
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zdenka Hertelyová | -- | 4465 | 2022-11-21 13:13:20 | | | |
| 2 | Amina Yu | -183 word(s) | 4282 | 2022-11-22 02:05:05 | | | | |
| 3 | Amina Yu | + 3 word(s) | 4285 | 2022-11-22 02:07:46 | | |
Video Upload Options
Breast cancer (BC)is etiologically, histopathologically and genetically a heterogeneous disease with both hereditary predispositions and non-hereditary factors. This is certainly true for BC as it refers to mammary carcinoma from ductal or lobular cells in the mammary epithelial tissue. Only a minor portion are sarcomas transformed from connective tissue and vessels. Malignant transformation in BC is the product of accumulations of consecutive mutations in critical regions of the genome that are normally involved in control of cell growth and division, DNA repair and programmed cell death. These mutations are partly inherited but mostly spontaneous. Contribution of genetic factors in BC has been indicated by familial occurrence which is estimated as 5–10% of all cases. High-penetrance genes which are linked with inherited BC susceptibility include BRCA1 and BRCA2, and more rarely TP53, PTEN, CDH1 and SKT11. Heterozygotic mutations in DNA repair genes BRCA1 (locus Ch17q21.31) or BRCA2 (Ch13q13.1) are the most common inherited conditions associated with BC. Absolute risk of BC for BRCA1 mutations reads ~50–65% in females and ~1% in males, while in BRCA2 mutations female risk ranges from 40% to 55% and reaches up to 9% in males. Familiar susceptibility to BC is also associated with mutations of lower penetrance genes as ATM (Ch11q22.3), PALB2 (Ch16p12.2) and CHEK2 (Ch22q12.1). Mutation in the androgen receptor gene (AR) has been found in cases of male BC. Susceptibility to sporadic BC cases can be linked with many more genes: e.g., sporadic invasive ductal variant of BC and lobular BC are associated with somatic mutation of genes RAD54L (Ch1p34.1) and CDH1 (Ch16q22.1), respectively. Other genes candidates associated with sporadic BC include: TP53 (Ch17p13.1), SLC22A1 (Ch11p15.4), PIK3CA (Ch3q26.32), ESR1 (Ch6q25.1-q25.2), RB1CC1 (Ch8q11.23), KRAS (12p12.1), AKT1 (14q32.33), RB1 (Ch13q14.2), PPM1D (Ch17q23.2), MYC (Ch8q24.21), FGFR1 and eventually ERBB2 (Ch17q12), CCND1 (Ch11q13.3), GATA3 (Ch10p14), MAP3K1 (Ch5q11.2) in certain lineages.
1. Diagnostics and Surveillance of the Breast Cancer (BC)
-
Mammography and breast NMRI (Magnetic Resonance Imaging) are useful non-invasive ways of how to exclude eventual other palpable breast lumps as abscess, cysts or fibroadenomas [5];
-
Biopsy is a preferred diagnostic tool. It can be done either as fine needle aspiration (FNA) or ultrasound-guided or stereotactic-navigated core needle biopsy (CNB). More recent minimally invasive breast biopsy or vacuum-assisted biopsies allow collection of several samples in one insertion instead of several punctures, which minimizes the spread of potentially malignant cells into surrounding tissue. Larger samples of tissue are obtained by classical surgery (probatory incisions or partial excisions or mamaectomy), as is done with tissue from regional lymph nodes. Tissue collected from breast tumour and sentinel lymph nodes is examined microscopically to determine the pathomorphological features and to classify them [7];
-
Histological proof of malignancy and assignment of histopathological phenotype has been a principal diagnostic method for a long time. It is supplemented by analysis of specific tumour cells products or markers to determine a molecular subtype of BC. Common biomarkers currently include oestrogen (ER) [8] and progesterone (PR) receptors [9], cytokeratins (CK) [10][11][12], human epidermal growth factor type 2 receptor (HER2) [13][14][15]. The BC samples obtained by biopsy and/or from post-surgery specimen can be currently processed by various methods (described in the following section). Genomic tests using individual or multigene assays can detect expression patterns of candidate genes associated with BC. All these methods should determine whether cancer is present, and if so, to identify the type of tumour, location, shape and spread of masses within or outside of the breast, respectively [3][10][16][17].
