Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rajneesh Verma | -- | 2169 | 2022-11-22 04:30:55 | | | |
| 2 | Peter Dong | Meta information modification | 2169 | 2022-11-22 05:15:43 | | | | |
| 3 | Conner Chen | + 4 word(s) | 2173 | 2022-11-22 08:18:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Verma, R.; Lee, Y.; Salamone, D.F.; Dong, P. Pluripotent Stem Cells in Livestock and Wildlife. Encyclopedia. Available online: https://encyclopedia.pub/entry/35628 (accessed on 08 February 2026).
Verma R, Lee Y, Salamone DF, Dong P. Pluripotent Stem Cells in Livestock and Wildlife. Encyclopedia. Available at: https://encyclopedia.pub/entry/35628. Accessed February 08, 2026.
Verma, Rajneesh, Younghyun Lee, Daniel F. Salamone, Peter Dong. "Pluripotent Stem Cells in Livestock and Wildlife" Encyclopedia, https://encyclopedia.pub/entry/35628 (accessed February 08, 2026).
Verma, R., Lee, Y., Salamone, D.F., & Dong, P. (2022, November 22). Pluripotent Stem Cells in Livestock and Wildlife. In Encyclopedia. https://encyclopedia.pub/entry/35628
Verma, Rajneesh, et al. "Pluripotent Stem Cells in Livestock and Wildlife." Encyclopedia. Web. 22 November, 2022.
Copy Citation
Induced pluripotent stem cell (iPSC) technology is an emerging technique to reprogram somatic cells into iPSCs that have revolutionary benefits in the fields of drug discovery, cellular therapy, and personalized medicine. In both mice and humans, embryonic stem cell lines (ESCs) have been established. However, this is not the case for farm and wild animals. Cryopreservation is an important and useful approach to preserve endangered wild and domestic species as well as their genetic material. Several studies have shown the potential of iPSCs to prevent the extinction of several valuable species, such as the snow leopard, Bengal tiger, drill monkey, and white rhinoceros
IPSC
endangered
farming
Ark
1. Introduction
Currently, we are experiencing the Holocene extinction or sixth mass extinction, which is directly due to human intervention [1][2]. Several groups around the world have tried to save the genetic blueprints of different animals in the form of cellular repositories or Frozen Arks. Such storage serves as the genetic capital to ensure the survival of endangered species as well as food production. The Frozen Ark’s ethos is to conserve knowledge before it is too late for future generations [2][3]. With time and technological advancements, the costs of preserving material and genome sequencing have declined over the past decade [1]. However, it is noteworthy that the Frozen Ark approach is not a substitute for preserving species but is a “Plan B” [1]. The Frozen Ark (www.frozenark.org accessed on 19 November 2015), established in 1996, aims at preserving the genetic material of endangered species. However, more recently, the “biobanks” have moved to preserve germplasm, tissue, blood, and DNA. Fertility preservation and reproductive technology are important tools for preserving endangered species and are closely linked to biobanking [1]. The integration of artificial insemination into conservation programs has been successful to some extent. Nevertheless, no wild species is currently being preserved using embryo-based or oocyte cryopreservation approaches [4], which might be attributed to inadequate knowledge regarding the species biology, expertise, facilities, and necessary funding for successful implementation [3].
The main aims of the Frozen Ark approach are as follows [3]:
- To provide a database of stored and accessible specimens.
- To enhance sample collection, processing, conservation, and distribution.
- To make biological material usable for conservation programs to help counter genetic erosion.
- To safeguard valuable genetic material for scientific research, advancing awareness, and benefiting humans.
- To disseminate information on the current global extinction crisis, its impact on genetic biodiversity across the planet, and the impact of genetic management of endangered species in their fight for survival.
2. PSCs in Livestock and Wildlife
In both mice and humans, embryonic stem cell lines (ESCs) have been established. However, this is not the case for farm and wild animals [5]. The emergence of induced pluripotent stem cell (iPSC) technology in 2006 (Figure 1) offered an alternative Pluripotent Stem Cells (PSCs) generation approach that can be translated to both farm and exotic animals [6][7][8][9][10][11]. Nuclear transfer reprogramming was the first stream. John Gurdon stated in 1962 that his lab had produced tadpoles from unfertilized eggs with a nucleus made from adult frogs’ intestinal cells. Ian Wilmut and colleagues announced the creation of Dolly, the first mammal created through somatic cloning of mammary epithelial cells, more than three decades later. These somatic cloning successes showed that somatic cell nuclei may be reprogrammed in oocytes and that even differentiated cells have all the genetic material needed for generating whole animals. Takashi Tada’s team demonstrated, in 2001, that ESCs also include elements that can reprogram somatic cells.
