Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexandre Vetcher | -- | 2533 | 2022-11-21 10:28:19 | | | |
| 2 | Vivi Li | Meta information modification | 2533 | 2022-11-23 05:16:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shchegolkov, A.V.; Shchegolkov, A.V.; Zemtsova, N.V.; Stanishevskiy, Y.M.; Vetcher, A.A. Advantages on Waste Management in Hydrogen Industry. Encyclopedia. Available online: https://encyclopedia.pub/entry/35538 (accessed on 15 January 2026).
Shchegolkov AV, Shchegolkov AV, Zemtsova NV, Stanishevskiy YM, Vetcher AA. Advantages on Waste Management in Hydrogen Industry. Encyclopedia. Available at: https://encyclopedia.pub/entry/35538. Accessed January 15, 2026.
Shchegolkov, Alexander V., Aleksei V. Shchegolkov, Natalia V. Zemtsova, Yaroslav M. Stanishevskiy, Alexandre A. Vetcher. "Advantages on Waste Management in Hydrogen Industry" Encyclopedia, https://encyclopedia.pub/entry/35538 (accessed January 15, 2026).
Shchegolkov, A.V., Shchegolkov, A.V., Zemtsova, N.V., Stanishevskiy, Y.M., & Vetcher, A.A. (2022, November 21). Advantages on Waste Management in Hydrogen Industry. In Encyclopedia. https://encyclopedia.pub/entry/35538
Shchegolkov, Alexander V., et al. "Advantages on Waste Management in Hydrogen Industry." Encyclopedia. Web. 21 November, 2022.
Copy Citation
The turn to hydrogen as an energy source is a fundamentally important task facing the global energetics, aviation and automotive industries. This step would reduce the negative man-made impact on the environment on the one hand, and provide previously inaccessible power modes and increased resources for technical systems, predetermining the development of an absolutely new life cycle for important areas of technology, on the other. The most important aspect in this case is the development of next-generation technologies for hydrogen industry waste management that will definitely reduce the negative impact of technology on the environment.
hydrogen storage (tank)
nanocomposite(s)
nanotubes
waste management
1. Introduction
Enhancement of environmental friendliness at all levels of energy resources employment is still a priority task that can be solved by considering hydrogen as an energy source [1]. Hydrogen is well-known as a carbon-free energy source, and its properties have been exhaustively studied by generations of scientists. Its wide application could potentially replace hydrocarbons and, accordingly, emissions of gaseous carbon in a variety of forms.
The application of hydrogen is based on the employment of fuel cells, which are efficient energy converters with significant potential for use in transport and other areas of energy production [2]. Fuel cells have an energy conversion efficiency of about 60–70%, which is significantly higher than for devices using the Carnot cycle. Currently, fuel cells have been demonstrated to be safe and efficient devices that can ensure a fast refueling process and energy efficiency [3]. Cathodic and anodic reaction—implemented on FC with a pronounced anode and cathode—is characterized by the fact that hydrogen is ionized, and its energy is released with the accumulation of electrons on the FC anode’s surface. At the same time, oxygen is reduced at the cathode, which indicates anodic oxidation and cathodic reduction [4].
It should be noted that the main material for creating fuel cells is titanium, which corrodes during operation. To reduce the intensity of the corrosion process, a cathodic deposition of tungsten trioxide on the titanium surface can be used [5].
There are several main directions for the use of hydrogen that are widespread at the moment. The most important applications of hydrogen are:
-
Chemical industry—synthesis of ammonia, methanol, and hydrocarbons, as well as the recovery of metals from their oxide form [6].
-
Energetics—an energy source for electric and thermal power engineering [9].
-
Petrochemistry—oil refining (hydrogenation purification of petroleum products—hydrodesulfurization) [10].
In this latter case, a distinction should be made between passenger cars [13] and commercial cargo and passenger transportation means [14][15].
The implementation of hydrogen as the main energy source in various types of buses with FC in their design features the fact that hydrogen is converted into electrical energy, and the by-product is water vapor, which condenses into water in the environment [16]. The approach presented—in which H2 is generated electrically to split water into O2 and H2 or by chemical conversion of methane to H2 (loop conversion of methane with steam on anti-coking compounds CeO2/La0.9Sr0.1Fe1−xNixO3) [17]—makes it possible to abandon the use of petroleum products such as motor oils. This will also have a positive and tangible impact on the ecological situation of megacities, as it will eliminate the need to recycle used engine oils. The transition to a hydrogen energy system is likely to be based on H2, obtained as a result of reforming natural gas or electrolysis.
