| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tatsuro Inoue | + 2749 word(s) | 2749 | 2020-12-08 07:42:18 | | | |
| 2 | Bruce Ren | Meta information modification | 2749 | 2020-12-15 03:53:22 | | | | |
| 3 | Bruce Ren | Meta information modification | 2749 | 2020-12-15 03:56:43 | | | | |
| 4 | Catherine Yang | Meta information modification | 2749 | 2021-09-30 03:28:53 | | |
Video Upload Options
Geriatric patients with hip fractures often experience overlap in problems related to nutrition, including undernutrition, sarcopenia, and frailty. Such problems are powerful predictors of adverse responses, although few healthcare professionals are aware of them and therefore do not implement effective interventions.
1. Introduction
Hip fractures are a global public health problem and result in hospitalization, disability, and death [1]. Globally, as the population ages, the number of hip fractures is increasing, and it is expected that 6.3 million people will suffer from hip fracture in 2050 [2]. Hip fracture patients have high mortality [3], experience prolonged disability [4], and require substantial costs for postoperative management [5]. Therefore, management after hip fracture is a critical issue to be resolved.
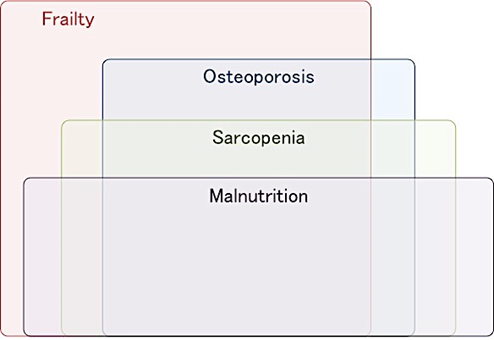
Hip fracture patients experience multiple geriatric nutritional problems, often including undernutrition, sarcopenia, and frailty at admission, all of which overlap (Figure 1), (Supplementary Figure S1–S3). These geriatric nutritional problems have significant impacts on disability, the occurrence of complications, and mortality after hip fracture. Therefore, interventions for these factors are a key strategy for improving postoperative clinical outcomes in patients with hip fracture.
Figure 1. The overlapping geriatric nutritional problems in patients with fragility hip fracture.
Conversely, the effect of interventions for geriatric nutritional problems in patients with hip fracture remains unclear. Nutritional therapy alone was not shown to reduce mortality [6]. Medical professionals often ignore undernutrition, sarcopenia, and frailty, and this unawareness inhibits improvements in clinical outcomes [7]. A focus must be placed on geriatric nutritional problems in hip fracture patients, and effective interventions should be considered.
. Undernutrition in Patients with Hip Fracture
2.1. Prevalence of Undernutrition
The prevalence of undernutrition with hip fracture is high and varies based on the evaluation tool used, ranging from about 7% to 26% (Table 1). The Mini Nutritional Assessment-Short Form (MNA-SF) [8][9][10][11][12][13][14][15] and the Mini Nutritional Assessment-Full Form (MNA-FF) [16][17][18][19] are the most commonly used tools for evaluating nutritional status in patients with hip fracture. The Malnutrition Screening Tool (MST) [20], Controlling Nutritional Status (CONUT) [21][22], Geriatric Nutritional Risk Index (GNRI) [23], Malnutrition Universal Screening Tool (MUST) [24], body mass index (BMI) [25][26], serum albumin [26][27], prealbumin [27], total protein [27], vitamin D [27] and lymphocyte count [16] are also used. These evaluation tools are useful for assessing the nutritional status of patients with hip fracture.
Table 1. Assessment of nutritional status, prevalence of undernutrition, and the impact of undernutrition on clinical outcomes in patients with hip fracture.