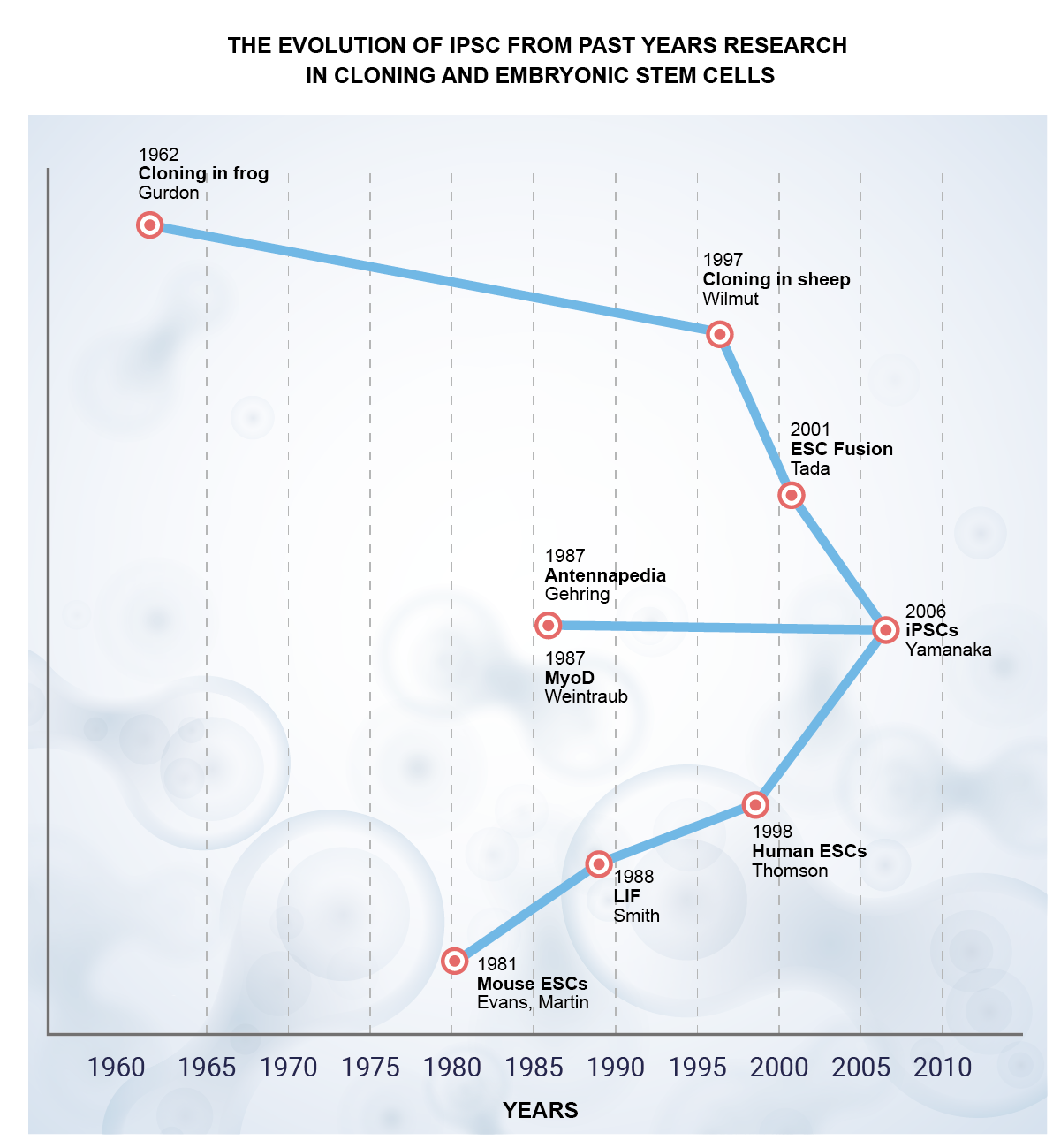
Figure 1. Pictorial presentation of the evolution of iPSC in 2006 from the past years of research on cloning and embryonic stem cells.
The identification of “master” transcription factors made the second stream. In 1987, it was discovered that the Drosophila transcription factor Antennapedia, when produced ectopically, causes the development of legs rather than antennae. In the same year, it was discovered that the mammalian transcription factor MyoD transformed fibroblasts into myocytes. The idea of a “master regulator”, a transcription factor that decides and influences the fate of certain lineages, was developed because of these findings. Several scientists have started looking for lone master regulators for different lineages. With a few exceptions, these attempts were unsuccessful.
The study of ESCs is the third and most significant research area. Austin Smith and others have developed culture conditions that permit the long-term maintenance of pluripotency since the first generation of mouse ESCs in 1981. Leukemia inhibitory factors are essential for maintaining mouse ESCs (LIF). Like this, the ideal culture conditions with basic fibroblast growth factor (bFGF) have been established from the first generation of human ESCs.
Capable of being derived from various types of accessible somatic cells, iPSCs offer an ethically acceptable and endless source of PSCs [12][13][14][15][16][17][18][19].
The first iPSCs were generated via retroviral transduction of Sox2, Oct4, c-Myc, and Klf4 into a donor cell genome [6][7]. However, recently, there have been attempts to develop non-integrating approaches that generate “clean iPSCs” with a pristine genome [9]. These approaches include viral delivery of RNA via the Sendai virus, transfection of modified mRNA [20], self-replicating mRNAs [20], episomal approaches, and protein-based reprogramming.
iPSC technology potentially acts as a major player in aiding with environmental protection and enhancing animal conservation [21]. This technology could provide a safety net to save current and future endangered species, or in the worst-case scenarios, could aid in de-extinction [15][16][17][18][19]. Currently, with limited funding, it must be considered that the iPSC technology may have broader applications. Manufacturing clean meat from iPSCs derived from domestic animals, such as cows and pigs [22][23], could reduce the environmental impact of commercial animal husbandry [9][10]. One can also think of the exploitation of iPSCs to obtain exotic animal products without harming the animals (Figure 2) [2]. For example, rhino horn or ivory that are produced in vitro could essentially compete with their black-market counterparts, which might lead to a reduction in poaching, and, in turn, protect the extinction of already endangered species [2]. The potential of iPSC technology with respect to the conservation of species and the environment is limited only by creativity and ambition.