The most widespread use of hydrogen is primarily in the field of motor transport, which needs environmentally friendly and affordable energy sources that are capable of replacing hydrocarbons. Another option that should be considered is the generation of energy at a thermal power plant [18], and in this case, one practice involves the partial mixing of hydrogen with methane or other gaseous fuels based on hydrocarbons [19].
The transition to hydrogen as the main type of fuel could form and transform the design of new types of internal combustion engine, and in particular, hydrogen rotary Wankel engines could find distribution [20]. Other types of vehicles in which hydrogen can be used include aircraft or air transport [21] and unmanned aerial vehicles [22]. Commercial hydrogen production currently depends mainly on steam natural gas reforming [23] and coal partial oxidation [24]. Clean production using both biomass and solar energy production methods is on its way [25].
Thus, there is a widespread practice of using hydrogen, which has the possibility of serving as a basis for an entire direction of research. For the successful dissemination of these achievements, however, several fundamentally important and significant problems need to be solved, including the safe generation and storage of hydrogen. At the same time, it should be borne in mind that polymer waste can be used as a source of cheap raw materials for producing hydrogen and related high-performance materials. There is also a fundamental possibility when using new control technologies related to artificial intelligence in the process of hydrogen synthesis and storage.
2. Design and Thermodynamics of HS Tank
HS is currently a “bottleneck” for the implementation of the use of hydrogen as renewable energy. A key challenge for the full development of hydrogen-based technologies is the safe, efficient, and economical storage of hydrogen.
Practical materials for HS should have the ability to undergo a reversible hydrogenation/dehydrogenation process, which is determined by the binding energy of hydrogen atoms. The PCT curve is a graph representing the dependence of pressure on composition at different temperatures. Figure 1 demonstrates a graphical interpretation of the Van ’t Hoff equation [26], which indicates the dependence of the logarithm of the equilibrium desorption pressure on the reciprocal temperature (T) (Figure 1a), as well as the dependence of the amount of hydrogen accumulation on pressure (Figure 1b).
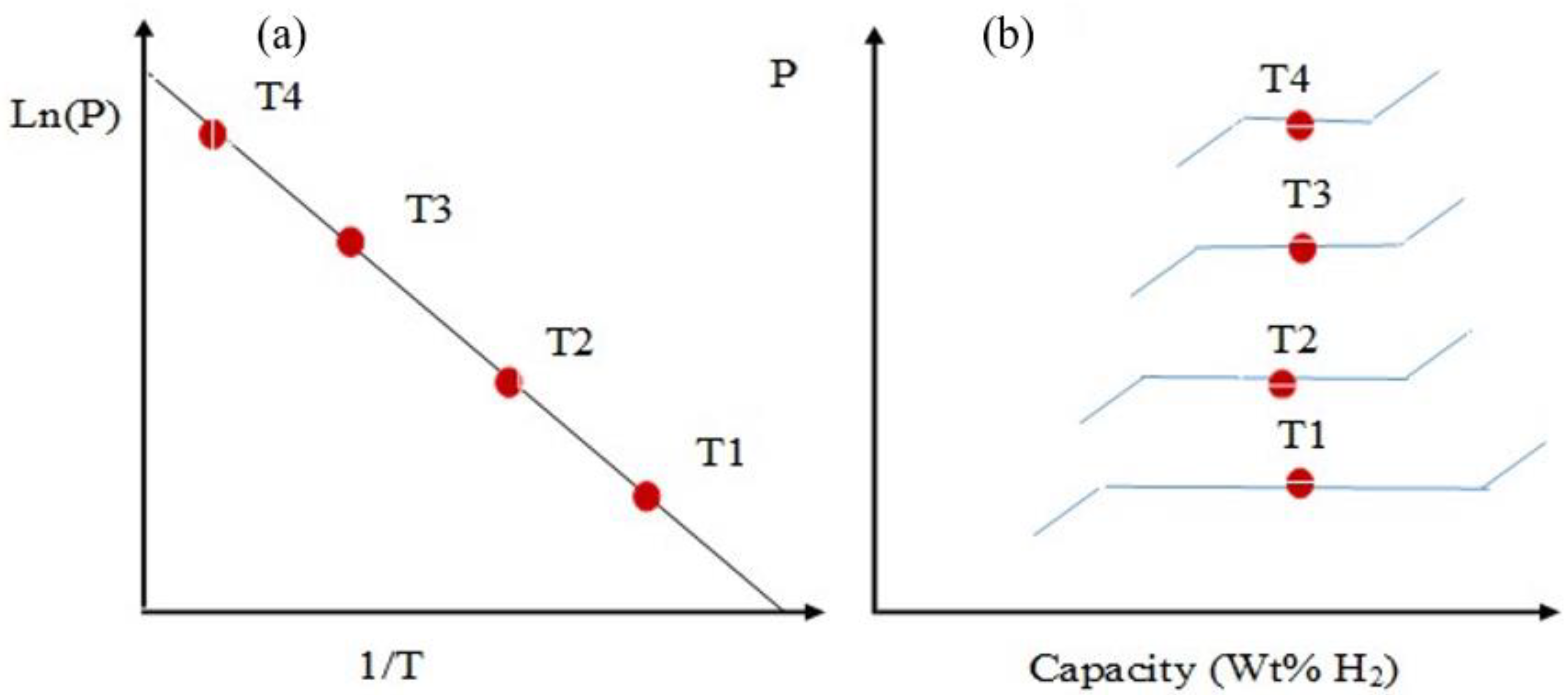
Figure 1. Van ’t Hoff diagram of metal hydride (MH) (a) and phase diagram (b).