|
Author, Year, Country |
Design, Setting |
Age (Years) Male/Female, n (%) |
Sample Size |
Evaluation Tool (Timing of Assessment) |
Prevalence of Undernutrition |
Outcome |
Main Results |
|
Miyanishi et al., 2010 [26] Japan |
Observational study, acute hospital |
Mean 79 24 (18.9)/103 (81.1) |
129 |
Serum albumin BMI |
Not stated |
Four-year mortality |
In, multiple logistic regression analysis, serum albumin level (OR 5.854, p < 0.001) and BMI (OR 1.169, p = 0.02) significantly influenced mortality. |
|
Koren-Hakim et al., 2012 [13] Israel |
Observational study, acute hospital |
Mean 83.5 (SD 6.0) 61 (28.4)/154 (71.6) |
215 |
MNA-SF (at admission and up to 48 h after admission) |
Well-nourished: 44.2% At risk: 44.2% Malnourished: 11.6% |
In-hospital complications Mortality (up to 36 months) |
Only comorbidity and low functioning can predict long-term mortality (a minimum of 12 up to 36 months). Nutritional status had no effect on outcomes. |
|
Gumieiro et al., 2012 [28] Brazil |
Prospective observational study, general hospital |
Mean 80.2 (SD 7.3) 20 (23.3)/66 (76.7) |
86 |
MNA-FF NRS-2002 (within the first 72 h of the patient’s admission) |
Not stated |
Gait status (patients who could walk or could not walk) and mortality at 6 months after hip fracture |
In a multivariate analysis, only the MNA-FF was associated with gait status (OR 0.773, 95% CI 0.663−0.901) and mortality 6 months after hip fracture (HR 0.869, 95% CI 0.757−0.998). |
|
Drevet et al., 2014 [29] France |
Prospective observational study, university hospital |
Mean 86.1 (SD 4.4) 15 (30)/35 (70) |
50 |
MNA-FF (no details provided) |
At risk for PEM: 58% PEM: 28% |
Activities of daily living Hospital stay |
PEM was associated with functional dependence (p = 0.002) and 8 days longer mean hospital stay (p = 0.012). |
|
Goisser et al., 2015 [17] Germany |
Prospective observational study, urban maximum care hospital |
Mean 84 (SD 5) (21)/(79) |
97 |
MNA-FF (preoperative nutritional status was evaluated retrospectively) |
At risk: 38% Malnourished: 17% |
Barthel Index after 6 months |
Malnourished patients suffered more from remaining losses in ADL ≥25% of initial Barthel Index points (p = 0.033), and regained their prefracture mobility level to a lesser extent (p = 0.020) than well-nourished patients. |
|
Bajada et al., 2015 [16] UK |
Retrospective observational study, general hospital |
Mean 79 years (range: 60–96 years) 19 (18)/89 (82) |
108 |
Serum albumin (normal level > 35 g/L) Lymphocyte count (normal 1−4.5 × 109 L) (on admission) |
No details provided |
Failure of internal fixation |
In binary logistic regression analysis, lymphocyte count, and albumin levels were independent predictors of failure of internal fixation. |
|
van Wissen et al., 2016 [18] Netherlands |
Retrospective cohort study, acute hospital |
Mean Malnourished: 85 (SD 5) At risk: 84 (SD 5) Well-nourished: 83 (SD 5) 61 (27.0)/165(73.0) |
226 |
MNA-FF (before surgery) |
Well-nourished: 4.9% At risk: 26.5% Malnourished: 68.6% |
Hospital stay Postoperative complications, Mortality (in-hospital and 1-year) |
Preoperative malnutrition is associated with in-hospital (OR 4.4; 95% CI 1.0, 20.4) and 1-year mortality (OR 2.7; 95% CI 1.1, 7.0). Malnutrition was not associated with any other outcome. |
|
Miu et al., 2017 [30] China |
Observational study, rehabilitation unit |
Mean 83.5 (SD 7.5) 74 (33.9)/44 (66.1) |
218 |
MNA-SF (within 72 h of admission) |
Well-nourished: 21.1% At risk: 52.6% Malnourished: 26.1% |
Functional status and place of residence at 6 months Hospital stay Mortality (in-hospital, 6 months) |
Functional recovery was slower in the malnourished group. In-patient mortality was higher in malnourished patients than in those at risk of malnourishment and well-nourished individuals. |
|
Helminen et al., 2017 [12] Finland |
Prospective observational study, acute hospital |
No details provided 169 (28.5)/425 (71.5) |
594 |
MNA-SF MNA-FF Serum albumin (preoperative period) |
MNA-SF Well-nourished: 53% At risk: 40% Malnourished: 7% MNA-FF Well-nourished: 35% At risk: 58% Malnourished: 7% Serum albumin <34 g/L: 46% |
Poorer mobility (transfer to more assisted living accommodation) Mortality (1 month, 4 months, and 1 year after fracture) |
Risk of malnutrition and malnutrition measured by MNA-FF predicted mobility and living arrangements within 4 months of hip fracture. At 1 year, risk of malnutrition predicted mobility and malnutrition predicted living arrangements when measured by the MNA-FF. Malnutrition, but not risk measured by the MNA-SF, predicted living arrangements at all time points. Neither measure predicted 1-month mobility. |
|
Vosoughi et al., 2017 [25] Iran |
Cross-sectional study, university hospital |
Mean 75.7 (SD 10.6) 318 (43.9)/406 (56.1) |
724 |
BMI (at admission) |
No details provided |
Mortality at 3 months and 1 year |
Multivariate logistic regression analysis recognized age (OR 1.08; 95% CI 1.05, 1.11), BMI (OR 0.88; 95% CI 0.82−0.96), and smoking (OR 1.76; 95% CI 1.05−2.96) as major independent risk factors for 1- and 3-year mortality. |
|