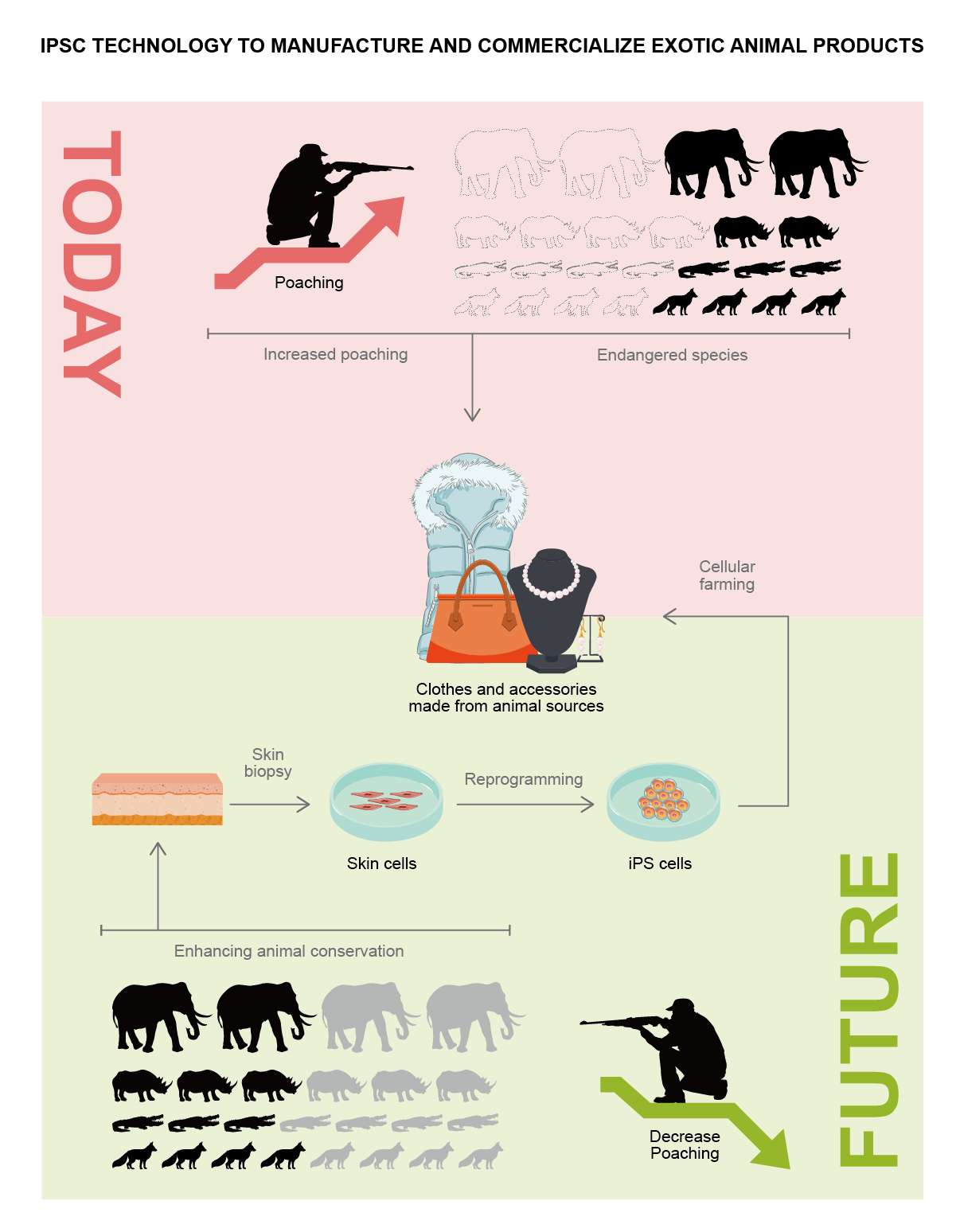
Figure 2. The use of iPSC technology for the production of exotic animal products could decrease animal poaching.
2.1. iPSCs, Bioreactors, and Bioprinting
We are now entering the “Holocene extinction” or “sixth mass extinction” as several species are being destroyed owing to human intervention. It has been reported that about 150–200 species of birds, mammals, insects, and plants become extinct each day [2]. Human activities and urbanization also require land, which leads to a reduction in the forest areas and irreversible damage to the forest ecosystems. The Food and Agriculture Organization of the United Nations (FAO) has reported that livestock are the main consumers of global land resources, earmarking about 80% of agricultural land [24], which leads to greenhouse gas emissions and deforestation [24]. In addition, the consistent use of antibiotics in animal feed has led to the development of antibiotic-resistant microbial strains, which further harm the human population [25]. Such environmental and health risks have shifted the consumers’ interests toward more environmentally sustainable animal products. One of the techniques to produce such products is known as “cellular agriculture”, which involves stem cell research. This technique is aimed at creating the animal-based products in vitro. More importantly, it does not involve harming or killing animals and potentially reduces the farming footprint in terms of land use and environmental impact. Animal stem cells are extracted via biopsy and replicated in vitro, followed by adequate modification to obtain the desired animal products. The desired cellular farming protocols have already been devised for a range of farm animals, facilitating the exploration of laboratory-generated animal products [26].
