| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stephan Ludwig | + 1846 word(s) | 1846 | 2020-12-07 04:27:50 | | | |
| 2 | Bruce Ren | Meta information modification | 1846 | 2020-12-15 03:33:49 | | |
Video Upload Options
Medical research is changing into direction of precision therapy, thus, sophisticated preclinical models are urgently needed. In human pathogenic virus research, the major technical hurdle is not only to translate discoveries from animals to treatments of humans, but also to overcome the problem of interspecies differences with regard to productive infections and comparable disease development. Transgenic mice provide a basis for research of disease pathogenesis after infection with human-specific viruses. Today, humanized mice can be found at the very heart of this forefront of medical research allowing for recapitulation of disease pathogenesis and drug mechanisms in humans.
1. Introduction
In times of a global pandemic, it is more obvious than ever that human pathogenic viruses are a major threat to global health. The emergence of human pathogenic viruses such as SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) or Zika virus highlights the urgent need of representative preclinical models to study virus entry, replication and spread within an organism as tools to develop efficient antiviral therapies and vaccinations. Aside from that, also several well-known human pathogenic viruses that are characterized in molecular detail like influenza virus, human immunodeficiency virus (HIV) or hepatitis viruses still remain a huge threat to public health.
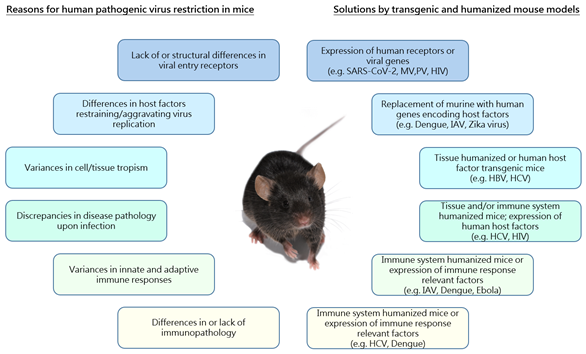
Mechanisms of infection, replication and disease pathology differ widely among different viruses. Suitability of cell culture systems or organoids to obtain comprehensive knowledge about viral characteristics or to test antiviral approaches is highly limited due to the lack of a functional immune system and vasculature. Thus, animal models still represent a unique opportunity to study viral replication processes and antiviral agents in vivo. Rodent models, and especially mice, are widely accepted and the preferred preclinical systems as they are readily available and exhibit low maintenance costs. However, viruses coevolve with their host organisms and, thus, often show a restricted species specificity frequently complicating the research of human pathogenic viruses in mouse models. This narrow host range is often based on the lack of surface entry receptors necessary for virus infection or due to remarkable differences in murine and human innate immune responses upon viral infection. Accompanying this, tissue tropism of human pathogenic viruses can differ significantly in mice causing differences in virus-induced pathology (Figure 1). Hence, mouse models often demonstrate limited susceptibility or permissiveness and, thus, results obtained from studies in murine models cannot necessarily be extrapolated to humans, which particularly complicates the validity of drug development and vaccination studies.
Figure 1. Reasons underlying restricted species specificity of human pathogenic viruses and solutions provided by the use of transgenic and humanized mouse models. Schematic overview of limitations in the use of mouse models for research of human pathogenic viruses (left) and examples of different transgenic/humanized mouse models providing solutions to overcome the respective restrictions (right). Associated boxes (limitations/solutions) are indicated by the use of the same color. MV: Measles virus; PV: Poliovirus; HIV: Human immunodeficiency virus; IAV: Influenza A virus; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Genetically modified and transgenic mouse models represent a sophisticated possibility to overcome interspecies differences in human pathogenic virus research such as species specificity and tissue tropism, allowing to investigate the function of specific human entry receptors, host factors or innate and adaptive immune responses in immunocompetent organisms. These models were of significant importance for scientific progress within the last decades and provided immeasurable insights into virus biology and disease pathogenesis. A decisive milestone was the development of immune system or tissue-humanized mouse models facilitating infection and replication of human pathogenic viruses in a humanized system. With recent advances in human cell or tissue engraftment in transgenic humanized mice, antiviral strategies and vaccines against many human pathogenic viruses already have been or will be evaluated in the future, towards identification of new drug innovations to treat and control human pathogenic infectious diseases.