It is necessary to consider the temperature regimes that are characteristic of the storage of liquid hydrogen. Transient heat transfer plays a leading role in multilayer insulation (MLI) in combination with a steam-cooled shield (SCS) used in liquid HS tanks. In [27], the profile of the transition temperature and the change in the heat flux of MLI and SCS were predicted and analyzed, which can help optimize the operating parameters for liquid HS.
Considering the technological aspects of HS in porous materials, a new design concept for a portable HS tank was identified [28]. Within the framework of this concept, a storage method is used in which low pressure and cryogenic temperature are realized. To maintain the cryogenic temperature, three-layer insulation was used, allowing for at least 12.5 days without the need for an external cooling circuit to maintain the optimum temperature. The HS tank is portable and can be used in various types of FS electric vehicles (FCEVs). As a tank filler, porous absorbents can be used, which form such storage conditions at which a temperature of 77 K and a pressure below 100 bar is maintained. The presented parameters are significantly lower than the internal pressure of 700 bar in commercial type IV tanks [29], thus improving safety and reducing the risk of explosion.
The safety of the use of HS tanks with TPRD under fire conditions can be improved by using composite materials [30]. HS tank model inputs include thermal parameters of the hydride and tank materials, fire heat flow into the tank, diameter TPRD, and initiation delay time TPRD. Non-stationary heat transfer from the environment through the tank wall and lining to hydrogen leads to decomposition of the composite resin for wrapping and melting of the lining. The lower limit of the diameter of the TPRD hole is sufficient to prevent the tank from bursting in the case of fire.
With respect to the option of storing hydrogen in liquid or solid form, storage in the solid state is preferable. This is due to the improvement in explosion and fire safety, and also provides better volumetric and gravimetric density, which improves the weight and size parameters of hydrogen accumulators. It should be noted that hydrogen in solid-state storage is bound by physicochemical forces [31]. The strength of the interaction between hydrogen and the carrier material varies from weak van der Waals interactions, which are characteristic of the physisorption binding of molecular hydrogen, to the strong chemisorption binding of atomic hydrogen [32]. The storage density of hydrogen can be improved by using hydride-type materials; hydrogen is packed with a HH distance of up to 170 kg/m3, which is more than twice the density of liquid hydrogen.
When storing hydrogen based on MH, it is necessary to use specialized heat exchangers or thermal control systems due to both endothermic and exothermic reactions taking place during the filling and unloading of MH tanks (Figure 2). In the absence of a heat exchanger or a suitable temperature range, the operation of the MP will have a negative influence, leading to the instability of the supply of hydrogen in FS systems. In [33], the influence of the tank surface temperature on the hydrogen consumption and hydrogen flow characteristics for the HS system MH of an electric vehicle operating on hydrogen FC was studied. Various temperature values were provided with the help of an external heat circulation device and a heat exchanger inside the MH tank. The FC operated in a power range from 200 to 600 W, and was regulated depending on the temperature and flow rate of the pumped reservoir [34].