It is noteworthy that cellular farming has been practiced for several decades. There have been several attempts previously to produce animal products without the use of live animals, such as recombinant proteins like insulin and rennet. However, cellular agriculture was first used to produce meat in 2012 by Mark Post and his team [27]. Their protocol required three months to generate the adequate quantity of muscle cells sufficient to produce a burger. However, the production cost of the burger was extremely high; the cost of the 85 g burger was $325,000 [28]. Nevertheless, their research provided a proof of concept, prompting many commercial firms to embark on lab-grown meat products [29].
The concept of cellular farming has been employed on other types of meats too, such as pork [28][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45]. Genovese and colleagues devised a protocol efficient skeletal muscle derivation from pig iPSCs. While this technique was entirely in vitro, the derivation of iPSCs still required cells from an animal source; thus, the product was not completely “animal-free” [28]. The in vitro culturing of iPSCs under serum-free conditions and in the absence of other animal products remains a major challenge. For in vitro cellular proliferation, most protocols require the use of animal products, such as fetal bovine serum (FBS), serum-derived products, or extracellular matrix [46]. The regulatory agencies demand the production of cells and any future iPSC meat or consumer products under xeno-free conditions [38]. Certain protocols have been devised for a feeder-free and xeno-free stem cell culturing to either eliminate or reduce the use of animal products in compliance with the regulatory constraints and to improve quality control processes [40][41][42]. Such media will not only eliminate the use of animal proteins, but also antibiotics and hormones.
Currently, cellular agriculture is highly costly [47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63]. It is estimated that the current cost of laboratory meat is ~$40,000 per kg, making it a very exclusive product. To reduce the cost, improved approaches, such as mass cell culture, are warranted.
Bioreactors facilitate suspension culture capable of producing abundant iPSCs and their derivatives within a few days [53]. Previous studies have demonstrated high mouse iPSC proliferation using stirred bioreactors [64] and scaling of human iPSCs using xeno-free media in bioreactors [64]. Furthermore, it is possible to collect and combine animal iPSCs from a bioreactor to mimic the actual processed meat product, which significantly lowers the final product cost than that obtained using conventional cell culture techniques [64].
In addition, to obtain highly ordered complex tissue, it is also necessary to insert the cells collected from an animal into a scaffold with specific vascularization and porosity [27]. While the generation of such complex tissues with micro vascularization is difficult, this problem may be overcome using the three-dimensional (3D) “bioprinting”. The process of hydrogel employs living cells that are suspended in hydrogel; this suspension can then be polymerized in the form of any complex 3D structure using computer-generated models [47][53][65]. A previous study has also demonstrated the generation of artificial skin constructs using human iPSCs inserted in alginate hydrogel [33]. The animal iPSC-derived fur and skin derived could be used as an alternative for natural fur and leather, which would be especially beneficial in the case of exotic animals, such as crocodiles [65][66][67][68][69][70][71][72][73]. To increase public awareness in this context, it is necessary to advertise the naturally obtained animal products, which, in turn, would help generate higher incentives for research on cellular farming and increase the demand for environmentally sustainable products [70][71].
The initial cell sample is fed with the nutrients and water required to grow and replicate [45]. Later, the cells are induced to differentiate into muscle, fat, and connective tissue that constitute meat. A support system (or scaffold) was then introduced to provide the cells with instructions on how to organize themselves into the correct 3D structure [65]. This whole process can be conducted in a grower (also known as a bioreactor) (Figure 3) [74].