2. Histopathological Forms of BC
- (i)
-
The classical nonspecific subtype is typical by pleomorphic cells with different shapes, sizes, and large non-uniform nuclei. In most cases, squamous and apocrine metaplasias, tissue necrosis and calcification are observed;
- (ii)
-
The apocrine subtype is associated with a very poor prognosis. Cells are large, with typically strongly acidophilic granular cytoplasm. The nuclei are distinct and vesicular [23];
- (iii)
-
Medullary carcinoma accounts for 3–5% of BC. Typically, women in their late 40s and early 50s are affected, and most commonly those who carry a BRCA1 gene mutation. It is often of triple-negative molecular pattern, but more responsive to chemotherapy and better prognosis than other ductal cancer;
- (iv)
-
Mucinous carcinoma, also called colloid carcinoma, accounts for less than 2% of BC. Tumours contains clusters of uniform epithelial tumour cells with mildly atypical nuclei that are loosely surrounded by excessive mucus;
- (v)
-
Papillary ductal carcinoma accounts for less than 1% of invasive BC. It is typical for older, postmenopausal women. Under a microscope, these cells resemble tiny fingers (papillae). Cells are typically small;
- (vi)
-
Tubular ductal carcinoma accounts for less than 2% of BC and is more common in women older than 50. The tumour cells are oval or elongated, well differentiated, randomly arranged, and lined with a single layer of epithelial cells and without the outer layer of myoepithelial cells. In all these last three phenotypes tumour cells are positive for ER and/or PR receptors and negative for the HER2 receptor [9].
- (i)
-
Classic (non-specific) subtype carries typical morphological features of lobular invasive carcinoma. Cells are small and uniformly distributed across the stroma, forming a typical Indian pattern. All, or at least part, of the pleomorphic subtype cells are considerably larger than those of the classical subtype and are characteristic for their eosinophilic cytoplasm. The nuclei of these cells are hyperchromatic, located eccentrically within the cell and with a very pronounced nucleolus. Absent expression of hormone receptors and high expression of tumour protein p53 and HER-2 receptor are also very typical for this subtype [25];
- (ii)
-
Tubulolobular subtype is a variant of classical lobular carcinoma. It is characterized by small tubular formations with and without a lumen and cells forming a linear pattern similar to the classical subtype. An in situ lesion is often present in this subtype;
- (iii)
-
Histiocytoid subtype consists of cells with a diffused growth pattern. Tumour cells are large, with a foamy cytoplasmic consistency that contains a significant number of granules. E-cadherin expression is negative for this subtype [23].