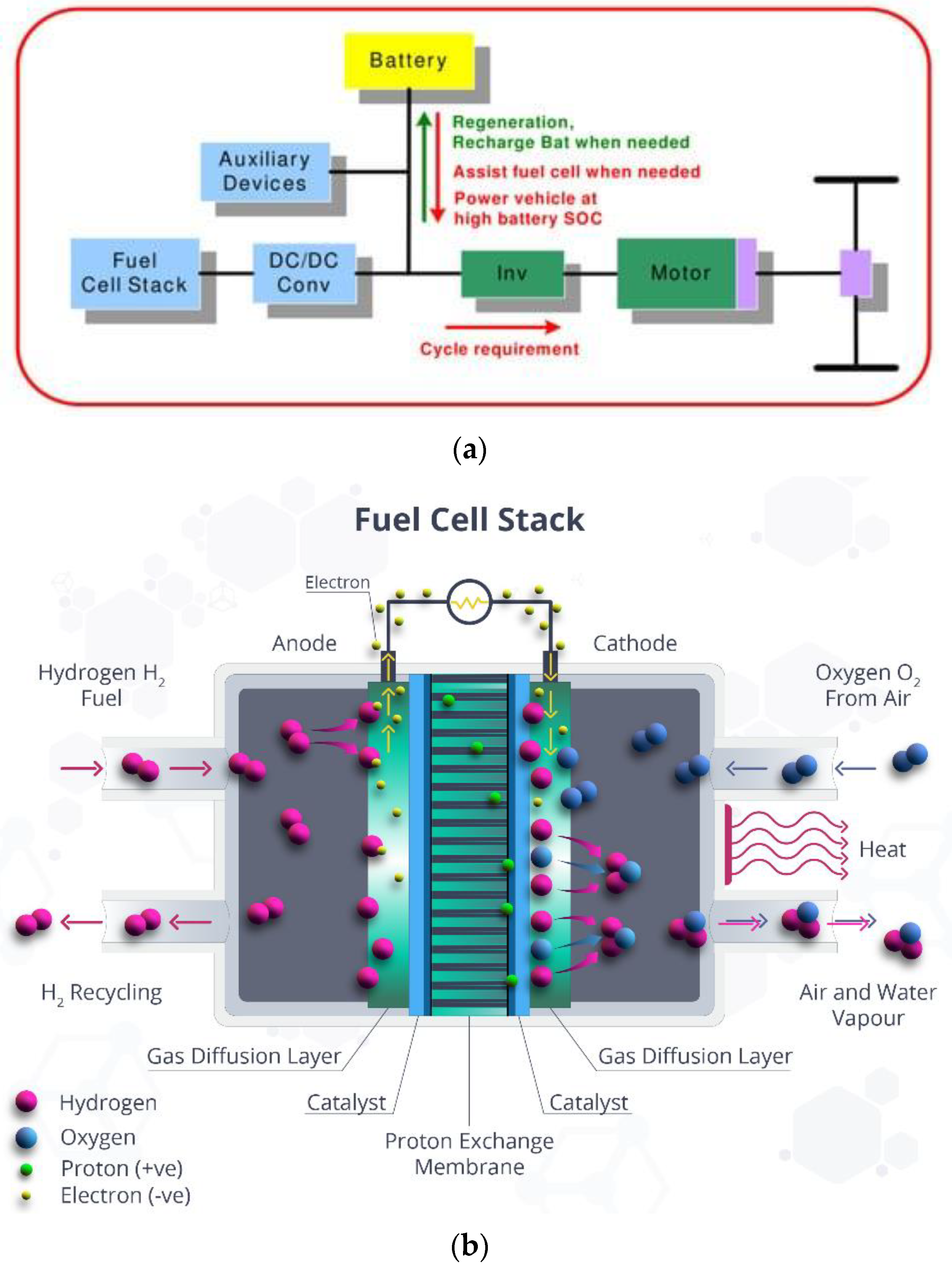
Figure 2. (a) Schematic of FC-based electric vehicle [29]. (b) The process of charging the cell with hydrogen.
MH cartridges based on the hydride of La0.75 Ce0.25 Ni5 can be used for HS [35]. The low thermal conductivity of MH is a limiting factor with respect to the technological problems of HS. To improve the thermal and physical characteristics of MH, metal foam with a porosity gradient can be used [36].
3. Composite Materials for HS—Thermoset Composites
The safe storage of a reasonable amount of hydrogen is associated with many problems related to the method and materials. Hydrogen accumulator materials can be of different types:
-
Dissociative material in which molecular hydrogen is dissociated into hydrogen atoms occupying internodes;
-
Materials with chemically bonded hydrogen;
-
Materials that adsorb molecular hydrogen, in which molecular hydrogen attaches to the surface due to weak interactions, such as the Van der Waals force or physical sorption.
The ability of certain materials to accumulate hydrogen depends on the structure and type of interaction with hydrogen. There are some new materials for HS. Storage of hydrogen in solid form can be briefly divided into the following categories:
-
MHs;
-
Hydrides based on light metals;
-
Chemical hydrides (complex hydrides);
-
Nanostructured materials (adsorption of molecular hydrogen).