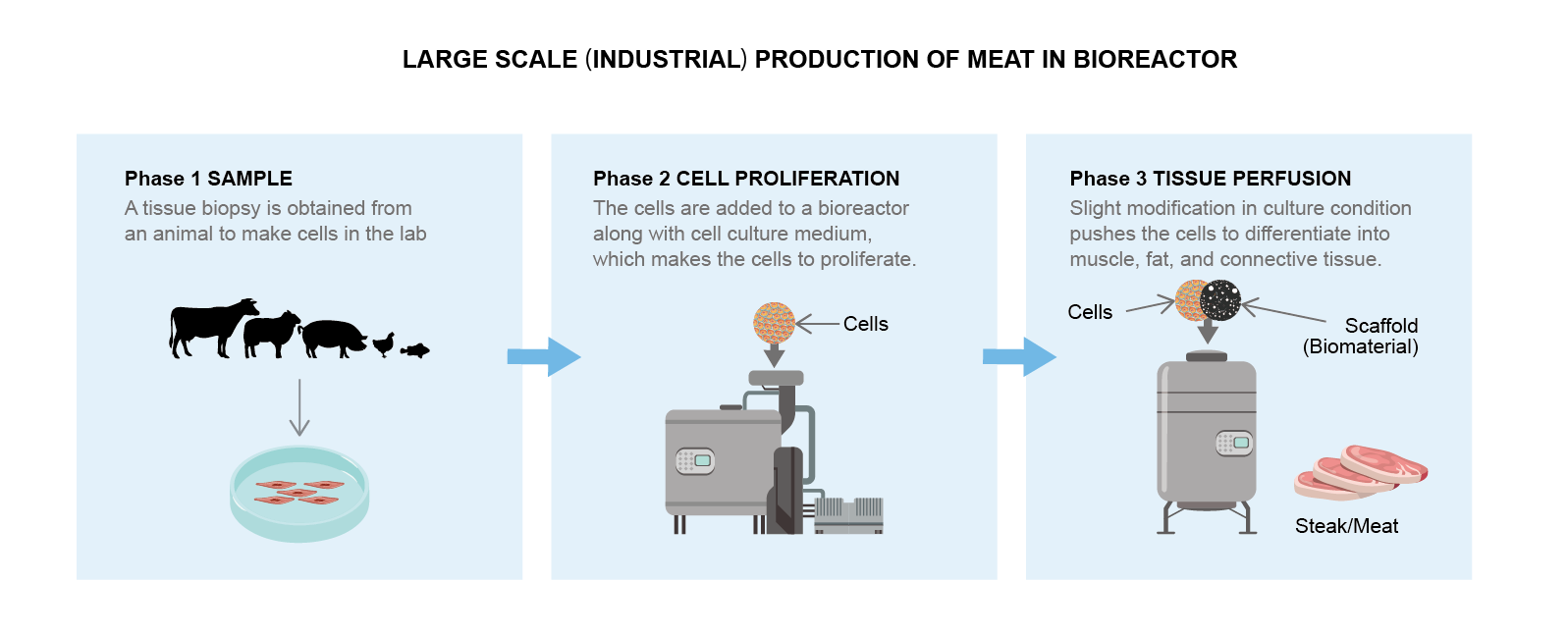
Figure 3. The production of meat in a bioreactor at the industrial scale.
2.2. Why Clean Meat?
The meat developed in vitro is termed as clean meat and has been referred to as a potential substitute for the conventional meat [28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44]. Traditional animal products are said to be unsustainable because the live source animals consume a large amount of feed, of which most of the generated energy is wasted by the animal for daily activities and the production of non-edible tissues [41]. Compared to the plant-based industries, the animal-based industries exhibit a more severe environmental footprint, especially in the context of water and land usage and greenhouse gas emission, with the worst environmental impact exhibited by the beef industry [41][42].
Initially, some investigators reported that chicken muscles could grow efficiently in the absence of live chickens [43][45][75]. Since then, many researchers have explored the possibility of producing meat in vitro. For the last 15 years, skeletal muscle stem cells have been used to generate cultured muscles for potential medical applications [44]. In another study, NASA used turkey cells to produce muscle culture and goldfish cells to produce the first edible lab-grown fish filet. Their study demonstrated that muscle strips could be produced by introducing a collagen matrix into the stem cell culture [76]. The emergence of the meat cultivation consortium led to the first meat cultivation symposium at the Norwegian Food Research Institute in Norway in 2008 for the exploration of potential applications of lab-grown muscle tissue [48]. Other studies have devised protocols to produce bone, skeletal muscle, fat, fibrous tissue, and cartilage [49][50][51][52][53][54][55][56]. Lab-grown meat, derived from the bovine stem cells, was first used to make a burger in 2013; however, the meat itself was very costly and requires around 10,000 individual muscle strips to mimic the natural product [57][58][59][60][61][62][63]. Even with the current progress, many puzzles still need to be solved to obtain the optimum meat substitutes for the general population using feasible methods [65][66][67][68][69][70][71][72][73][75][77][78][79][80][81][82][83][84].
References
- Forget Noah! Scientists Set Up a ‘Frozen Ark’ to Preserve the DNA of Endangered Animals Ahead of the Sixth Mass Extinction. 2015. Available online: https://www.dailymail.co.uk/sciencetech/article-3325421/Forget-Noah-Scientists-set-Frozen-Ark-preserve-DNA-endangered-animals-ahead-sixth-mass-extinction.html (accessed on 19 November 2015).
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, 1400253.
- Benirschke, K. The frozen ZOO concept. Zoo Biol. 1984, 3, 325–328.
- The Cloning Revolution, Part 2. 2008. Available online: https://www.independent.co.uk/news/science/the-cloning-revolution-part-2-811224.html (accessed on 18 April 2018).
- Hildebrandt, T.B.; Hermes, R.; Colleoni, S.; Diecke, S.; Holtze, S.; Renfree, M.B.; Stejskal, J.; Hayashi, K.; Drukker, M.; Loi, P.; et al. Embryos and embryonic stem cells from the white rhinoceros. Nat. Commun. 2018, 9, 2589.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676.
- Boland, M.J.; Hazen, J.L.; Nazor, K.L.; Rodriguez, A.R.; Gifford, W.; Martin, G.; Kupriyanov, S.; Baldwin, K.K. Adult mice generated from induced pluripotent stem cells. Nature 2009, 461, 91–94.
