Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Said Gharby | -- | 4508 | 2022-11-18 13:25:12 | | | |
| 2 | Jessie Wu | Meta information modification | 4508 | 2022-11-21 06:12:53 | | | | |
| 3 | Jessie Wu | -18 word(s) | 4490 | 2022-11-21 06:18:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gharby, S.; Oubannin, S.; Bouzid, H.A.; Bijla, L.; Ibourki, M.; Gagour, J.; Koubachi, J.; Sakar, E.H.; Majourhat, K.; Lee, L.; et al. Antioxidant Compounds Extracted from Plants for Vegetable Oils. Encyclopedia. Available online: https://encyclopedia.pub/entry/35305 (accessed on 07 February 2026).
Gharby S, Oubannin S, Bouzid HA, Bijla L, Ibourki M, Gagour J, et al. Antioxidant Compounds Extracted from Plants for Vegetable Oils. Encyclopedia. Available at: https://encyclopedia.pub/entry/35305. Accessed February 07, 2026.
Gharby, Saïd, Samira Oubannin, Hasna Ait Bouzid, Laila Bijla, Mohamed Ibourki, Jamila Gagour, Jamal Koubachi, El Hassan Sakar, Khalid Majourhat, Learn-Han Lee, et al. "Antioxidant Compounds Extracted from Plants for Vegetable Oils" Encyclopedia, https://encyclopedia.pub/entry/35305 (accessed February 07, 2026).
Gharby, S., Oubannin, S., Bouzid, H.A., Bijla, L., Ibourki, M., Gagour, J., Koubachi, J., Sakar, E.H., Majourhat, K., Lee, L., Harhar, H., & Bouyahya, A. (2022, November 18). Antioxidant Compounds Extracted from Plants for Vegetable Oils. In Encyclopedia. https://encyclopedia.pub/entry/35305
Gharby, Saïd, et al. "Antioxidant Compounds Extracted from Plants for Vegetable Oils." Encyclopedia. Web. 18 November, 2022.
Copy Citation
Oil oxidation is the main factor limiting vegetable oils’ quality during storage, as it leads to the deterioration of oil’s nutritional quality and gives rise to disagreeable flavors. These changes make fat-containing foods less acceptable to consumers. To deal with this problem and to meet consumer demand for natural foods, vegetable oil fabricators and the food industry are looking for alternatives to synthetic antioxidants to protect oils from oxidation. In this context, natural antioxidant compounds extracted from different parts (leaves, roots, flowers, and seeds) of medicinal and aromatic plants (MAPs) could be used as a promising and sustainable solution to protect consumers’ health.
extraction
vegetable oils
MAPs
1. Medicinal and Aromatic Plants Extracts for Vegetable Oils Enrichment
Medicinal and aromatic plants (MAPs) are considered perfect sources of natural antioxidants, such as phenolic substances, usually referred to as polyphenols, which are ubiquitous components of plants and herbs [1]. More than 8000 phenolic compounds have been reported as naturally occurring substances from plants [2]. Other types of substances in plants, such as phenolic acids, phenolic triterpenes, carotenoids, diterpenes, and flavonoids, are interesting bioactive compounds with several health properties (antioxidant, antimicrobial, antifungal, and anti-inflammatory activities) [2]. MAPs serve as an indigenous source of new compounds with therapeutic value and can also be involved in drug development [3]. Herb extracts were used as natural food additives in ancient traditions to improve sensory characteristics thanks to their health properties. The principal components found in plants correspond to four important biochemical classes namely polyphenols, terpenes, glycosides, and alkaloids [4], and many natural antioxidant compounds. These are now used in medical and pharmaceutical products as substitutes for artificial antioxidants, which are suspected to be a major cause of carcinogenesis [2]. The use of MAPs in foods is an excellent strategy to enhance the