3. Molecular Subtypes of BC
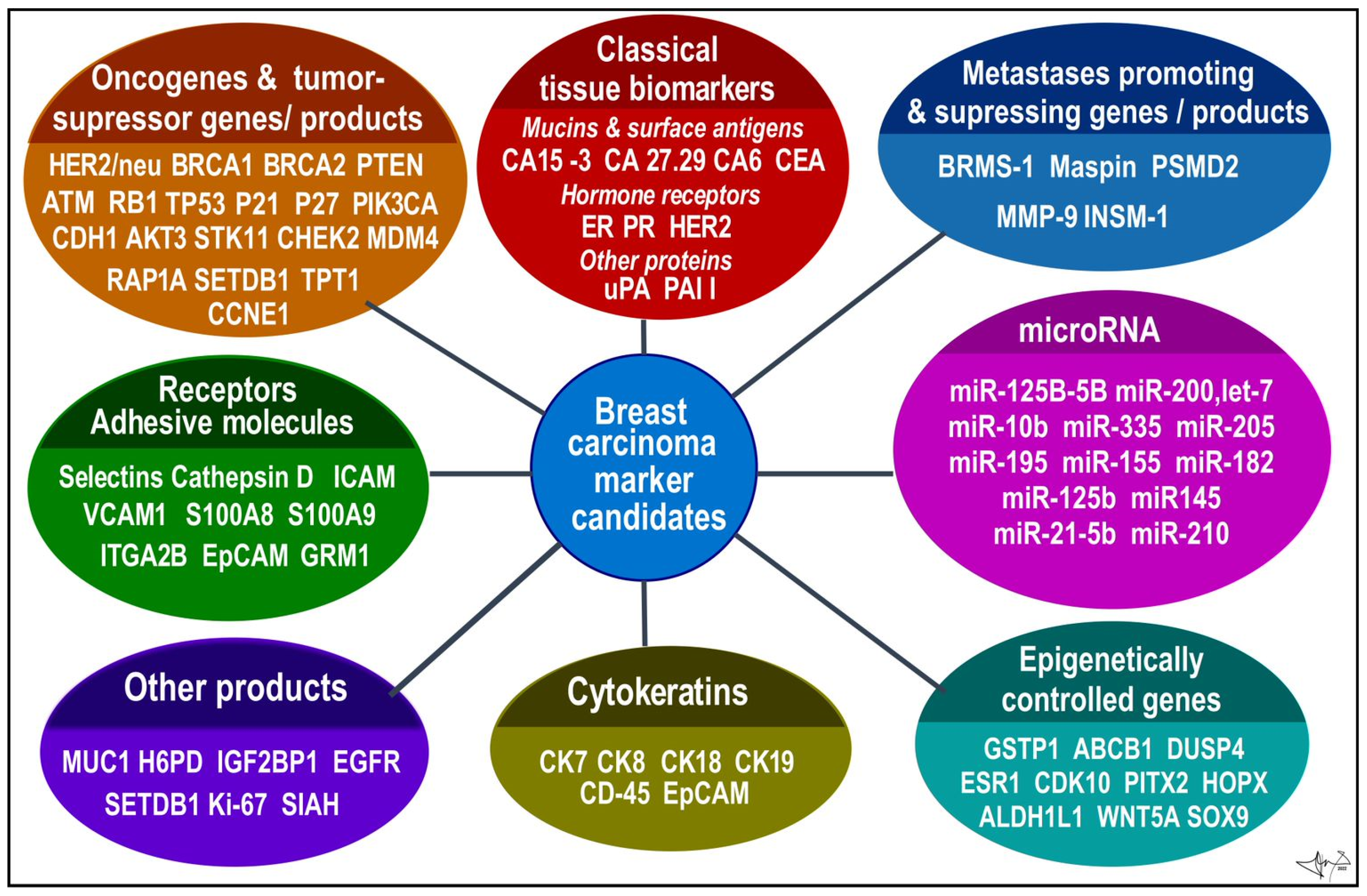
References
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol Res. 2017, 50, 33.
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27.
- Hagemann, I.S. Molecular Testing in Breast Cancer: A Guide to Current Practices. Arch. Pathol. Lab. Med. 2016, 140, 815–824.
- Nicolini, A.; Ferrari, P.; Duffy, M.J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. 2018, 52 (Pt 1), 56–73.
- Gøtzsche, P.C.; Jørgensen, K.J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013, 6, CD001877.
- Morrow, M.; Waters, J.; Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 2011, 378, 1804–1811.
- Pesapane, F.; Suter, M.B.; Rotili, A.; Penco, S.; Nigro, O.; Cremonesi, M.; Bellomi, M.; Jereczek-Fossa, B.A.; Pinotti, G.; Cassano, E. Will traditional biopsy be substituted by radiomics and liquid biopsy for breast cancer diagnosis and characterisation? Med. Oncol. 2020, 37, 29.