Mazzola et al., 2017 [14] Italy |
Prospective observational study, university hospital |
Mean 84.0 (SD 6.6) 106 (25.5)/309 (74.5) |
415 |
MNA-SF (within 24 h of admission) |
Well-nourished: 36.6% At risk: 44.6% Malnourished: 18.8% |
Postoperative delirium |
Multivariate regression analysis showed that those at risk of malnutrition (OR 2.42; 95% CI = 1.29–4.53) and those overtly malnourished (OR 2.98; 95% CI = 1.43–6.19) were more likely to develop postoperative delirium. |
|
Inoue et al., 2017 [15] Japan |
Prospective observational study, three acute hospitals |
Mean 82.7 (SD 9.2) 69 (10.1)/165 (80.9) |
204 |
MNA-SF (first few days after admission before surgery) |
Well-nourished: 27.0% At risk: 48.0% Malnourished: 25.0% |
FIM at discharge |
In multiple regression analyses, MNA-SF was a significant independent predictor for FIM at discharge (well-nourished vs. malnourished, β = 0.86, p < 0.01). |
|
Nishioka et al., 2018 [11] Japan |
Retrospective observational cohort study, convalescent rehabilitation units |
Mean 85 years (21.8)/(78.2) |
110 |
MNA-SF (on admission and at discharge) |
Only malnourished patients at admission were included |
FIM at discharge Discharged to home |
Multivariable analysis revealed a significant association between improvement in nutritional status and higher FIM score at discharge (β = 7.377, p = 0.036). No association with discharge to home. |
|
Stone et al., 2018 [27] USA |
Retrospective observational cohort study, acute hospital |
Not stated 241(39.7)/366(60.3) |
607 |
Albumin Prealbumin Total protein Vitamin D |
Not stated |
Thirty-day readmission |
The model incorporated four nutritional makers (albumin, prealbumin, total protein, and vitamin D) with an internally cross-validated C-statistic of 0.811 (95% CI; 0.754, 0.867). |
|
Zanetti et al., 2018 [19] Italy |
Observational study, acute hospital |
Mean 84.7 (SD 7.4) 259 (21.4)/952 (78.6) |
1211 |
MNA-FF (within 72 h from admission)
|
Mean MNA-FF score: 22.3 (SD 5.1) |
Three, six and twelve-month mortality |
Poor nutritional status was significantly associated with 3, 6, and 12- month mortality after adjustment for potential confounders. |
|
Kotera et al. 2019 [22] Japan |
Retrospective observational cohort study, acute hospitals |
Mean 87 (SD 6) Not stated |
607 |
GNRI CONUT
|
GNRI Normal: 0.8% Light: 3.0% Moderate: 5.7% Severe: 14.4% CONUT Normal: 1.6% Light: 2.7% Moderate: 8.1% Severe: 38.9% |
Mortality of 180 days |
The GNRI value in the nonsurvivors was significantly lower than that in the survivors. The CONUT value in the nonsurvivors was significantly higher than that in the survivors. |
|
Yagi et al., 2020 [21] Japan |
Retrospective observational cohort study, community-based hospital |
Median 86 years (interquartile range 80–90) (19.9)/(80.1) |
211 |
CONUT (admission day) |
Malnourished (CONUT score >1): 78.7% |
Postoperative complications |
Multivariable analysis found that the CONUT score was an independent risk factor for postoperative complications (OR 1.21; 95% CI = 1.01–1.45). |
|
Hao et al., 2020 [23] USA |
Retrospective observational study (secondary analysis), 47 sites in North America |
Mean 82 (SD 7) (27)/(73) |
290 |
25-hydroxyvitamin D GNRI (preoperative) |
25-hydroxyvitamin D (ng/mL) ≥30: 17% 20 to <30: 37% 12 to <20: 34% <12: 12% GNRI No risk: 33 Some risk: 33 Major/moderate risk: 34 |
Mortality and mobility at 30 and 60 days after surgery |
Compared with patients with <12 ng/mL, those with higher 25(OH)D concentrations had higher rates of walking at 30 days (p = 0.031): 12 to <20 ng/mL (adjusted OR 2.61; 95% CI 1.13, 5.99); 20 to <30 ng/mL (3.48; 1.53, 7.95); ≥30 ng/mL (2.84; 1.12, 7.20). There was also greater mobility at 60 days (p = 0.028) in patients with higher 25(OH)D compared with the reference group (<12 ng/mL). GNRI <92 showed an overall trend to reduce mobility (adjusted p = 0.056) at 30 but not at 60 days. There was no association of vitamin D or GNRI with mortality at either time. |
|
Han et al., 2020 [24] UK |
Retrospective observational study, National Health Service hospital |
Mean 83.8 (SD 8.6) 349(28.2)/890(71.8) |
1239 |
MUST |
Low risk Medium risk High risk |
Mobilization (starting rehabilitation within 1 day after surgery) Pressure ulcers In-patient mortality Change in discharge destination |
Compared with the well-nourished group, malnourished individuals showed increased risk for failure to mobilize within 1 day of surgery (OR 1.6; 95% CI 1.0–2.7), pressure ulcers (OR 5.5, 95% CI, 1.8–17.1), in-patient mortality (OR 2.3; 95% CI, 1.1–4.8), and discharge to residential/nursing care (OR 2.8; 95% CI, 1.2–6.6). |
Abbreviations: BMI, body mass index; OR, odds ratio; SD, standard deviation; MNA-SF, Mini Nutritional Assessment-Short Form; MNA-FF, Mini Nutritional Assessment-Full Form; NRS-2002, Nutrition Risk Screening 2002; CI, confidence interval; HR, hazard ratio; FIM, Functional Independence Measure; PEM, protein energy malnutrition; ADL, activities of daily living; GNRI, Geriatric Nutritional Risk Index; CONUT, Controlling Nutritional Status; MUST, Malnutrition Universal Screening Tool.