flavor and the aroma of various foods since plant extracts are rich in phytochemicals, which are of particular importance due to their health-promoting effects [4][5]. Plants extracts have been exploited to enrich vegetable oils (VOs) with natural antioxidants, as discussed in Salta et al. [6]. For instance, oregano in cottonseed oil, rosemary, and sage extracts in both palm oil and rapeseed oil, ethanolic extract of summer savory in sunflower oil, methanolic extract of tea leaves and oat extracts in cottonseed oil, and spinach powder in soybean oil. Likewise, leafy vegetable extracts (cabbage, coriander leaves, hongone, and spinach) in sunflower, as well as olive leaves, which are very studied to enrich edible oils such as olive oil [6], virgin olive oil [7], and other VOs (sunflower, soybean, palm, etc.) [6][8]. Olive leaves are rich in oleuropein a natural product of the secoiridoid group [9], known for its blood pressure-lowering effect and most abundant phenolic compounds in olive leaves [10][11]. Many studies were conducted to enrich oils with olive leaf extracts [12][13][14]. Extracts from species belonging to the Lamiaceae family have been reported in several studies for their antioxidative activity [15]. Rosemary was used in traditional medicine as a stimulant and mild analgesic, and it has been considered one of the most functional herbs for treating poor circulation, inflammatory diseases, headaches, and physical and mental fatigue [5]. Its extracts have been used in food preservation, as they prevent oxidation and microbial contamination [15] and also as an additive to enrich VOs, rosemary extract’s effectiveness was evaluated generally for oils during deep fat-fraying by oils such as soybean and palm oils [16][17] and also for a mixture of sunflower, soybean, and palm oils [18]. Thymus species are well recognized for their antispasmodic, sedative, antioxidant, and antibacterial characteristics and are frequently used in the food sector as herbal teas, aromatic, flavoring agents (condiment and spice), and medicinal plants. The preservative effect of thyme (Thymus schimperi R.) was evaluated on soybean oil, butter, and meat, and it was found to increase the induction time of the foods [19]. Phenolic acids, flavonoids, and phenolic monoterpenes, bioactive compounds from thyme extract were used to flavor corn refined oil enhancing its oxidative stability and antioxidant activity [20]. Oregano covers approximately 60 species known as oregano in the world [21]. High content of phenolic compounds and essential oils in oregano confers to the plant its strong antioxidant character [22], as well as other biological activities such as antimicrobial activities [23]. It was macerated in olive oil in order to improve its enrichment with antioxidants from the plant [24], and also its essential oil was used to flavor olive oil [25]. Laurel is a plant species from the Lauraceae family, native to the Mediterranean region, dried leaves, also known as bay leaves, and essential oil are used as a valuable spice and flavoring agent in the culinary and food industry [26][27].
Laurel essential oil effects on virgin olive oil were studied by Taoudiat et al. [28]. These authors reported that the oxidative stability of oil samples supplemented with plant extracts was improved compared to samples without the addition of herbal plant extracts. Other plants were investigated to enrich and improve oils, such as pomegranate, pistachio, walnut, savory, etc. Table 1 summarizes different plants, oils enriched, and the main results of the enrichment reported.
Table 1. Most plants used for vegetable oils enrichment.