This review aims to provide an overview of different options to genetically engineer and humanize mice. We will discuss several examples of human pathogenic viruses that have been studied in translational, human-relevant preclinical systems (see Table 1). 2. Transgenic Mice Expressing Viral Genes
Due to the absence of permissive mouse models for several human viruses, transgenic mouse strains were developed incorporating essential individual viral genes or even whole virus genomes within their own genome to investigate virus pathogenicity and characterize specific properties of viral genes.
Some of the most intensively studied transgenic mouse models expressing viral genomes have been generated to analyze hepatitis B and C virus (HBV, HCV)-induced liver pathology and virus replication. Hepatitis B and C infection share a common phenotype of liver pathology induced by chronic necroinflammation resulting in liver cirrhosis and hepatocellular carcinoma (HCC). HBV cccDNA (covalently closed circular DNA) persists in infected host cells and the virus can uniquely integrate its genome into the DNA of the infected cell. Most integration events retain the HBx antigen [1]. In sharp contrast, HCV is a positive-strand RNA virus that replicates through a negative-stranded intermediate in the cytoplasm. Persistence of HCV is thus facilitated by RNA intermediates that remain in infected host cells. Since murine hepatocytes do not express HBV- and HCV-specific receptors, intrahepatic infection and virus spread cannot be analyzed in wild type mice [2][3].
In order to study the role of individual HBV genes in the development of hepatopathology, immunotolerant HBV-transgenic mice were generated by germline modifications. While the expression of the viral envelope protein did not induce hepatocellular cytopathology, adoptive transfer of virus–antigen primed splenocytes resulted in hepatocellular injury and immunopathology caused by MHC I-restricted antigen-presentation to CD8+ T lymphocytes and envelope-specific antibodies [4]. These data provided the first hints for virus-induced immunopathology based on adaptive immune responses directed against hepatocytes expressing viral genes. Many transgenic lineages that express individual HBV genes such as the envelope [5][6][7], core [8], precore [9] and X [10][11] genes under the control of HBV- or murine liver-specific promoters have been described in the last decades. This led to substantial improvement in understanding the properties of the respective viral genes. Afterwards, transgenic mouse models were generated expressing the full viral replicon, which resulted in high-yield HBV replication in murine kidneys and liver [12]. Using these HBV transgenic mice, efficacy of antiviral agents such as HBV-specific inhibitors, small interfering RNAs (siRNAs), and cytokines have been tested [13][14][15][16]. Other transgenic mice, induced by microinjection of viral persistence-inducing replication intermediate cccDNA [17][18] or by adeno-associated virus in vivo transduction of the HBV replicon, were used to study antiviral agents or vaccination approaches for acute or chronic infections [19][20]. More recent approaches used HBV transgenic mice to investigate the therapeutic potential of CRISPR/Cas9 techniques for use in chronic HBV gene therapy [21][22].
Several groups have generated transgenic mice expressing individual HCV proteins or viral polyproteins to study the effect on liver pathology, steatosis and HCC manifestations [23][24][25][26][27][28][29]. However, since the overexpression of HCV proteins in these transgenic mice differs compared to low level expression during natural infection, it is unclear whether the obtained findings are transferable to the pathology observed in humans [30]. Along that line, it was shown that inflammation-associated hepatocarcinogenesis depends on the host genetic background of the respective transgenic mouse model [31]. Thus, these models helped to elucidate the pathophysiology of HCV gene products, but they are limited by their inability to support HCV replication based on the lack of negative-stranded RNA expression. Compared to transgenic mouse models for HBV, expression of the HCV genome did not result in viral progeny production or virus genome replication. Thus, these models do not allow investigation of antiviral agents or vaccination approaches.
Since murine hepatocytes do not express the respective virus entry receptors for HBV and HCV, the abovementioned studies did neither allow for virus infection studies nor resemble the whole infection process via the natural route and virus spread as observed within human tissues. Though limited in their applicability for virus infection studies, these models were groundbreaking for initial hepatotropic virus research related to the mechanisms of HBV and HCV pathogenesis.
In line with HBV and HCVtg mice, several attempts have been made to generate HIVtg mice resulting in substantial understanding of virus–host interactions and clinical manifestation of acquired immune deficiency syndrome (AIDS) symptoms or the development of Kaposi’s sarcoma [32][33][34][35][36]]. However, HIVtg mice showed discrepancies in tissue- and immunopathology (reviewed in [37]). Along that line, mice do neither express respective entry receptors nor the co-factors needed for efficient viral replication, and, thus, do not allow analyses of virus replication, antiviral strategies or vaccination approaches [38].