2.2. Impact of Undernutrition on Clinical Outcomes
A large number of observational studies reported a significant association between undernutrition and clinical outcomes in patients with hip fracture. Most studies set mortality [28][29] or ADL [30] as clinical outcomes and the occurrence of postoperative complications, length of hospital stay [29], discharge disposition [24], readmission [27], mobility , and failure after internal fixation [16] as additional outcomes. Inoue et al. and Goisser et al. reported that undernutrition, as evaluated via the MNA-SF and MNA-FF, respectively, was a significant predictor of improved ADL at discharge from acute hospitals and six months postsurgery. Nishioka et al. revealed that improvement in nutritional status via MNA-SF screening during hospitalization in a convalescent hospital was associated with ADL at discharge. Miu and Lam [30] reported that, compared with at-risk and well-nourished patients, malnourished patients screened via the MNA-SF had a higher rate of in-hospital mortality. Gumieiro et al. [28] reported that the MNA-FF score was a predictor of mortality after six months. Vosoughi et al. [25] reported that BMI was an independent risk factor of mortality at one and three years. Conversely, Koren-Hakim et al. [13] reported that the MNA-SF score was not associated with mortality at 36 months. Overall, most of the studies found an association between nutritional status and clinical outcomes in hip fracture patients.
Several studies examining the appropriate nutritional screening tools recommended the use of the MNA-SF for hip fracture patients. The European Society for Clinical Nutrition and Metabolism also recommended the MNA-SF and the Malnutrition Universal Screening Tool and the Nutritional Risk Score 2002 (NRS-2002), which is known as a validated nutritional screening tool [31]. In their comparisons of these validated screening tools, Inoue et al. [32] and Koren-Hakim et al. [33] reported that the MNA-SF was a good predictor of ADL at discharge from an acute hospital, readmission during six months, and mortality at 36 months. In a study comparing the MNA-FF and NRS-2002 [28], only the MNA-FF could predict walking ability and mortality after six months. These results suggested that the use of the MNA-SF or MNA-FF is appropriate for predicting clinical outcomes in patients with hip fracture.
2.3. Highlights of Undernutrition in Hip Fracture
Evaluation of nutritional status is important, because undernutrition is a significant risk factor for clinical outcomes in hip fracture patients. The MNA-SF and MNA-FF are the most commonly used tools for nutritional status evaluation and were reported to be significant independent predictors of clinical outcomes. The MNA-SF is a simple and quick nutritional screening tool for nutritional status [34]. Furthermore, calf circumference rather than BMI can be used in the scoring of the MNA-SF, which is an advantage because of the difficulty in accurately measuring body weight on admission for patients with hip fracture. Moreover, the scoring for the MNA-SF includes the following components: functional, psychological, and cognitive aspects. Thus, the MNA-SF can accurately reflect the characteristics of elderly patients with hip fracture and might be the most appropriate nutritional screening tool for clinical outcomes in patients with hip fracture.
3. Nutritional Intervention for Patients with Hip Fracture
Based on the current evidence, the effectiveness of nutritional therapy alone for hip fracture patients is unclear. A systematic review of nutritional interventions for hip fracture patients reported only low-quality evidence to reduce complications and no clear effect on mortality. Many intervention studies examined the effect of oral administration of protein [35][36][37][38][39][40][41][42], β-hydroxy-β-methylbutyrate [43], vitamin D [44][45][46], whey protein [47][48], or combined calcium β-hydroxy-β-methylbutyrate (CaHMB), vitamin D, and protein intake [49] on clinical outcomes. One randomized controlled trial for hip fracture patients conducted an intervention to calculate energy requirements by measuring the resting energy expenditure using an indirect calorimeter [50]. In individual randomized controlled trials, the group that received the nutritional intervention had better outcomes than the control group in terms of occurrence of complications [50], severity of pressure ulcers [38], length of hospital stay [39], readmission rate [44], nutritional status [36], muscle strength [48], muscle mass [41][43], and wound-healing period [49]. Conversely, there was no significant difference in nutritional status [35][39] or mortality [37] between the group that received a nutritional intervention alone and the control group. The effects of nutritional intervention on ADL are not consistent [39][40][41][48]. There were no intervention studies that reported enhanced rehabilitation used in combination with nutritional therapy. These discrepancies might suggest that nutritional interventions alone are insufficient to improve clinical outcomes.
4. Combined Nutritional Intervention with Rehabilitation Exercise
A combination of nutrition and exercise interventions is effective for elderly patients with sarcopenia. A combination of amino acid intake and exercise improved muscle strength, muscle mass, and ADL of community-dwelling women with sarcopenia [51] and sarcopenic patients with cerebrovascular disease [52]. A meta-analysis reported that the combination of nutrition and exercise had a positive effect on physical function in community-dwelling elderly individuals [53]. Combined nutrition and exercise interventions promoted muscle protein synthesis compared with each of these interventions alone [54]. Thus, these combination interventions for hip fracture patients may contribute to improved clinical outcomes.