| Plant Common Name | Scientific Name | Part Used | Oil Enriched | Concentration | Main Results | Reference |
|---|---|---|---|---|---|---|
| Olive tree | Olea europaea L. | Leaves | Sunflower oil | 200 mg of TPC of methanol extract/kg of oil | Increase in TPC (nd-155 mg CAE), AA (282–504 mg TE) and OS (1.3–2 h). | [6] |
| 400–2400 ppm (juice) |
Improvement of oil quality during heating process (viscosity, acid value, peroxide value). | [29] | ||||
| Corn oil | 1000–1500 ppm (ethanol-water extract) |
TPC (18.00 ± 0.09–172.57 ± 0.53 ppm), AA (1.72–23.85%), TCC (nd-3.64 ± 0.01 mg β carotene/kg-oil) | [30] | |||
| Refined olive oil | plus 500 µL of extract (ethanol-extract) | Increase in total polyphenol area (from 0.1 ± 0.1 to 22.5 ± 0.4) | [8] | |||
| Olive oil | 1 g of milled leaves/10 mL of oil | Enrichement of oil with 14.45 ± 3.35 µg/mL of Oleuropein. | [13] | |||
| 20 kg of fruits with 5 L of water olive leaves extract (OLE) | OLE enhanced TPC about 10% (150.9 ± 11.3 μg GAE/g of oil) | [31] | ||||
| 3% of leaves extract (methanol extract) | Increase in TPC and antioxidant activity | [32] | ||||
| Refined olive oil | 400 ppm of chlorophyll pigment (ethanol extract) | Incresase in chlorophyll content of oil enriched (1.46 ± 0.08 to 4.13 ± 0.02 mg/kg) | [12] | |||
| Refined Soybean oil Palm oil Maize oil Rapeseed oil Extra virgin oil olive oil |
200 and 400 μg/mL of phenols (ethanol extract) | Additional stability and impovement quality parameters and transfert of oleuropein to target oils | [14] | |||
| Rosemary | Rosmarinus officinalis L. | Leaves | Chia oil | 1000 mg/kg (ethanol-eau extract) | Improvement of the oxidative stability From an induction period of 0.43 ± 0.01 h to 1.30 ± 0.06 h |
[33] |
| Flax oil | 1000 mg/kg (ethanol-eau extract) | Improvement of the oxidative stability From an induction period of 0.37 ± 0.02 h to 1.17 ± 0.20 h |
||||
| Hemp oil | 20 mg of rosemary leaves extract (ethanol, methanol; acetone; ether)/100 g of oil | Improvement of the oxidative stability From a peroxide value of 105.93 ± 0.12 mEqO2/kg to 98.70 ± 0.50 mEqO2/kg for enriched hemp oil |
[34] | |||
| Sunflower and soybean mixture oil | Ethanol extract (Concentration not determined)- | Improvement of the oxidative stability Enriched oils keeps the lower peroxide value, acidity and saturated fatty acids |
[18] | |||
| Commercial rosemary extract with a very high carnosic acid content of 70% | Soybean oil | 400 mg/kg of commercial rosemary extract with a very high carnosic acid content of 70% | Improvement of the oxidative stability From an induction period of 2.2 ± 0.22 h to 3.4 ± 0.18 h From a peroxide value of 23.72 ± 0.51 mEqO2/kg 17.32 ± 0.15 mEqO2/kg |
[35] | ||
| Cotton oil | Improvement of the oxidative stability From an induction period of 1.88 ± 0.2 h to 3.35 ± 0.15 h From a peroxide value of 19.47 ± 0.18 mEqO2/kg 16.53 ± 0.24 mEqO2/kg |
|||||
| Rice bran oil | Improvement of the oxidative stability From an induction period of 3.83 ± 0.07 h 6.22 ± 0.21 h From a peroxide value of 29.45 ± 0.61 mEqO2/kg 19.00 ± 0.19 mEqO2/kg |
|||||
| Leaves | Virgin olive oil | 5% (w/v) of leaves/oil | Increase in the content of free fatty acids from 0.42 ± 0.01 g/100 g to 0.57 ± 0.02 g/100 g From an induction period of 3.75 h to 4.5 h |
[36] | ||
| Oregano | Origanum vulgare L. | Leaves | Soybean oil | 0.01%, 0.03% and 0.07% (Ethanol/water (7/3) extract) | Improvement of oxidative stability (tON/°C = 155.22 ± 0.42 at 0.01% to 159.35 ± 0.69 at 0.07%) | [37] |
| Sunflower oil | 400 ppm (Aqueous–ethanolic extract) | Increase in antioxidant activity | [38] | |||