- Zhao, X.Y.; Li, W.; Lv, Z.; Liu, L.; Tong, M.; Hai, T.; Hao, J.; Guo, C.L.; Ma, Q.W.; Wang, L.; et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009, 461, 86–90.
- Du, X.; Feng, T.; Yu, D.; Wu, Y.; Zou, H.; Ma, S.; Feng, C.; Huang, Y.; Ouyang, H.; Hu, Z.; et al. Barriers for deriving transgene-free pig iPS cells with episomal vectors. Stem Cells 2015, 33, 3228–3238.
- Fan, N.; Chen, J.; Shang, Z.; Dou, H.; Ji, G.; Zou, Q.; Wu, L.; He, L.; Wang, F.; Liu, K.; et al. Piglets cloned from induced pluripotent stem cells. Cell Res. 2013, 23, 162–166.
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532.
- Liu, H.; Zhu, F.; Yong, J.; Zhang, P.; Hou, P.; Li, H.; Jiang, W.; Cai, J.; Liu, M.; Cui, K.; et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 2008, 3, 587–590.
- Han, X.; Han, J.; Ding, F.; Cao, S.; Lim, S.S.; Dai, Y.; Zhang, R.; Zhang, Y.; Lim, B.; Li, N. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res. 2011, 21, 1509–1512.
- Nagy, K.; Sung, H.K.; Zhang, P.; Laflamme, S.; Vincent, P.; Agha-Mohammadi, S.; Woltjen, K.; Monetti, C.; Michael, L.P.; Smith, L.C.; et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. Rep. 2011, 7, 693–702.
- Ben-Nun, I.F.; Montague, S.C.; Houck, M.L.; Tran, H.T.; Garitaonandia, I.; Leonardo, T.R.; Wang, Y.C.; Charter, S.J.; Laurent, L.C.; Ryder, O.A.; et al. Induced pluripotent stem cells from highly endangered species. Nat. Methods 2011, 8, 829–831.
- Selvaraj, V.; Wildt, D.; Pukazhenthi, B. Induced pluripotent stem cells for conserving endangered species? Nat. Methods 2011, 8, 805–807.
- Verma, R.; Holland, M.K.; Temple-Smith, P.; Verma, P.J. Inducing pluripotency in somatic cells from the snow leopard (Panthera uncia), an endangered felid. Theriogenology 2012, 77, 220–228.e2.
- Lu, Y.; West, F.D.; Jordan, B.J.; Mumaw, J.L.; Jordan, E.T.; Gallegos-Cardenas, A.; Beckstead, R.B.; Stice, S.L. Avian-induced pluripotent stem cells derived using human reprogramming factors. Stem Cells Dev. 2012, 21, 394–403.
- Verma, R.; Liu, J.; Holland, M.K.; Temple-smith, P.; Williamson, M.; Verma, P.J. Nanog is an essential factor for induction of pluripotency in somatic cells from endangered fields. Biores. Open Access 2013, 2, 72–76.
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630.
- Chronowska, E.Z. Induced pluripotent stem (iPS) cells in domestic animals recent achievements—A review. Anim. Sci. Pap. Rep. 2013, 31, 89–99.
- Esteban, M.A.; Xu, J.; Yang, J.; Peng, M.; Qin, D.; Li, W.; Jiang, Z.; Chen, J.; Deng, K.; Zhong, M.; et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J. Biol. Chem. 2009, 284, 17634–17640.
- Bogliotti, Y.S.; Wu, J.; Vilarino, M.; Okamura, D.; Soto, D.A.; Zhong, C.; Sakurai, M.; Sampaio, R.V.; Suzuki, K.; Izpisua Belmonte, J.C.; et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2090–2095.
- Pearson, T.R.H.; Brown, S.; Murray, L.; Sidman, G. Greenhouse gas emissions from tropical forest degradation: An underestimated source. Carbon Balance Manag. 2017, 12, 3.
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A review of current bacterial resistance to antibiotics in food animals. Front. Microbiol. 2022, 13, 822689.
- Treich, N. Cultured meat: Promises and challenges. Environ. Resour. Econ. 2021, 79, 33–61.
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301.
- Kumar, P.; Sharma, N.; Sharma, S.; Mehta, N.; Verma, A.K.; Chemmalar, S.; Sazili, A.Q. In-vitro meat: A promising solution for sustainability of meat sector. J. Anim. Sci. Technol. 2021, 66, 693–724.
- Clean Meat. Available online: https://www.fairr.org/article/clean-meat/ (accessed on 4 April 2019).
- Bryant, C.; Barnett, J. Consumer acceptance of cultured meat: A systematic review. Meat Sci. 2018, 143, 8–17.
- Burton, N.M.; Vierck, J.; Krabbenhoft, L.; Bryne, K.; Dodson, M.V. Methods for animal satellite cell culture under a variety of conditions. Methods Cell Sci. 2000, 22, 51–61.
- Calkins, C.R.; Hodgen, J.M.A. Fresh look at meat flavor. Meat Sci. 2007, 77, 63–80.