5. Advanced Strategies for Improvement of Clinical Outcomes
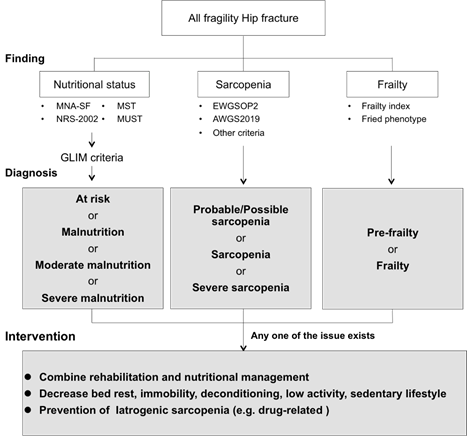
To improve clinical outcomes effectively, medical professionals should be aware of geriatric nutritional problems in hip fracture patients (Figure 2). On the basis of geriatric nutritional evaluation, we must be careful about iatrogenic sarcopenia. Iatrogenic sarcopenia is caused by hospitalization and is drug-related. Hospitalization-related iatrogenic sarcopenia is caused by physicians, nurses, and other medical professionals [55][56]. Iatrogenic sarcopenia mainly comprises inactivity- and nutritional-related factors. Inactivity-related iatrogenic sarcopenia is mainly caused by unnecessary inactivity during the perioperative period. In hospitalized hip fracture patients, approximately 99% of the day consists of sedentary time [57]. The incidence of sarcopenia in acute hospitals is approximately 15%, and the duration of bed rest is associated with the incidence of sarcopenia [58]. In patients in rehabilitation hospitals, increased time away from bed is more effective in improving ADL [59]. Medical professionals should pay close attention to iatrogenic sarcopenia, and avoiding unnecessary bed rest, immobility, and deconditioning in patients could prevent activity-related sarcopenia.
Figure 2. The specific strategies of geriatric nutritional evaluation and advanced intervention for patients with fragility hip fracture. Abbreviations: MNA-SF, Mini Nutritional Assessment-Short Form; MST, Malnutrition Screening Tool; NRS-2002, Nutrition Risk Screening 2002; MUST, Malnutrition Universal Screening Tool; EWGSOP, European Working Group on Sarcopenia in Older People; AWGS, Asian Working Group for Sarcopenia.
In hip fracture patients, nutritional-related iatrogenic sarcopenia requires a comprehensive approach. Only 17.5% of patients meet their energy requirements in the first week after hip surgery [60]. Additionally, multiple factors are associated with reduced food intake after fractures [61][62], and it is clear that interventions that merely administer supplements are insufficient for improving clinical outcomes. Bell et al. [63] reported that intensive individualized, multidisciplinary (orthopedic and geriatric physician, nursing staff, physiotherapists and occupational therapists, dietitian, pharmacist, etc.) interventions reduced barriers to food intake; food intake increased in the group with multidisciplinary intervention (mean 1489.0 kcal/day, protein intake of 1.13 g/body weight) compared with the group with conventional care (mean 707.4 kcal/day, protein intake of 0.60 g/body weight) in hip fracture patients. Additionally, medical professionals should pay attention to sarcopenic dysphagia accompanied by deterioration in nutritional status after hip surgery. A multidisciplinary, comprehensive pragmatic intervention trial is required for hip fractures with overlapping undernutrition, sarcopenia, and frailty. Compared with randomized controlled trials, pragmatic trials can be routinely conducted with less stringent inclusion and exclusion criteria. Therefore, selection bias can be controlled, and the results can be easily generalized to routine clinical practice. Comprehensive multidisciplinary interventions are necessary to prevent nutritional-related iatrogenic sarcopenia in patients with hip fracture.
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos. Int. 2004, 15, 897–902, doi:10.1007/s00198-004-1627-0.
- Cooper, C.; Campion, G.; Melton, L.J. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289, doi:10.1007/BF01623184.
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16, 6–10, doi:10.1007/s00198-004-1702-6.
- Shyu, Y.-I.L.; Chen, M.-C.; Liang, J.; Wu, C.-C.; Su, J.-Y. Predictors of functional recovery for hip fractured elders during 12 months following hospital discharge: A prospective study on a Taiwanese sample. Osteoporos. Int. 2004, 15, 475–482, doi:10.1007/s00198-003-1557-2.
- Braithwaite, R.S.; Col, N.F.; Wong, J.B. Estimating hip fracture morbidity, mortality and costs. J. Am. Geriatr. Soc. 2003, 51, 364–370, doi:10.1046/j.1532-5415.2003.51110.x.
- Avenell, A.; Handoll, H. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst. Rev. 2016, doi:10.1002/14651858.cd001880.
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646, doi:10.1016/S0140-6736(19)31138-9.
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, doi:10.1371/journal.pmed.1000100.
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423, doi:10.1093/ageing/afq034.
- National Heart Lung and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 17 November 2020).
- Nishioka, S.; Wakabayashi, H.; Momosaki, R. Nutritional Status Changes and Activities of Daily Living after Hip Fracture in Convalescent Rehabilitation Units: A Retrospective Observational Cohort Study from the Japan Rehabilitation Nutrition Database. J. Acad. Nutr. Diet. 2018, 118, 1270–1276, doi:10.1016/j.jand.2018.02.012.
- Helminen, H.; Luukkaala, T.; Saarnio, J.; Nuotio, M. Comparison of the Mini-Nutritional Assessment short and long form and serum albumin as prognostic indicators of hip fracture outcomes. Injury 2017, 48, 903–908, doi:10.1016/j.injury.2017.02.007.