| Extra virgin olive oil | 10, 20 and 40 g of extract obtained by infusion/L | Improvement of oxidative stability | [39] | |||
| Laurel | Laurus nobilis L. | Essential oil | Extra-virgin Olive oil | 0.01% of essential oil (volume of essential oil/volume of extra virgin olive oil) |
Improvement of oxidative stability | [28] |
| Thyme | Thymus schimperi R. | Leaves and flowers | Soybean oil | 0.1 and 0.2% (Ethanol extract) | Increase in the induction time of soybean oil from 1.92 to 3.25 h Increase in the protection factor from 1.00 ± 0.042 to 1.69 ± 0.010 |
[19] |
| Thymus vulgaris L. | Soybean oil | 0.01%, 0.03% and 0.07% | Improvement of the oxidative stability (From tON of 145.86 ± 0.47 to 156.86 ± 0.84 at 0.07%) |
[37] | ||
| Refined corn oil | 5 g/40 mL of oil | Increase in the TPC from 23.63 mg/100 g to 53.99 mg/100 g Increase in antioxidant activity from 100.66 mg GAE/ 100 g to 185.22 mg GAE/100 g |
[40] | |||
| Basil | Ocimum basilicum L. | Leaves | Olive oil | 150 g of basil leaves/1 L of oil | Increase in Linalool and Eugenol ercentages | [41] |
| Soybean oil | 3000 mg of basil ethanol extract/kg of oil | Improvement of oxidative stability | [42] | |||
| Sunflower oil | 100 ppm and 400 ppm ofaqueous–ethanolic extract | Increase in antioxidant activity at 400 ppm | [38] | |||
| Pomegranate | Punica granatum L. | Juice | Pomegranate seed oil | (0%, 25%, 50%, 75%, and 100%) of juice | TPC (0.72–6.4 mg gallic acid/g) at 100% | [43] |
| Pistachio | Pistacia spp. | Kernels | Virgin pistachio oil Walnut oil |
- | TPC = 407 ± 7 mg/kg gallic acid DPPH = 13 ± 1 44 ± 3 mmol/kg Trolox Improvement of the oxidative stability |
[44] |
| Walnut | Juglans nigra L. | Virgin pistachio oil Walnut oil |
- | TPC = 339 ± 6 mg/kg gallic acid DPPH = 44 ± 3 mmol/kg Trolox Improvement of the oxidative stability |
[44] | |
| Peppermint | Mentha piperita L. | Leaves | Refined rapeseed and Sunflower oils | 100 ppm–400 ppm of aqueous–ethanolic extract | Decreasing in DPPH antioxidant activity for rapeseed oil Increase in DPPH antioxidant activity for sunflower oil |
[38] |
| Savory | Satureja thymbra L. | Refined rapeseed and Sunflower oils | Higher antioxidant activity at 400 ppm | [38] | ||
| Sage | Salvia officinalis L. | Sunflower oil | Higher antioxidant activity at 100 ppm than oil supplemented by BHA | [38] | ||
| Catnip | Nepeta cataria L. | Leaves and flowering parts | Sunflower oil | 600 and 1200 ppm of acetone extract |
Increase the production of hydroperoxides for both concentrations An increase in the formation of hexanal for 600 ppm and a decrease for 1200 ppm |
[45] |
| Hyssop | Hyssopus officinalis L. | An increase in the production of hydroperoxides for 600 ppm and a decrease for 1200 ppm A decrease in the formation of hexanal for both 600 ppm and 1200 ppm |
||||
| Lemon balm | Melissa officinalis L. | Decrease the production of hydroperoxides for both 600 and 1200 ppm A decrease in the formation of hexanal for both 600 ppm and 1200 ppm |
||||
| Pepper | Capsicum annuum L. | - | Extra virgin olive oil | 10, 20 and 40 g of powder/L of oil | Improvement of oxidative stability | [39] |
| Garlic | Allium sativum L. | - | 20, 30 and 40 g of powder/of oil | Improvement of oxidative stability | [39] |
AA = Antioxidant activity, BHA = Butylated hydroxyanisole, CAE = Catechin acid equivalent, DPPH = 2,2-diphenyl-1-picrylhydrazyl, GAE = Galic acid equivalent, TCC = Total
2. Extraction Methods of Antioxidants from Medicinal Plants and Enrichment of Vegetable Oils
2.1. Extraction Methods of Antioxidants from Medicinal and Aromatic Plants
After the collection of MAPs, the extraction of the antioxidant substances represents the first step in the enrichment of oil (Figure 1) [46][47].