2. Future Directions
Successful establishment of transgenic and especially transgenic humanized mice provided immeasurable opportunities to advance medical research on infectious diseases that were previously inaccessible. The transgenic mouse models described in this review are, amongst others, groundbreaking for the understanding of human pathogenic virus infections and their disease pathology. Along that line, these mouse models provide the possibility to study emerging virus-induced diseases, as discussed for SARS-CoV-2. Since some viruses have the unique ability to cross species boundaries and adapt to new hosts, humanized mouse models will continue to play a crucial role in understanding newly emerging or human-adapting viruses, in the future. However, existing models are still far from being perfect. A mouse model that can be applied to study all known and newly emerging human pathogenic viruses while completely mimicking virus-induced disease pathogenesis does not exist and researchers still need to consider the scientific questions in the respective areas of the disease to select the best suitable animal model. While virus entry receptor or antiviral host factor humanized mice are a great model to study virus infection, replication and the role of single human genes involved in antiviral immune responses, they are not suitable to evaluate disease pathogenesis or efficacy of antiviral approaches as the complexity of human immune responses is not necessarily reflected. Especially the analysis of possible vaccination candidates or antiviral approaches additionally requires immune system humanized mice. Amongst these, HUMAMICE are highly sophisticated humanized models that allow e.g., to study antigen specificity of human immune cells, however, without additional humanization, these animals are not susceptible for infection with human pathogenic viruses such as SARS-CoV-2.
The ideal future model for most human pathogenic virus infections will be an animal model with a reconstituted human immune system presenting antigens in the human HLA context, constitutively expressing human cytokines necessary for efficient immune cell development. In addition, specific human organs or humanized cell types would be needed to mimic viral transmission routes and disease pathology. Such a model does not exist yet, but with the current advances in technology it might be only a matter of time to have it available to study human specific virus infection complexity and to develop more efficient vaccine and treatment strategies. Furthermore, there still is the sustained need to identify and replace human-specific factors that are absent in mice but are needed for optimal human cell differentiation and function to decrease or even prevent the development of graft versus host disease that still occurs in many of the human immune system engrafted models.
Thus, key improvements in specific areas are still required, however, with careful characterization, the future will yield more appropriate preclinical models, supporting more rapid and relevant translational drug discovery and development.
References
- Feitelson, M.A.; Lee, J. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007, 252, 157–170.
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 2012, doi:10.7554/eLife.00049.
- Li, H.; Zhuang, Q.; Wang, Y.; Zhang, T.; Zhao, J.; Zhang, Y.; Zhang, J.; Lin, Y.; Yuan, Q.; Xia, N.; et al. HBV life cycle is restricted in mouse hepatocytes expressing human NTCP. Cell. Mol. Immunol. 2014, 11, 175–183, doi:10.1038/cmi.2013.66.
- Moriyama, T.; Guilhot, S.; Klopchin, K.; Moss, B.; Pinkert, C.A.; Palmiter, R.D.; Brinster, R.L.; Kanagawa, O.; Chisari, F.V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science 1990, 248, 361–364, doi:10.1126/science.1691527.
- Babinet, C.; Farza, H.; Morello, D.; Hadchouel, M.; Pourcel, C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science 1985, 230, 1160–1163, doi:10.1126/science.3865370.
- Burk, R.D.; DeLoia, J.A.; elAwady, M.K.; Gearhart, J.D. Tissue preferential expression of the hepatitis B virus (HBV) surface antigen gene in two lines of HBV transgenic mice. J. Virol. 1988, 62, 649–654, doi:10.1128/jvi.62.2.649-654.1988.
- Chisari, F.V.; Pinkert, C.A.; Milich, D.R.; Filippi, P.; Mclachlan, A.; Palmiter, R.D.; Brinster, R.L. A Transgenic Mouse Model of the Chronic Hepatitis B Surface Antigen Carrier State. Science 1985, 230, 1157–1160.
- Guidotti, L.G.; Martinez, V.; Loh, Y.T.; Rogler, C.E.; Chisari, F.V. Hepatitis B virus nucleocapsid particles do not cross the hepatocyte nuclear membrane in transgenic mice. J. Virol. 1994, 68, 5469–5475, doi:10.1128/jvi.68.9.5469-5475.1994.
- Milich, D.R.; Jones, J.E.; Hughes, J.L.; Prices, J.; Raney, A.K.; Mclachlan, A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. USA 1990, 87, 6599–6603.