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Grosman, B.; Frishman, S.; Salai, M.; Beloosesky, Y. The relationship between nutritional status of hip fracture operated elderly patients and their functioning, comorbidity and outcome. Clin. Nutr. 2012, 31, 917–921, doi:10.1016/j.clnu.2012.03.010.
- Mazzola, P.; Ward, L.; Zazzetta, S.; Broggini, V.; Anzuini, A.; Valcarcel, B.; Brathwaite, J.S.; Pasinetti, G.M.; Bellelli, G.; Annoni, G. Association Between Preoperative Malnutrition and Postoperative Delirium after Hip Fracture Surgery in Older Adults. J. Am. Geriatr. Soc. 2017, 65, 1222–1228, doi:10.1111/jgs.14764.
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Pre-fracture nutritional status is predictive of functional status at discharge during the acute phase with hip fracture patients: A multicenter prospective cohort study. Clin. Nutr. 2017, 36, 6–11, doi:10.1016/j.clnu.2016.08.021.
- Bajada, S.; Smith, A.; Morgan, D. Pre-operative nutritional serum parameters as predictors of failure after internal fixation in undisplaced intracapsular proximal femur fractures. Injury 2015, 46, 1571–1576, doi:10.1016/j.injury.2015.05.001.
- Goisser, S.; Schrader, E.; Singler, K.; Bertsch, T.; Gefeller, O.; Biber, R.; Bail, H.J.; Sieber, C.C.; Volkert, D. Malnutrition According to Mini Nutritional Assessment Is Associated with Severe Functional Impairment in Geriatric Patients before and up to 6 Months after Hip Fracture. J. Am. Med. Dir. Assoc. 2015, 1–7, doi:10.1016/j.jamda.2015.03.002.
- van Wissen, J.; van Stijn, M.F.M.; Doodeman, H.J.; Houdijk, A.P.J. Mini nutritional assessment and mortality after hip fracture surgery in the elderly. J. Nutr. Health Aging 2016, 20, 964–968, doi:10.1007/s12603-015-0630-9.
- Zanetti, M.; Gortan Cappellari, G.; Ratti, C.; Ceschia, G.; Murena, L.; De Colle, P.; Barazzoni, R. Poor nutritional status but not cognitive or functional impairment per se independently predict 1 year mortality in elderly patients with hip-fracture. Clin. Nutr. 2019, 38, 1607–1612, doi:10.1016/j.clnu.2018.08.030.
- Milte, R.; Miller, M. Dietetic care of hip fracture patients across Australia: Are we doing enough? Nutr. Diet. 2011, 68, 214–220, doi:10.1111/j.1747-0080.2011.01538.x.
- Yagi, T.; Oshita, Y.; Okano, I.; Kuroda, T.; Ishikawa, K.; Nagai, T.; Inagaki, K. Controlling nutritional status score predicts postoperative complications after hip fracture surgery. BMC Geriatr. 2020, 20, 1–7, doi:10.1186/s12877-020-01643-3.
- Kotera, A. Geriatric Nutritional Risk Index and Controlling Nutritional Status Score can predict postoperative 180-day mortality in hip fracture surgeries. JA Clin. Rep. 2019, 5, doi:10.1186/s40981-019-0282-6.
- Hao, L.; Carson, J.L.; Schlussel, Y.; Noveck, H.; Shapses, S.A. Vitamin D deficiency is associated with reduced mobility after hip fracture surgery: A prospective study. Am. J. Clin. Nutr. 2020, 112, 613–618, doi:10.1093/ajcn/nqaa029.
- Han, T.S.; Yeong, K.; Lisk, R.; Fluck, D.; Fry, C.H. Prevalence and consequences of malnutrition and malnourishment in older individuals admitted to hospital with a hip fracture. Eur. J. Clin. Nutr. 2020, doi:10.1038/s41430-020-00774-5.
- Vosoughi, A.R.; Emami, M.J.; Pourabbas, B.; Mahdaviazad, H. Factors increasing mortality of the elderly following hip fracture surgery: Role of body mass index, age, and smoking. Musculoskelet. Surg. 2017, 101, 25–29, doi:10.1007/s12306-016-0432-1.
- Miyanishi, K.; Jingushi, S.; Torisu, T. Mortality after hip fracture in Japan: The role of nutritional status. J. Orthop. Surg. (Hong Kong) 2010, 18, 265–270, doi:10.1177/230949901001800301.
- Stone, A.V.; Jinnah, A.; Wells, B.J.; Atkinson, H.; Miller, A.N.; Futrell, W.M.; Lenoir, K.; Emory, C.L. Nutritional markers may identify patients with greater risk of re-admission after geriatric hip fractures. Int. Orthop. 2018, 42, 231–238, doi:10.1007/s00264-017-3663-3.
- Gumieiro, D.N.; Rafacho, B.P.M.; Gonçalves, A.F.; Tanni, S.E.; Azevedo, P.S.; Sakane, D.T.; Carneiro, C.A.S.; Gaspardo, D.; Zornoff, L.A.M.; Pereira, G.J.C.; et al. Mini Nutritional Assessment predicts gait status and mortality 6 months after hip fracture. Br. J. Nutr. 2012, 1–5, doi:10.1017/S0007114512003686.