- Chen, D.; Li, W.; Du, M.; Wu, M.; Cao, B. Sequencing and characterization of divergent marbling levels in the beef cattle (Longissimus dorsi Muscle) transcriptome. Asian-Australas. J. Anim. Sci. 2015, 28, 158–165.
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654.
- Cui, H.X.; Guo, L.P.; Zhao, G.P.; Liu, R.R.; Li, Q.H.; Zheng, M.Q.; Wen, J. Method using a co-culture system with high-purity intramuscular preadipocytes and satellite cells from chicken pectoralis major muscle. Poult. Sci. 2018, 97, 3691–3697.
- D’Alessandro, A.; Rinalducci, S.; Marrocco, C.; Zolla, V.; Napolitano, F.; Zolla, L. Love me tender: An Omics window on the bovine meat tenderness network. J. Proteom. 2012, 75, 4360–4380.
- Damez, J.-L.; Clerjon, S. Quantifying and predicting meat and meat products quality attributes using electromagnetic waves: An overview. Meat Sci. 2013, 95, 879–896.
- Dodson, M.V.; Allen, R.E.; Du, M.; Bergen, W.G.; Velleman, S.G.; Poulos, S.P.; Fernyhough-Culver, M.; Wheeler, M.B.; Duckett, S.K.; Young, M.R.I.; et al. Invited review: Evolution of meat animal growth research during the past 50 years: Adipose and muscle stem cells. J. Anim. Sci. 2015, 93, 457–481.
- Du, M.; Wang, B.; Fu, X.; Yang, Q.; Zhu, M.-J. Fetal programming in meat production. Meat Sci. 2015, 109, 40–47.
- Edelman, P.D.; McFarland, D.C.; Mironov, V.A.; Matheny, J.G. Commentary: In vitro-cultured meat production. Tissue Eng. 2005, 11, 659–662.
- Flachowsky, G.; Meyer, U.; Sudekum, K.H. Invited review: Resource inputs and land, water and carbon footprints from the production of edible protein of animal origin. Arch. Anim. Breed. 2018, 61, 17–36.
- Consumer Interest towards Clean Meat. Available online: https://dash.harvard.edu/handle/1/34901168 (accessed on 7 March 2018).
- Gaydhane, M.K.; Mahanta, U.; Sharma, C.S.; Khandelwal, M.; Ramakrishna, S. Cultured meat: State of the art and future. Biomanuf. Rev. 2018, 3, 1.
- Goodwin, J.N.; Shoulders, C.W. The future of meat: A qualitative analysis of cultured meat media coverage. Meat Sci. 2013, 95, 445–450.
- Gorraiz, C.; Beriain, M.J.; Chasco, J.; Insausti, K. Effect of aging time on volatile compounds, odor, and flavor of cooked beef from pirenaica and Friesian bulls and heifers. J. Food Sci. 2002, 67, 916–922.
- Genovese, N.; Domeier, T.; Telugu, B.; Micheal Roberts, R. Enhanced development of skeletal myotubes from porcine induced pluripotent stem cells. Sci. Rep. 2017, 7, 41833.
- Heher, P.; Maleiner, B.; Pruller, J.; Teuschl, A.H.; Kollmitzer, J.; Monforte, X.; Wolbank, S.; Redl, H.; Runzler, D.; Fuchs, C. A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain. Acta Biomater. 2015, 24, 251–265.
- Farm Animal Suffering Leaves a Bad Taste in Your Mouth. Psychology Today. 2016. Available online: https://works.bepress.com/harold-herzog/98/ (accessed on 24 August 2016).
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319.
- Hopkins, P.D.; Dacey, A. Vegetarian meat: Could technology save animals and satisfy meat eaters? J. Agric. Environ. Ethics 2008, 21, 579–596.
- Jordan, G.; Thomasius, R.; Schröder, H.; Wulf, J.S.; Schluter, O.; Sumpf, B.; Maiwald, M.; Schmidt, H.; Kronfeldt, H.D.; Scheuer, R.; et al. Non-invasive mobile monitoring of meat quality. J. Verbr. Lebensm. 2009, 4, 7–14.
- King, J.A.; Miller, W.M. Bioreactor development for stem cell expansion and controlled differentiation. Curr. Opin. Chem. Biol. 2007, 11, 394–398.
- Landau, S.; Guo, S.; Levenberg, S. Localization of engineered vasculature within 3D tissue constructs. Front. Bioeng. Biotechnol. 2018, 6, 2.
- Langelaan, M.L.P.; Boonen, K.J.M.; Polak, R.B.; Baaijens, F.P.T.; Post, M.J.; van der Schaft, D.W.J. Meet the new meat: Tissue engineered skeletal muscle. Trends Food Sci. Technol. 2010, 21, 59–66.
- Miller, M.F.; Carr, M.A.; Ramsey, C.B.; Crockett, K.L.; Hoover, L.C. Consumer thresholds for establishing the value of beef tenderness. J. Anim. Sci. 2001, 79, 3062–3068.
- Mitchell, A.D. Impact of research with cattle, pigs, and sheep on nutritional concepts: Body composition and growth. J. Nutr. 2007, 137, 711–714.
- Moritz, M.S.M.; Verbruggen, S.E.L.; Post, M.J. Alternatives for large-scale production of cultured beef: A review. J. Integr. Agric. 2015, 14, 208–216.