- Kim, C.M.; Koike, K.; Saito, I.; Miyamura, T.; Jay, G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature 1991, 351, 317–320, doi:10.1038/351317a0.
- Lee, T.H.; Finegold, M.J.; Shen, R.F.; DeMayo, J.L.; Woo, S.L.; Butel, J.S. Hepatitis B virus transactivator X protein is not tumorigenic in transgenic mice. J. Virol. 1990, 64, 5939–5947, doi:10.1128/jvi.64.12.5939-5947.
- Guidotti, L.G.; Matzke, B.; Schaller, H.; Chisari, F.V. High-level hepatitis B virus replication in transgenic mice. J. Virol. 1995, 69, 6158–6169, doi:10.1128/jvi.69.10.6158-6169.
- Weber, O.; Schlemmer, K.H.; Hartmann, E.; Hagelschuer, I.; Paessens, A.; Graef, E.; Deres, K.; Goldmann, S.; Niewoehner, U.; Stoltefuss, J.; et al. Inhibition of human hepatitis B virus (HBV) by a novel non-nucleosidic compound in a transgenic mouse model. Antivir. Res. 2002, 54, 69–78, doi:10.1016/S0166-3542(01)00216-9.
- Wieland, S.F.; Guidotti, L.G.; Chisari, F.V. Intrahepatic Induction of Alpha/Beta Interferon Eliminates Viral RNA-Containing Capsids in Hepatitis B Virus Transgenic Mice. J. Virol. 2000, 74, 4165–4173, doi:10.1128/jvi.74.9.4165-4173.2000.
- Julander, J.G.; Colonno, R.J.; Sidwell, R.W.; Morrey, J.D. Characterization of antiviral activity of entecavir in transgenic mice expressing hepatitis B virus. Antivir. Res. 2003, 59, 155–161, doi:10.1016/S0166-3542(03)00109-8.
- Hwang, J.-R.; Park, S.-G. Mouse models for hepatitis B virus research. Lab. Anim. Res. 2018, 34, 85, doi:10.5625/lar.2018.34.3.85.
- Yang, P.L.; Althage, A.; Chung, J.; Chisari, F.V. Hydrodynamic injection of viral DNA: A mouse model of acute hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 2002, 99, 13825–13830, doi:10.1073/pnas.202398599.
- Huang, L.-R.; Wu, H.-L.; Chen, P.-J.; Chen, D.-S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 2006, 103, 17862–17867.
- Dion, S.; Bourgine, M.; Godon, O.; Levillayer, F.; Michel, M.-L. Adeno-Associated Virus-Mediated Gene Transfer Leads to Persistent Hepatitis B Virus Replication in Mice Expressing HLA-A2 and HLA-DR1 Molecules. J. Virol. 2013, 87, 5554–5563, doi:10.1128/jvi.03134-12.
- Yang, D.; Liu, L.; Zhu, D.; Peng, H.; Su, L.; Fu, Y.X.; Zhang, L. A mouse model for HBV immunotolerance and immunotherapy. Cell. Mol. Immunol. 2014, 11, 71–78, doi:10.1038/cmi.2013.43.
- Lin, S.R.; Yang, H.C.; Kuo, Y.T.; Liu, C.J.; Yang, T.Y.; Sung, K.C.; Lin, Y.Y.; Wang, H.Y.; Wang, C.C.; Shen, Y.C.; et al. The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Mol. Ther. Nucleic Acids 2014, 3, e186, doi:10.1038/mtna.2014.38.
- Li, H.; Sheng, C.; Liu, H.; Wang, S.; Zhao, J.; Yang, L.; Jia, L.; Li, P.; Wang, L.; Xie, J.; et al. Inhibition of HBV expression in HBV transgenic mice using AAV-delivered CRISPR-SaCas9. Front. Immunol. 2018, 9, 1–9, doi:10.3389/fimmu.2018.02080.
- Frelin, L.; Brenndörfer, E.D.; Ahlén, G.; Weiland, M.; Hultgren, C.; Alheim, M.; Glaumann, H.; Rozell, B.; Milich, D.R.; Bode, J.G.; et al. The hepatitis C virus and immune evasion: Non-structural 3/4A transgenic mice are resistant to lethal tumour necrosis factor α mediated liver disease. Gut 2006, 55, 1475–1483, doi:10.1136/gut.2005.085050.