Intermetallic compounds can be used as hydrogen accumulators [37][38]. This is due to the peculiarities of their atomic structure, in which interstices with the optimal binding energy for hydrogen are observed, forming the process of absorption or desorption under conditions close to standard. In this regard, for the storage of hydrogen, the class of compounds of the Laves phase with the formula unit (AB) needs to be taken into consideration [39]. Because they change from one to the other on heating and cooling (usually C14 at high temperatures and C15 at low temperatures), hydrogen absorption–desorption can be thought of as a phase change process. Structure C15 is an fcc structure containing six atoms per unit cell, while structures C14 and C36 are hexagonal structures containing 12 and 24 atoms per unit cell. Figure 3 demonstrates the crystal structures of C14- and C15-type alloys. Ideally, the lattice parameters are closely related in each structure and between structures. However, in real MH alloys with a predominance of C14, the c/a ratio is slightly lower than the theoretical value (223--√≅1633) [40]. Three types of position are available for tetrahedral hydrogen filling positions (A2B2, AB3 and B4) for both C14 and C15 structures, as shown in Figure 3. There are no octahedral positions at all in the Laves phases, so further discussion will focus only on tetrahedral positions [41].
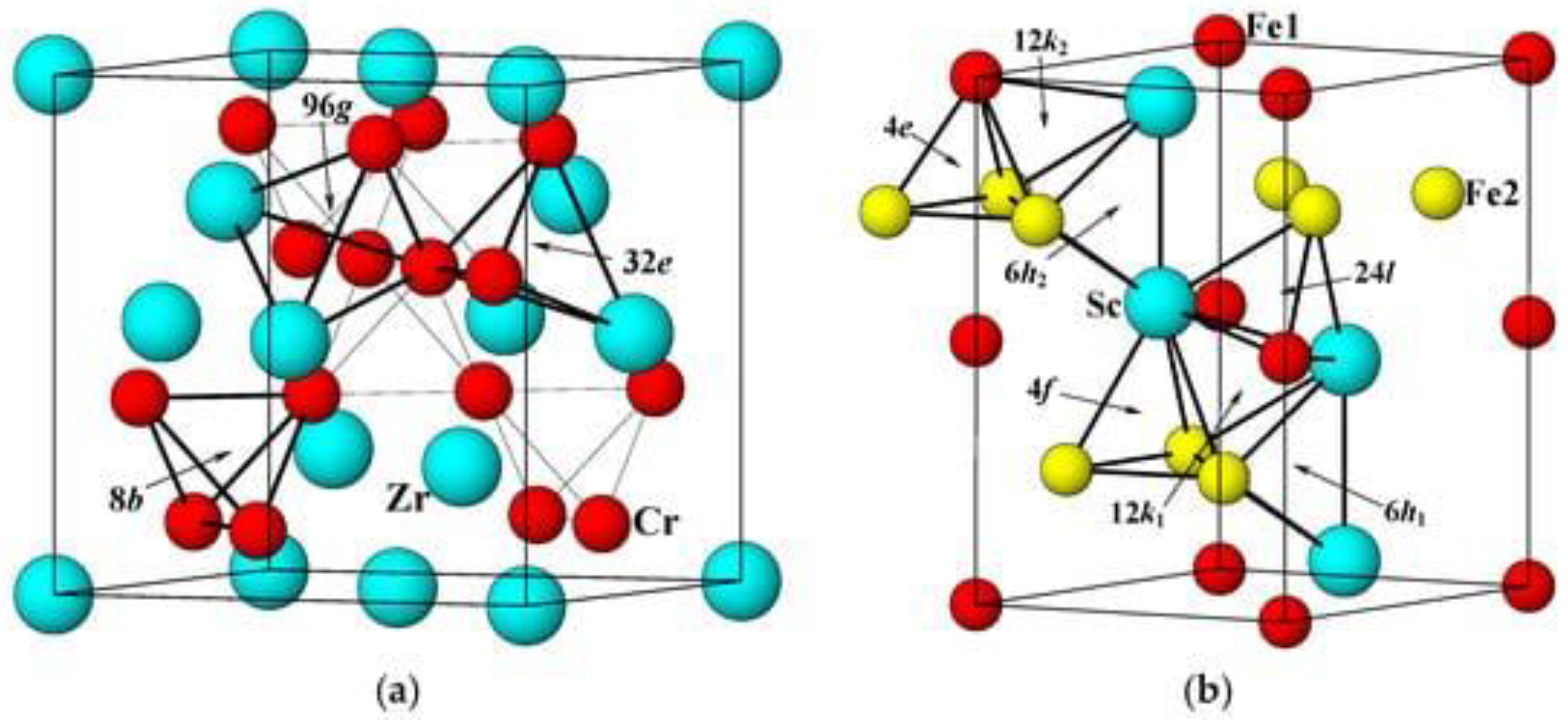
Figure 3. Elementary cells for the structures ZrCr2 (C15) (a) and ScFe2 (C14) (b). Various tetrahedral hydrogen filling sites (A2B2, AB3 and B4) are indicated by arrows [34].
