing lithium resources has become increasingly important. Among these technologies, the electrochemical lithium recovery (ELR) system is a rapid and eco-friendly extraction method that has been studied recently. In this study, an ELR system using a spinel-type LiMn2O4 (LMO) is briefly reviewed. As LMO electrodes have high Li+ selectivity and stability compared to other lithium battery cathodes, they have been widely used as positive electrodes for the ELR system.
1. Introduction
With the recent rapid growth of the electric vehicle industry, the lithium battery industry has grown at the same time [1]. However, there have been concerns about an undersupply of lithium as a resource, and a possible scenario for the shortage in the mid-2020s was reported [2][3]. Therefore, it has been argued that there is a need for a rapid extraction technology that can recover lithium from various sources [4]. The currently commercialized lithium recovery technology is lime soda evaporation, which is suitable for the mass production of lithium from brine sources. Unfortunately, it is a time-consuming process (12–18 months) with a low recovery rate and causes industrial pollution [5]. Thus, electrochemical lithium recovery (ELR) systems have been studied to solve these problems [6][7][8].
2. The ELR system
The ELR system is an electrochemical process that separates and concentrates lithium-ion (Li+) by using lithium selective electrodes [9]. Figure 1 shows the operational principle of the ELR system using the spinel LiMn2O4 electrode. Generally, the system has an electrochemistry cell structure that consists of the working electrode (lithium-ion battery cathode) and the counter electrode. The system operates by repeated conduction of the following two steps. In Step 1, the Li+ brine water source is inserted between the electrodes so that the system cell spontaneously discharges. This results in Li+ intercalation into the lithium-ion battery cathode and the oxidation reaction on the counter electrode. After Step 1, the exhausted brine source is replaced by the recovery solution. When the charging current is applied (Step 2), Li+ de-intercalates from the LiMn2O4 electrode as the reduction reaction occurs on the counter electrode. Li+ then diffuses into the recovery solution, which concentrates the product. These two steps are conducted repeatedly until a Li+ recovery solution with high concentration and purity is produced.
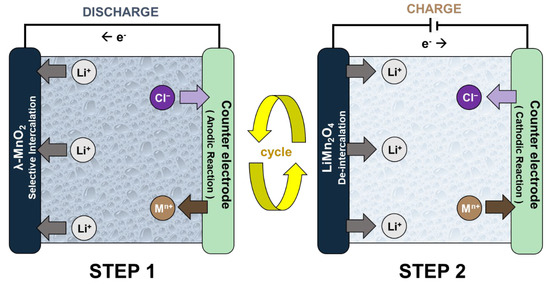
Figure 1. The operational principle of the electrochemical lithium recovery (ELR) systems.
The representative cathode electrodes used for the ELR system are olivine LFP (LiFePO4) and spinel LMO (LiMn2O4 or λ-MnO2) [5][10]. Both have sufficient reactivity with Li+ and proper potential windows for lithium recovery. Yet compared to the LFP electrode, the LMO electrode shows much higher stability for repeated cycles with higher selectivity for Li+, especially in the high Mg2+ concentration solution [11]. Accordingly, the LMO based ELR system has been studied more intensively than LFP and the LMO is considered a suitable material.
LiMn2O4 has a spinel structure which is corresponding with the Fd3m space group [12]. Spinel structure consists of three-dimensional pores that cause the steric effect. These small pores, which transport only Li+, enable selective recovery of Li+ [5][13]. LiMn2O4 has tetrahedral 8a sites for the Li+, octahedral 16d sites for the Mn3+ and Mn4+, and face-centered cubic (FCC) 32e sites for the oxygen anions [14]. Intercalation phenomena in the ELR system can be described by the redox mechanism [15][16]. At the electrochemical reduction step, the valence of the manganese cations is changed from +4 to +3 and Li+ intercalates in the tetrahedral 8a sites. Contrary to this step, at the electrochemical oxidation step, the valence of the manganese cations is changed from +3 to +4 and Li+ de-intercalates [17].