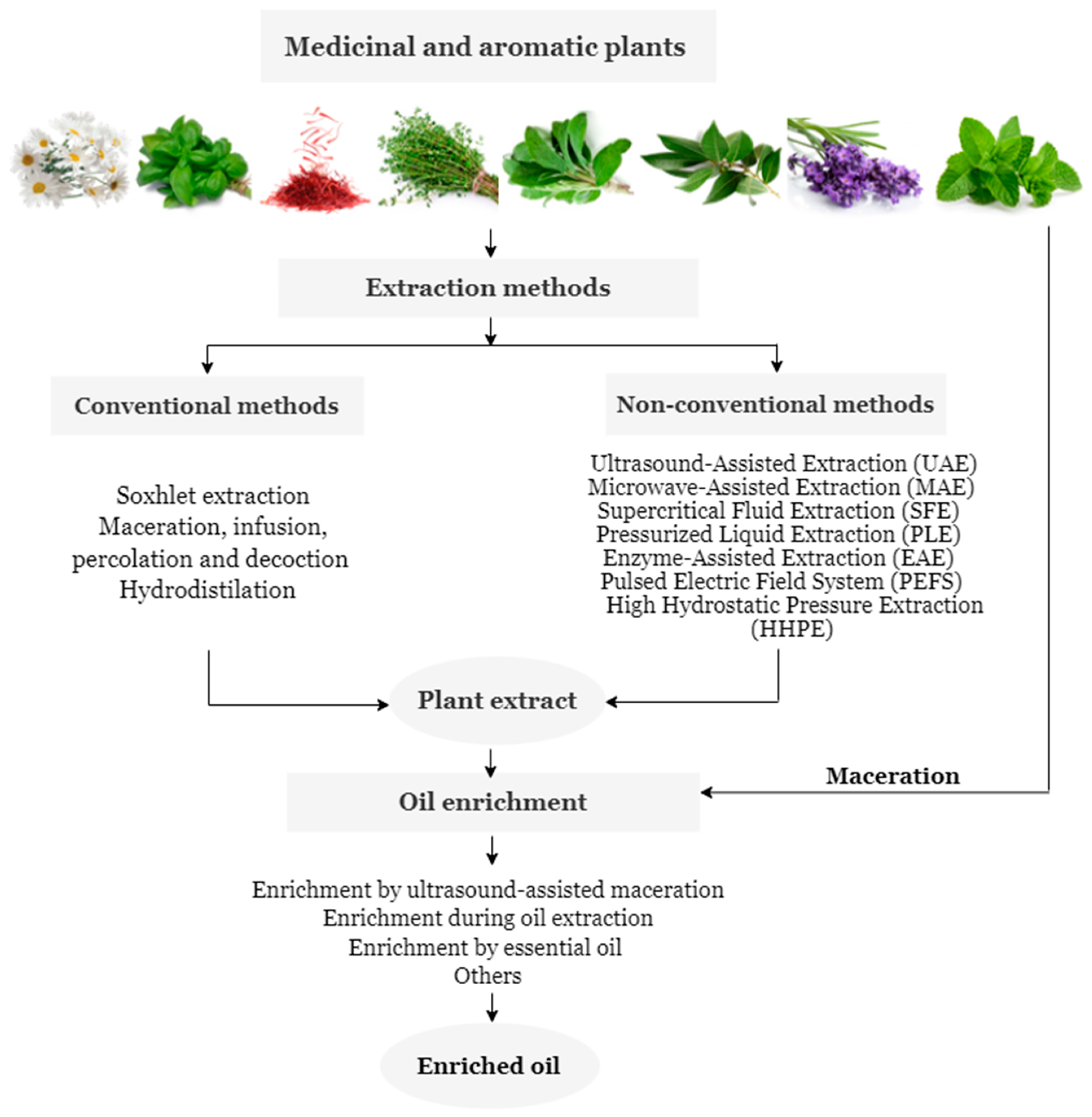
Figure 1. Extraction and enrichment methods of antioxidants from MAPs.