High-temperature MH, such as MgH2, is considered one of the most promising HS technologies [42]. However, there are two main bottlenecks, including the low rate of H2 absorption and the low power of the MH reactor. In this regard, heat removal from the MG tank plays a crucial role in the HS process. The results show that the charging time is significantly reduced by increasing the number of air channels, as the heat transfer rate is significantly improved. When the initial coolant temperature rises, the charging time increases; however, as the Reynolds number of the coolant increases and the hydrogen inlet pressure increases, the absorption process accelerates. The recommended configuration of the heat exchanger is introduced taking into account both the loading time and production constraints. It is shown that the loading of a new multi-zone hydrogen energy storage using four air channels is approximately 30 min, which provides a more applicable hydrogen fuel system.
Ref [43] presents experimental studies concerning the absorption of H2 in a solid-state HS device based on LmNi4.91Sn0.15 with integrated cooling tubes (ECT). MH with ECT 36 and 60 loaded with 2.75 kg LmNi served as the basis for a hydrogen accumulator that implements various modes of supply pressure (10–35 bar), absorption temperature (20–30 °C) and coolant flow (2.2–30 L/min).
It has been found that at any given absorption temperature, the rate of H2 absorption and the amount of absorbed H2 increase when the H2 supply pressure rises to about 35 bar. Assuming the supply of H2 at a pressure of 35 bar and an absorption temperature of 30 °C, using oil as a coolant at a flow rate of 3.2 L/min, the maximum absorption of hydrogen is ≈1.2 wt.% for 10 min for 36 ECT and 8 min for 60 ECT. Under absorption conditions with a supply pressure of 25 bar, a water flow rate of 30 L/min and an absorption temperature of 30 °C, the absorption time in the reactor with 60 ECT was reduced to 5 min. Most metals are able to absorb hydrogen reversibly. Undoubtedly, the MG reactor (MR) is the main device used to achieve the desired stability and integrated operation of the HS system.
It has been found that at any given absorption temperature, the rate of hydrogen absorption and the amount of hydrogen absorbed increases as the hydrogen supply pressure rises to about 35 bar. Assuming a hydrogen supply pressure of 35 bar and an absorption temperature of 30 °C, using oil as the heat transfer medium at a flow rate of 3.2 L/min, the maximum absorbed hydrogen is ≈1.18 wt% per 10 min for 36 ECT and 8 min for 60 ECT. Under absorption conditions with a supply pressure of 25 bar, water flow 30 L/min and absorption temperature 30 °C, the absorption time in the reactor from 60 ECT is reduced to 5 min. The majority of metals can reversibly absorb hydrogen. Undoubtedly, the MH reactor (MHR) is the main device used to achieve the stable and integrated operation of HS systems desired.
Furthermore, each of the materials of this class in the form of nanocomposites will be considered to give a reasonable explanation for the improvement in the storage conditions of hydrogen as an energy source.
References
- Yang, Y.; Tong, L.; Yin, S.; Liu, Y.; Wang, L.; Qiu, Y.; Ding, Y. Status and challenges of applications and industry chain technologies of hydrogen in the context of carbon neutrality. J. Clean. Prod. 2022, 376, 134347.
- Nimir, W.; Al-Othman, A.; Tawalbeh, M.; Al Makky, A.; Ali, A.; Karimi-Maleh, H.; Karimi, F.; Karaman, C. Approaches towards the development of heteropolyacid-based high temperature membranes for PEM fuel cells. Int. J. Hydrogen Energy, 2021; in press.
- Modarres, M.; Kaminskiy, M.; Krivtsov, V.V. Reliability Engineering and Risk Analysis: A Practical Guide, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017.
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026.
- Shchegolkov, A.V.; Lipkin, M.S.; Shchegolkov, A.V. Preparation of WO3 Films on Titanium and Graphite Foil for Fuel Cell and Supercapacitor Applications by Electrochemical (Cathodic) Deposition Method. Russ. J. Gen. Chem. 2022, 92, 1161–1167.
- Hu, Y.; Xue, N.; Zhang, Y.; Hu, P. An eco-friendly vanadium precipitation method through solution-phase hydrogen reduction with nickel catalysis. J. Taiwan Inst. Chem. Eng. 2022, 134, 104334.
- Machado, G.; Coelho, C. Vertically-aligned carbon nanotube at low pressure by cold-wall thermal CVD using a two-phase deposition step. Carbon Trends 2021, 5, 100087.