- Pawlikowski, B.; Vogler, T.O.; Gadek, K.; Olwin, B.B. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev. Dyn. 2017, 246, 359–367.
- Post, M.J. Cultured beef: Medical technology to produce food. J. Sci. Food Agric. 2014, 94, 1039–1041.
- Post, M.J.; Hocquette, J.F. Chapter 16—New sources of animal proteins: Cultured Meat. In New Aspects of Meat Quality, 1st ed.; Purslow, P.P., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 425–441.
- Purslow, P.P. New developments on the role of intramuscular connective tissue in meat toughness. Annu. Rev. Food Sci. Technol. 2014, 5, 133–153.
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geissler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521.
- Qiu, F.; Xie, L.; Ma, J.-E.; Luo, W.; Zhang, L.; Chao, Z.; Chen, S.; Nie, Q.; Lin, Z.; Zhang, X. Lower Expression of SLC27A1 enhances intramuscular fat deposition in chicken via down-regulated fatty acid oxidation mediated by CPT1A. Front. Physiol. 2017, 8, 449.
- Shafa, M.; Day, B.; Yamashita, A.; Meng, G.; Liu, S.; Krawetz, R.; Rancourt, D.E. Derivation of iPSCs in stirred suspension bioreactors. Nat. Methods 2012, 9, 465–466.
- Rodriguez, B.L.; Larkin, L.M. 12—Functional three-dimensional scaffolds for skeletal muscle tissue engineering. In Functional 3D Tissue Engineering Scaffolds, 1st ed.; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 279–304.
- Rafii, S.; Butler, J.M.; Ding, B.S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325.
- Roberts, R.M.; Yuan, Y.; Genovese, N.; Ezashi, T. Livestock models for exploiting the promise of pluripotent stem cells. ILAR J. 2015, 56, 74–82.
- Ben-Arye, T.; Levenberg, S. Tissue engineering for clean meat production. Front. Sustain. Food Syst. 2019, 3, 46.
- Sack, M.; Hofbauer, A.; Fischer, R.; Stoger, E. The increasing value of plant-made proteins. Curr. Opin. Biotechnol. 2015, 32, 163–170.
- Sarlio, S. Towards Healthy and Sustainable Diets: Perspectives and Policy to Promote the Health of People and the Planet, 1st ed.; Springer: Berlin/Heidelberg Germany, 2018.
- Schmidinger, K. Worldwide Alternatives to Animal Derived Foods-Overview and Evaluation Models: Solution to Global Problems Caused by Livestock. Ph.D. Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, February 2012.
- Schröder, M.J.A.; McEachern, M.G. Consumer value conflicts surrounding ethical food purchase decisions: A focus on animal welfare. Int. J. Consum. Stud. 2004, 28, 168–177.
- Shahidi, F. Lipid-derived flavors in meat products. In Meat Processing: Improving Quality, 1st ed.; Kerry, J., Kerry, J., Ledward, D., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 105–121.
- Mattick, C.S. Cellular agriculture: The coming revolution in food production. Bull. Atom. Sci. 2018, 74, 32–35.
- Wang, G.; Kim, W.K.; Cline, M.A.; Gilbert, E.R. Factors affecting adipose tissue development in chickens: A review. Poult. Sci. 2017, 96, 3687–3699.
- Guo, B.; Dalrymple, B.P. Chapter 11—Transcriptomics of Meat Quality. In New Aspects of Meat Quality, 1st ed.; Purslow, P.P., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 259–320.
- Sharma, S.; Thind, S.S.; Kaur, A. In vitro meat production system: Why and how? J. Food Sci. Technol. 2015, 52, 7599–7607.
- Slade, P. If you build it, will they eat it? Consumer preferences for plant-based and cultured meat burgers. Appetite 2018, 125, 428–437.
- Specht, E.A.; Welch, D.R.; Rees Clayton, E.M.; Lagally, C.D. Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry. Biochem. Eng. J. 2018, 132, 161–168.
- An Analysis of Culture Medium Costs and Production Volumes for Cell-Based Meat. 2020. Available online: https://www.gfi.org/files/sci-tech/clean-meat-production-volume-and-medium-cost.pdf (accessed on 9 February 2020).
- Trivedi, D.K.; Hollywood, K.A.; Rattray, N.J.W.; Ward, H.; Trivedi, D.K.; Greenwood, J.; Ellis, D.I.; Goodacre, R. Meat, the metabolites: An integrated metabolite profiling and lipidomics approach for the detection of the adulteration of beef with pork. Analyst 2016, 141, 2155–2164.
- Van der Gucht, O. Cultured Meat: Current State of the Art and Future Challenges. Master’s Thesis, Ghent University, Ghent, Belgium, 2018.
- Van der Weele, C.; Driessen, C. Emerging profiles for cultured meat; ethics through and as design. Animals 2013, 3, 647–662.
- Verbeke, W.; Marcu, A.; Rutsaert, P.; Gaspar, R.; Seibt, B.; Fletcher, D.; Barnett, J. ‘Would you eat cultured meat?’ Consumers’ reactions and attitude formation in Belgium, Portugal, and the United Kingdom. Meat Sci. 2015, 102, 49–58.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
22 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