- Drevet, S.; Bioteau, C.; Mazière, S.; Couturier, P.; Merloz, P.; Tonetti, J.; Gavazzi, G. Prevalence of protein-energy malnutrition in hospital patients over 75 years of age admitted for hip fracture. Orthop. Traumatol. Surg. Res. 2014, 100, 669–674, doi:10.1016/j.otsr.2014.05.003.
- Miu, K.Y.D.; Lam, P.S. Effects of nutritional status on 6-month outcome of hip fractures in elderly patients. Ann. Rehabil. Med. 2017, 41, 1005–1012, doi:10.5535/arm.2017.41.6.1005.
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421, doi:10.1016/S0261-5614(03)00098-0.
- Inoue, T.; Misu, S.; Tanaka, T.; Kakehi, T.; Ono, R. Acute phase nutritional screening tool associated with functional outcomes of hip fracture patients: A longitudinal study to compare MNA-SF, MUST, NRS-2002 and GNRI. Clin. Nutr. 2019, 38, 220–226, doi:10.1016/j.clnu.2018.01.030.
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Anbar, R.; Gross Nevo, R.F.; Schlesinger, A.; Frishman, S.; Salai, M.; Beloosesky, Y. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin. Nutr. 2015, 3–8, doi:10.1016/j.clnu.2015.07.014.
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485; discussion 485–487.
- Botella-Carretero, J.I.; Iglesias, B.; Balsa, J.A.; Zamarrón, I.; Arrieta, F.; Vázquez, C. Effects of oral nutritional supplements in normally nourished or mildly undernourished geriatric patients after surgery for hip fracture: A randomized clinical trial. JPEN J. Parenter. Enter. Nutr. 2008, 32, 120–128, doi:10.1177/0148607108314760.
- Botella-Carretero, J.I.; Iglesias, B.; Balsa, J.A.; Arrieta, F.; Zamarrón, I.; Vázquez, C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: A randomized clinical trial. Clin. Nutr. 2010, 29, 574–579, doi:10.1016/j.clnu.2010.01.012.
- Espaulella, J.; Guyer, H.; Diaz-Escriu, F.; Mellado-Navas, J.A.; Castells, M.; Pladevall, M. Nutritional supplementation of elderly hip fracture patients. A randomized, double-blind, placebo-controlled trial. Age Ageing 2000, 29, 425–431, doi:10.1093/ageing/29.5.425.
- Houwing, R.H.; Rozendaal, M.; Wouters-Wesseling, W.; Beulens, J.W.J.; Buskens, E.; Haalboom, J.R. A randomised, double-blind assessment of the effect of nutritional supplementation on the prevention of pressure ulcers in hip-fracture patients. Clin. Nutr. 2003, 22, 401–405, doi:10.1016/S0261-5614(03)00039-6.
- Myint, M.W.W.; Wu, J.; Wong, E.; Chan, S.P.; To, T.S.J.; Chau, M.W.R.; Ting, K.H.; Fung, P.M.; Au, K.S.D. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: A single blind randomised controlled trial. Age Ageing 2013, 42, 39–45, doi:10.1093/ageing/afs078.
- Neumann, M.; Friedmann, J.; Roy, M.A.; Jensen, G.L. Provision of high-protein supplement for patients recovering from hip fracture. Nutrition 2004, 20, 415–419, doi:10.1016/j.nut.2004.01.004.
- Tidermark, J.; Ponzer, S.; Carlsson, P.; Söderqvist, A.; Brismar, K.; Tengstrand, B.; Cederholm, T. Effects of protein-rich supplementation and nandrolone in lean elderly women with femoral neck fractures. Clin. Nutr. 2004, 23, 587–596, doi:10.1016/j.clnu.2003.10.006.
- Wyers, C.E.; Reijven, P.L.M.; Breedveld-Peters, J.J.L.; Denissen, K.F.M.; Schotanus, M.G.M.; Van Dongen, M.C.J.M.; Eussen, S.J.P.M.; Heyligers, I.C.; Van Den Brandt, P.A.; Willems, P.C.; et al. Efficacy of Nutritional Intervention in Elderly after Hip Fracture: A Multicenter Randomized Controlled Trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1429–1437, doi:10.1093/gerona/gly030.
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas 2017, 101, 42–50, doi:10.1016/j.maturitas.2017.04.010.
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Platz, A.; Orav, E.J.; Stähelin, H.B.; Willett, W.C.; Can, U.; Egli, A.; Mueller, N.J.; Looser, S.; et al. Effect of High-Dosage Cholecalciferol and Extended Physiotherapy on Complications After Hip Fracture. Arch. Intern. Med. 2010, 170, 813–820, doi:10.1001/archinternmed.2010.67.
- Papaioannou, A.; Kennedy, C.C.; Giangregorio, L.; Ioannidis, G.; Pritchard, J.; Hanley, D.A.; Farrauto, L.; Debeer, J.; Adachi, J.D. A randomized controlled trial of vitamin D dosing strategies after acute hip fracture: No advantage of loading doses over daily supplementation. BMC Musculoskelet. Disord. 2011, 12, doi:10.1186/1471-2474-12-135.