- Chan, K.; Maznam, N.; Hazan, M.; Ahmad, R.; Sa’Ari, A.; Azman, N.; Mamat, M.; Rahman, M.; Tanemura, M.; Yaakob, Y. Multi-walled carbon nanotubes growth by chemical vapour deposition: Effect of precursor flowing path and catalyst size. Carbon Trends 2022, 6, 100142.
- Rejeb, O.; Alirahmi, S.M.; Assareh, E.; Assad, M.E.H.; Jemni, A.; Bettayeb, M.; Ghenai, C. Innovative integrated solar powered polygeneration system for green Hydrogen, Oxygen, electricity and heat production. Energy Convers. Manag. 2022, 269, 116073.
- Speight, J.G. Effects in Refining. In High Acid Crudes; Gulf Professional Publishing: Houston, TX, USA, 2014; Chapter 4; pp. 77–109.
- Jafari, H.; Safarzadeh, S.; Azad-Farsani, E. Effects of governmental policies on energy-efficiency improvement of hydrogen fuel cell cars: A game-theoretic approach. Energy 2022, 254, 124394.
- Ku, A.Y.; Reddi, K.; Elgowainy, A.; McRobie, J.; Li, J. Liquid pump-enabled hydrogen refueling system for medium and heavy duty fuel cell vehicles: Station design and technoeconomic assessment. Int. J. Hydrogen Energy 2022, 47, 25486–25498.
- Boretti, A. Comparison of fuel economies of high efficiency diesel and hydrogen engines powering a compact car with a flywheel based kinetic energy recovery systems. Int. J. Hydrogen Energy 2010, 35, 8417–8424.
- Coleman, D.; Kopp, M.; Wagner, T.; Scheppat, B. The value chain of green hydrogen for fuel cell buses—A case study for the Rhine-Main area in Germany. Int. J. Hydrogen Energy 2020, 45, 5122–5133.
- Charters, D. A comparison of energy vectors in powering hybrid buses. Renew. Energy Focus 2016, 17, 73–74.
- Emonts, B.; Schiebahn, S.; Görner, K.; Lindenberger, D.; Markewitz, P.; Merten, F.; Stolten, D. Re-energizing energy supply: Electrolytically-produced hydrogen as a flexible energy storage medium and fuel for road transport. J. Power Sources 2017, 342, 320–326.
- Zhao, K.; Fang, X.; Cui, C.; Kang, S.; Zheng, A.; Zhao, Z. Co-production of syngas and H2 from chemical looping steam reforming of methane over anti-coking CeO2/La0.9Sr0.1Fe1−xNixO3 composite oxides. Fuel 2022, 317, 123455.
- Peláez-Peláez, S.; Colmenar-Santos, A.; Pérez-Molina, C.; Rosales, A.-E.; Rosales-Asensio, E. Techno-economic analysis of a heat and power combination system based on hybrid photovoltaic-fuel cell systems using hydrogen as an energy vector. Energy 2021, 224, 120110.
- Karimkashi, S.; Kahila, H.; Kaario, O.; Larmi, M.; Vuorinen, V. Numerical study on tri-fuel combustion: Ignition properties of hydrogen-enriched methane-diesel and methanol-diesel mixtures. Int. J. Hydrogen Energy 2020, 45, 4946–4962.
- Wang, H.; Ji, C.; Shi, C.; Yang, J.; Ge, Y.; Wang, S.; Chang, K.; Meng, H.; Wang, X. Parametric modeling and optimization of the intake and exhaust phases of a hydrogen Wankel rotary engine using parallel computing optimization platform. Fuel 2022, 324, 124381.
- Gomez, A.; Smith, H. Liquid hydrogen fuel tanks for commercial aviation: Structural sizing and stress analysis. Aerosp. Sci. Technol. 2019, 95, 105438.
- Shi, C.; Zhang, Z.; Ji, C.; Li, X.; Di, L.; Wu, Z. Potential improvement in combustion and pollutant emissions of a hydrogen-enriched rotary engine by using novel recess configuration. Chemosphere 2022, 299, 134491.
- Collodi, G. Hydrogen Production via Steam Reforming with CO2 Capture. Chem. Eng. Trans. 2010, 19, 37–42.
- Santhanam, K.S.; Press, R.J.; Miri, M.J.; Bailey, A.V.; Takacs, G.A. Introduction to Hydrogen Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017.
- Takeda, S.; Nam, H.; Chapman, A. Low-carbon energy transition with the sun and forest: Solar-driven hydrogen production from biomass. Int. J. Hydrogen Energy 2022, 47, 24651–24668.