- Sommer, S.; Fuqua, S.A. Estrogen receptor and breast cancer. Semin. Cancer Biol. 2001, 11, 339–352.
- Berse, B.; Lynch, J.A. Molecular diagnostic testing in breast cancer. Semin. Oncol. Nurs. 2015, 31, 108–121.
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35.
- Weissenstein, U.; Schumann, A.; Reif, M.; Link, S.; Toffol-Schmidt, U.D.; Heusser, P. Detection of circulating tumor cells in blood of metastatic breast cancer patients using a combination of cytokeratin and EpCAM antibodies. BMC Cancer 2012, 12, 206.
- Xenidis, N.; Perraki, M.; Kafousi, M.; Apostolaki, S.; Bolonaki, I.; Stathopoulou, A.; Kalbakis, K.; Androulakis, N.; Kouroussis, C.H.; Pallis, T.; et al. Predictive and Prognostic Value of Peripheral Blood Cytokeratin-19 mRNA-Positive Cells Detected by Real-Time Polymerase Chain Reaction in Node-Negative Breast Cancer Patients. J. Clin. Oncol. 2006, 24, 3756–3762.
- Litton, J.K.; Burstein, H.J.; Turner, N.C. Molecular Testing in Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book. 2019, 39, e1–e7.
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429.
- Nitta, H.; Kelly, B.D.; Allred, C.; Jewell, S.; Banks, P.; Dennis, E.; Grogan, T.M. The assessment of HER2 status in breast cancer: The past, the present, and the future. Pathol. Int. 2016, 66, 313–324.
- Coleman, C. Early Detection and Screening for Breast Cancer. Semin. Oncol. Nurs. 2017, 33, 141–155.
- Tarighati, E.; Keivan, H.; Mahani, H. A review of prognostic and predictive biomarkers in breast cancer. Clin. Exp. Med. 2022. Online ahead of print.
- Yao, F.; Yan, C.; Zhang, Y.; Shen, L.; Zhou, D.; Ni, J. Identification of blood protein biomarkers for breast cancer staging by integrative transcriptome and proteome analyses. J. Proteom. 2021, 230, 103991.
- Yoon, E.C.; Schwartz, C.; Brogi, E.; Ventura, K.; Wen, H.; Darvishian, F. Impact of biomarkers and genetic profiling on breast cancer prognostication: A comparative analysis of the 8th edition of breast cancer staging system. Breast J. 2019, 25, 829–837.
- Patel, B. A review of breast cancer and hormonal therapy. Int. J. Pharm. Sci. Res. 2019, 10, 519–527.
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer:ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220.
- Gote, V.; Nookala, A.R.; Bolla, P.K.; Pal, D. Drug Resistance in Metastatic Breast Cancer: Tumor Targeted Nanomedicine to the Rescue. Int. J. Mol. Sci. 2021, 22, 4673.
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31.
- Santarpia, L.; Bottai, G.; Kelly, C.M.; Győrffy, B.; Szekely, B.; Pusztai, L. Deciphering and Targeting Oncogenic Mutations and Pathways in Breast Cancer. Oncologist. 2016, 21, 1063–1078.
- Fumagalli, C.; Ranghiero, A.; Gandini, S.; Corso, F.; Taormina, S.; De Camilli, E.; Rappa, A.; Vacirca, D.; Viale, G.; Guerini-Rocco, E.; et al. Inter-tumor genomic heterogeneity of breast cancers: Comprehensive genomic profile of primary early breast cancers and relapses. Breast Cancer Res. 2020, 22, 107.
- Mamouch, F.; Berrada, N.; Aoullay, Z.; Khanoussi, B.E.L.; Errihani, H. Inflammatory Breast Cancer: A Literature Review. World J. Oncol. 2018, 9, 129–135.
- Lim, B.; Woodward, W.A.; Wang, X.; Reuben, J.M.; Ueno, N.T. Inflammatory breast cancer biology: The tumour microenvironment is key. Nat. Rev. Cancer. 2018, 18, 485–499.