- Bachrach-Lindström, M.; Unosson, M.; Ek, A.C.; Arnqvist, H.J. Assessment of nutritional status using biochemical and anthropometric variables in a nutritional intervention study of women with hip fracture. Clin. Nutr. 2001, 20, 217–223, doi:10.1054/clnu.2000.0383.
- Chevalley, T.; Hoffmeyer, P.; Bonjour, J.P.; Rizzoli, R. Early serum IGF-I response to oral protein supplements in elderly women with a recent hip fracture. Clin. Nutr. 2010, 29, 78–83, doi:10.1016/j.clnu.2009.07.003.
- Niitsu, M.; Ichinose, D.; Hirooka, T.; Mitsutomi, K.; Morimoto, Y.; Sarukawa, J.; Nishikino, S.; Yamauchi, K.; Yamazaki, K. Effects of combination of whey protein intake and rehabilitation on muscle strength and daily movements in patients with hip fracture in the early postoperative period. Clin. Nutr. 2016, 35, 943–949, doi:10.1016/j.clnu.2015.07.006.
- Ekinci, O.; Yanlk, S.; Terzioǧlu Bebitoǧlu, B.; Yllmaz Akyüz, E.; Dokuyucu, A.; Erdem, Ş. Effect of Calcium β-Hydroxy-β-Methylbutyrate (CaHMB), Vitamin D, and Protein Supplementation on Postoperative Immobilization in Malnourished Older Adult Patients with Hip Fracture. Nutr. Clin. Pract. 2016, 31, 829–835, doi:10.1177/0884533616629628.
- Anbar, R.; Beloosesky, Y.; Cohen, J.; Madar, Z.; Weiss, A.; Theilla, M.; Koren Hakim, T.; Frishman, S.; Singer, P. Tight Calorie Control in geriatric patients following hip fracture decreases complications: A randomized, controlled study. Clin. Nutr. 2014, 33, 23–28, doi:10.1016/j.clnu.2013.03.005.
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23, doi:10.1111/j.1532-5415.2011.03776.x.
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, P.T.; Nishino, P.T.; Kuzuhara, P.T.; Tomioka, P.T. Effects of a leucine-enriched amino acid supplement on muscle mass , muscle strength , and physical function in post-stroke patients with sarcopenia : A randomized controlled trial. Nutrition 2019, 58, 1–6, doi:10.1016/j.nut.2018.05.028.
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for Treating Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–553.e16, doi:10.1016/j.jamda.2017.03.019.
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384, doi:10.1152/japplphysiol.91397.2008.
- Wakabayashi, H. Rehabilitation nutrition in general and family medicine. J. Gen. Fam. Med. 2017, 18, 153–154, doi:10.1002/jgf2.116.
- Nagano, A.; Nishioka, S.; Wakabayashi, H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J. Nutr. Health Aging 2019, 23, 256–265, doi:10.1007/s12603-018-1150-1.
- Davenport, S.J.; Arnold, M.; Hua, C.; Schenck, A.; Batten, S.; Taylor, N.F. Physical Activity Levels During Acute Inpatient Admission After Hip Fracture are Very Low. Physiother. Res. Int. 2015, 2050, 174–181, doi:10.1002/pri.1616.
- Martone, A.M.; Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Bari, M. Di; Maggio, M.; Manca, G.M.; Marzetti, E.; et al. The incidence of sarcopenia among hospitalized older patients: Results from the Glisten study. J. Cachexia Sarcopenia Muscle 2017, 8, 907–914, doi:10.1002/jcsm.12224.
- Murayama, I.; Asai, T.; Misu, S.; Yamauchi, M.; Miura, A.; Ikemura, T.; Takehisa, T.; Takehisa, Y. Is increased “stay away from bed” time associated with improved clinical rehabilitation outcomes in Japanese rehabilitation hospitals? A prospective observational study and clinical practice. Aging Clin. Exp. Res. 2019, doi:10.1007/s40520-019-01269-5.
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Inadequate Postoperative Energy Intake Relative to Total Energy Requirements Diminishes Acute Phase Functional Recovery from Hip Fracture. Arch. Phys. Med. Rehabil. 2019, 100, 32–38, doi:10.1016/j.apmr.2018.06.012.
- Foss, N.B.; Jensen, P.S.; Kehlet, H. Risk factors for insufficient perioperative oral nutrition after hip fracture surgery within a multi-modal rehabilitation programme. Age Ageing 2007, 36, 538–543, doi:10.1093/ageing/afm079.
- Mudge, A.M.; Ross, L.J.; Young, A.M.; Isenring, E.A.; Banks, M.D. Helping understand nutritional gaps in the elderly (HUNGER): A prospective study of patient factors associated with inadequate nutritional intake in older medical inpatients. Clin. Nutr. 2011, 30, 320–325, doi:10.1016/j.clnu.2010.12.007.
- Bell, J.J.; Bauer, J.D.; Capra, S.; Pulle, R.C. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients—Results of a pragmatic intervention. Clin. Nutr. 2014, 1–7, doi:10.1016/j.clnu.2013.12.003.