- Kawamura, T.; Furusaka, A.; Koziel, M.J.; Chung, R.T.; Wang, T.C.; Schmidt, E.V.; Liang, T.J. Transgenic expression of hepatitis C virus structural proteins in the mouse. Hepatology 1997, 25, 1014–1021, doi:10.1002/hep.510250437.
- Koike, K.; Moriya, K.; Ishibashi, K.; Matsuura, Y.; Suzuki, T.; Saito, I.; Iino, S.; Kurokawa, K.; Miyamura, T. Expression of hepatitis C virus envelope proteins in transgenic mice. J. Gen. Virol. 1995, 76, 3031–3038, doi:10.1099/0022-1317-76-12-3031.
- Alonzi, T.; Agrati, C.; Costabile, B.; Cicchini, C.; Amicone, L.; Cavallari, C.; della Rocca, C.; Folgori, A.; Fipaldini, C.; Poccia, F.; et al. Steatosis and intrahepatic lymphocyte recruitment in hepatitis C virus transgenic mice. J. Gen. Virol. 2004, 85, 1509–1520, doi:10.1099/vir.0.19724-0.
- Lerat, H.; Honda, M.; Beard, M.R.; Loesch, K.; Sun, J.; Yang, Y.; Okuda, M.; Gosert, R.; Xiao, S.Y.; Weinman, S.A.; et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology 2002, 122, 352–365, doi:10.1053/gast.2002.31001.
- Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998 Sep;4(9):1065-7. doi: 10.1038/2053. PMID: 9734402.
- Jia, F.; Diao, P.; Wang, X.; Hu, X.; Kimura, T.; Nakamuta, M.; Nakamura, I.; Shirotori, S.; Sato, Y.; Moriya, K.; et al. Dietary Restriction Suppresses Steatosis-Associated Hepatic Tumorigenesis in Hepatitis C Virus Core Gene Transgenic Mice. Liver Cancer 2020, 9, 529–548, doi:10.1159/000508308.
- Burm, R.; Collignon, L.; Mesalam, A.A.; Meuleman, P. Animal models to study hepatitis C virus infection. Front. Immunol. 2018, 9, 1032, doi:10.3389/fimmu.2018.01032.
- Klopstock, N.; Katzenellenbogen, M.; Pappo, O.; Sklair-Levy, M.; Olam, D.; Mizrahi, L.; Potikha, T.; Galun, E.; Goldenberg, D. HCV tumor promoting effect is dependent on host genetic background. PLoS ONE 2009, 4, e5025, doi:10.1371/journal.pone.0005025.
- Hanna, Z.; Kay, D.G.; Cool, M.; Jothy, S.; Rebai, N.; Jolicoeur, P. Transgenic Mice Expressing Human Immunodeficiency Virus Type 1 in Immune Cells Develop a Severe AIDS-Like Disease. J. Virol. 1998, 72, 121, doi:10.1128/JVI.72.1.121-132.1998.
- Leonard, J.M.; Abramczuk, J.W.; Pezen, D.S.; Rutledge, R.; Belcher, J.H.; Hakim, F.; Shearer, G.; Lamperth, L.; Travis, W.; Fredrickson, T.; et al. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 1988, 242, 1665, doi:10.1126/science.3201255.
- Goudreau, G.; Carpenter, S.; Beaulieu, N.; Jolicoeur, P. Vacuolar myelopathy in transgenic mice expressing human immunodeficiency virus type 1 proteins under the regulation of the myelin basic protein gene promoter. Nat. Med. 1996, 2, 655–661, doi:10.1038/nm0696-655.
- Vogel, J.; Hinrichs, S.H.; Reynolds, R.K.; Luciw, P.A.; Jay, G. The HIV tat gene induces dermal lesions resembling Kaposi’s sarcoma in transgenic mice. Nature 1988, 335, 606–611, doi:10.1038/335606a0.
- Brady, H.J.; Abraham, D.J.; Pennington, D.J.; Miles, C.G.; Jenkins, S.; Dzierzak, E.A. Altered cytokine expression in T lymphocytes from human immunodeficiency virus Tat transgenic mice. J. Virol. 1995, 69, 7622–7629.
- Hatziioannou, T.; Evans, D.T. Animal models for HIV/AIDS research. Nat. Rev. Microbiol. 2012, 10, 852–867.
- Bieniasz, P.D.; Cullen, B.R. Multiple Blocks to Human Immunodeficiency Virus Type 1 Replication in Rodent Cells. J.Virol. 2000, 74, 9868–9877.