- Dubar, S.; Boukrid, M.; Bouquet, D.E.; Joliniere, J.; Guillou, L.; Vo, Q.D.; Major, A.; Ben Ali, N.; Khomsi, F.; Feki, A. Paget’s Breast Disease: A Case Report and Review of the Literature. Front Surg. 2017, 4, 51.
- Adams, S.J.; Kanthan, R. Paget’s disease of the male breast in the 21st century: A systematic review. Breast 2016, 29, 14–23.
- Fentiman, I.S.; Fourquet, A.; Hortobagyi, G.N. Male breast cancer. Lancet 2016, 367, 595–604.
- Li, J.; Guan, X.; Fan, Z.; Ching, L.M.; Li, Y.; Wang, X.; Cao, W.M.; Liu, D.X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767.
- He, Z.; Chen, Z.; Tan, M.; Elingarami, S.; Liu, Y.; Li, T.; Deng, Y.; He, N.; Li, S.; Fu, J.; et al. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. 2020, 53, e12822.
- Najjar, S.; Allison, K.H. Updates on breast biomarkers. Virchows Arch. 2022, 480, 163–176.
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Onc. 2018, 2, 4.
- Perou, C.; Sørlie, T.; Eisen, M.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752.
- Sauter, E.R. Reliable Biomarkers to Identify New and Recurrent Cancer. Eur. J. Breast Health 2017, 13, 162–167.
- Mcguire, A.; Brown, J.A.L.; Kerin, M.J. Metastatic breast cancer: The potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev. 2015, 34, 145–155.
- Wang, Z.; Liu, L.; Li, Y.; Song, Z.; Jing, Y.; Fan, Z.; Zhang, S. Analysis of CK5/6 and EGFR and Its Effect on Prognosis of Triple Negative Breast Cancer. Front Oncol. 2021, 10, 575317.
- Arancibia, T.; Morales-Pison, S.; Maldonado, E.; Jara, L. Association between single-nucleotide polymorphisms in miRNA and breast cancer risk: An updated review. Biol. Res. 2021, 54, 26.
- Daniel, A.R.; Hagan, C.R.; Lange, C.A. Progesterone receptor action: Defining a role in breast cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 359–369.
- ONLINE MENDELIAN INHERITANCE IN MAN, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number:114480, World Wide Web URL. Available online: https://omim.org/ (accessed on 5 May 2022).
- Orafa, Z.; Karimi, N.; Keyvani, S.; Oloomi, M. Quantitative CK19 biomarker detection in breast cancer cell lines. J. Med. Life 2022, 15, 188–195.
- Fazilat-Panah, D.; Vakili Ahrari Roudi, S.; Keramati, A.; Fanipakdel, A.; Sadeghian, M.H.; Shandiz, F.H.; Shahidsales, S.; Javadinia, S.A. Changes in Cytokeratin 18 during Neoadjuvant Chemotherapy of Breast Cancer: A Prospective Study. Iran J. Pathol. 2020, 15, 117–126.
- Lukong, K.E. Understanding breast cancer—The long and winding road. BBA Clin. 2017, 7, 64–77.
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front Pharmacol. 2021, 11, 632079.
- Duffy, M.J.; Mcgowan, P.M.; Harbeck, N.; Thomssen, C.; Schmitt, M. uPA and PAI-1 as biomarkers in breast cancer: Validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014, 16, 428.
- Kittaneh, M.; Montero, A.J.; Glück, S. Molecular profiling for breast cancer: A comprehensive review. Biomark Cancer. 2013, 5, 61–70.
- Holowatyj, A.N.; Ruterbusch, J.J.; Ratnam, M.; Gorski, D.H.; Cote, M.L. HER2 status and disparities in luminal breast cancers. Cancer Med. 2016, 5, 2109–2116.
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer 2016, 8, 93–107.