ELR systems using the LMO electrode are reviewed in this study. The timeline in Figure 2 briefly summarizes the contents of this paper. The ELR research using the LMO electrode can be classified and discussed by four categories: system proposal, LMO electrode modification, system analysis, and application. After the discussion of the literature, ELR system performance was organized and discussed further. Based on the trend and development process of the ELR system, we suggested the perspectives of this research.
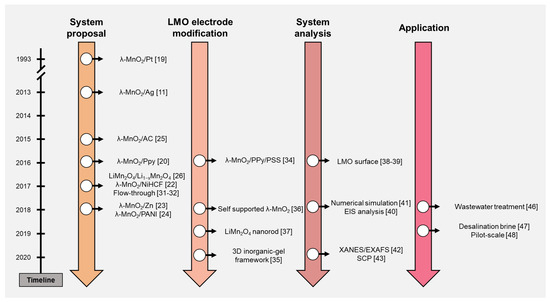
Figure 2. Timeline of the electrochemical lithium recovery (ELR) system research.
References
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research—Global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179.
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243.
- Wanger, T.C. The Lithium future-resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206.
- Calvo, E.J. Electrochemical methods for sustainable recovery of lithium from natural brines and battery recycling. Curr. Opin. Electrochem. 2019, 15, 102–108.
- Xu, X.; Chen, Y.; Wan, P.; Gasem, K.; Wang, K.; He, T.; Adidharma, H.; Fan, M. Extraction of lithium with functionalized lithium ion-sieves. Prog. Mater. Sci. 2016, 84, 276–313.
- Yoon, H.; Lee, J.; Kim, S.; Yoon, J. Review of concepts and applications of electrochemical ion separation (EIONS) process. Sep. Purif. Technol. 2019, 215, 190–207.
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. Adv. Mater. 2020, 32. [PubMed]
- Srimuk, P.; Su, X.; Yoon, J.; Aurbach, D.; Presser, V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 2020, 5, 517–538.
- Pasta, M.; Battistel, A.; La Mantia, F. Batteries for lithium recovery from brines. Energy Environ. Sci. 2012, 5, 9487.
- Xu, P.; Hong, J.; Qian, X.; Xu, Z.; Xia, H.; Tao, X.; Xu, Z.; Ni, Q.Q. Materials for lithium recovery from salt lake brine. J. Mater. Sci. 2020.
- Lee, J.; Yu, S.-H.; Kim, C.; Sung, Y.-E.; Yoon, J. Highly selective lithium recovery from brine using a λ-MnO2–Ag battery. Phys. Chem. Chem. Phys. 2013, 15, 7690. [PubMed]
- Ooi, K.; Miyai, Y.; Katoh, S.; Maeda, H.; Abe, M. Topotactic Li+ Insertion to λ-MnO2 in the Aqueous Phase. Langmuir 1989, 5, 150–157.
- Ooi, K.; Miyai, Y.; Katoh, S.; Maeda, H.; Abe, M. Lithium-ion Insertion/Extraction Reaction with λ-MnO2 in the Aqueous Phase. Chem. Lett. 1988, 17, 989–992.
- Greedan, J.E.; Raju, N.P.; Wills, A.S.; Morin, C.; Shaw, S.M.; Reimers, J.N. Structure and Magnetism in λ-MnO2. Geometric Frustration in a Defect Spinel. Chem. Mater. 1998, 10, 3058–3067.
- Ooi, K.; Miyai, Y.; Sakakihara, J. Mechanism of Li Insertion in Spinel-Type Manganese Oxide. Redox and Ion-Exchange Reactions. Langmuir 1991, 7, 1167–1171.
- Feng, Q.; Miyai, Y.; Kanoh, H.; Ooi, K. Li+ Extraction/Insertion with Spinel-Type Lithium Manganese Oxides. Characterization of Redox-Type and Ion-Exchange-Type Sites. Langmuir 1992, 8, 1861–1867.
- Liu, W.; Kowal, K.; Farrington, G.C. Mechanism of the Electrochemical Insertion of Lithium into LiMn2O4 Spinels. J. Electrochem. Soc. 1998, 145, 459–465.