- Lakhlifi, A.; Dahoo, P.R.; Picaud, S.; Mousis, O. A simple van’t Hoff law for calculating Langmuir constants in clathrate hydrates. Chem. Phys. 2015, 448, 53–60.
- Jiang, W.; Sun, P.; Li, P.; Zuo, Z.; Huang, Y. Transient thermal behavior of multi-layer insulation coupled with vapor cooled shield used for liquid hydrogen storage tank. Energy 2021, 231, 120859.
- Nguyen, D.H.; Kim, J.H.; Vo, T.T.N.; Kim, N.; Ahn, H.S. Design of portable hydrogen tank using adsorption material as storage media: An alternative to Type IV compressed tank. Appl. Energy 2022, 310, 118552.
- Su, Y.; Lv, H.; Zhou, W.; Zhang, C. Review of the Hydrogen Permeability of the Liner Material of Type IV On-Board Hydrogen Storage Tank. World Electr. Veh. J. 2021, 12, 130.
- Molkov, V.; Dadashzadeh, M.; Kashkarov, S.; Makarov, D. Performance of hydrogen storage tank with TPRD in an engulfing fire. Int. J. Hydrogen Energy 2021, 46, 36581–36597.
- Ahamed, M.I.; Shakeel, N.; Anwar, N.; Khan, A.; Asiri, A.M.; Dzudzevic-Cancar, H. 4—Graphene-based nanocomposite for hydrogen storage application. In Micro and Nano Technologies, Nanomaterials for Hydrogen Storage Applications; Sen, F., Khan, A., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–78. ISBN 9780128194768.
- Zhang, L.; Ren, D.; Ding, W. High hydrogen storage ability of a decorated g-C3N4 monolayer decorated with both Mg and Li: A density functional theory (DFT) study. Int. J. Hydrogen Energy 2022, 47, 28548–28555.
- Özdoğan, E.; Hüner, B.; Süzen, Y.O.; Eşiyok, T.; Uzgören, I.N.; Kıstı, M.; Uysal, S.; Selçuklu, S.B.; Demir, N.; Kaya, M.F. Effects of tank heating on hydrogen release from metal hydride system in VoltaFCEV Fuel Cell Electric Vehicle. Int. J. Hydrogen Energy, 2022; in press.
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296.
- Malleswararao, K.; Aswin, N.; Kumar, P.; Dutta, P.; Murthy, S.S. Experiments on a novel metal hydride cartridge for hydrogen storage and low temperature thermal storage. Int. J. Hydrogen Energy 2022, 47, 16144–16155.
- Bai, X.-S.; Yang, W.-W.; Yang, Y.-J.; Zhang, K.-R.; Yang, F.-S. Multi-variable optimization of metal hydride hydrogen storage reactor with gradient porosity metal foam and evaluation of comprehensive performance. Int. J. Hydrogen Energy 2022, 47, 35340–35351.
- Banerjee, S.; Mukhopadhyay, P. Interstitial Ordering. In Pergamon Materials Series; Elsevier: Pergamon, Turkey, 2007; Chapter 8; Volume 12, pp. 717–781.
- Matysina, Z.A.; Gavrylyuk, N.A.; Kartel, M.T.; Veziroglu, A.; Veziroglu, T.N.; Pomytkin, A.P.; Schur, D.V.; Ramazanov, T.S.; Gabdullin, M.T.; Zolotarenko, A.D.; et al. Hydrogen sorption properties of new magnesium intermetallic compounds with MgSnCu4 type structure. Int. J. Hydrogen Energy 2021, 46, 25520–25532.
- Chang, S.; Young, K.-H.; Ouchi, T.; Meng, T.; Nei, J.; Wu, X. Studies on Incorporation of Mg in Zr-Based AB2 Metal Hydride Alloys. Batteries 2016, 2, 11.
- Young, K.-H.; Nei, J.; Wan, C.; Denys, R.V.; Yartys, V.A. Comparison of C14- and C15-Predomiated AB2 Metal Hydride Alloys for Electrochemical Applications. Batteries 2017, 3, 22.
- Eisapour, A.H.; Eisapour, M.; Talebizadehsardari, P.; Walker, G.S. An innovative multi-zone configuration to enhance the charging process of magnesium based metal hydride hydrogen storage tank. J. Energy Storage 2021, 36, 102443.
- Anbarasu, S.; Muthukumar, P.; Mishra, S.C. Tests on LmNi4.91Sn0.15 based solid state hydrogen storage device with embedded cooling tubes—Part A: Absorption process. Int. J. Hydrogen Energy 2014, 39, 3342–3351.
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
944
Revisions:
2 times
(View History)
Update Date:
23 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