Extraction efficiency is influenced by several factors, such as the extraction temperature, the concentration of the extraction solvent, the extraction pH, and the extraction time, among others [48][49][50]. Solvent is one of the most critical factors, the selection of these products is based on the chemical nature and polarity of the antioxidant compounds to be extracted. The selection of solvents can be generally divided into two groups. These are polar and moderately polar solvents, just like water, methanol, ethanol, propanol, acetone, and their aqueous mixtures for the extraction of water-soluble antioxidants like phenolic compounds, flavonoids, and anthocyanins [51][52]. While familiar organic solvents, like mixtures of hexane with acetone, methanol, ethanol, or mixtures of ethyl acetate with acetone, methanol, and ethanol, have been used for the extraction of fat-soluble antioxidants, namely carotenoids [53][54]. The most commonly used extraction methods can be grouped into conventional (hot water bath, maceration, and Soxhlet extraction) [55], and non-conventional procedures [56]. The first is traditional methods, with high solvent consumption, accomplished at the level of small research or by small production companies [57]. Non-conventional methods are modern and use high energy inputs/processing capacity to improve the efficiency and/or selectivity of the extraction [58], (ultrasound, microwave, pressurized liquids, enzymatic hydrolysis, high hydrostatic pressure, supercritical fluids, and pulsed electrical field) [59].
2.1.1. Conventional Extraction Methods
-
Soxhlet extraction
The Soxhlet method is the most frequent method for the extraction of bioactive compounds from vegetables [60]. The Soxhlet extractor was invented by Franz von Soxhlet in 1879 [61]. The main application of this apparatus is in chemistry to dissolve weakly soluble compounds from solid matrices. It permits an unattended and unmanaged operation and efficiently recycles a slight volume of solvent to dissolve a greater volume of material [60]. Soxhlet extraction depends widely on the properties of the matrix and particle size as internal diffusion can be a limiting step of the extraction, solvents used during the Soxhlet extraction must have the necessary properties such as selectivity, solvation, distribution coefficient, density, interfacial tension, recoverability, and chemical reactivity. A co-solvent can be added to raise the polarity of the liquid phase [62]. Among the advantages of Soxhlet extraction, is that the sample is repeatedly brought into contact with a solvent. This allows the shifting of the transfer equilibrium. In addition, the system remains at a relatively high extraction temperature due to the effect of the heat applied to the distillation flask, reaching some extraction cavities. Also, there is no need for filtration after leaching [63]. However, Soxhlet extraction has a number of disadvantages, such as the long extraction time (6 h), exposure to dangerous and flammable liquid organic solvents, and the possibility of toxic emissions throughout extraction. Solvents used in the extraction system must be of high purity, which can increase the extraction price. This procedure is not considered eco-friendly and could participate in the pollution problem compared to a conventional extraction method like supercritical fluid extraction [64]. The perfect sample for Soxhlet extraction is also constrained to a dry, finely separated solid [57] as well as many factors such as solvent-to-sample ratio, temperature, and agitation speed need to be taken into account for this technique [65].
-
Maceration, infusion, percolation, and decoction
Maceration requires soaking plants (coarse or powdered) in a container sealed with a solvent (called a menstruum) and left at room temperature for a minimum period of 3 days with frequent agitation until the soluble matter has dissolved [66]. The mixture is then filtered, and the solid residue is pressed to extract most of the occluded solutions, the filtered and pressed liquid obtained is mixed and separated from impurities by filtration. The final filtered liquid is evaporated and concentrated [67].
Infusion and decoction share the same principle with maceration; both are immersed in boiled or cold water [66]. In contrast, the maceration time is shorter in the case of infusion. For decoction, the sample is boiled in a given volume of water for a specified time. Decoction is only suitable for the extraction of thermostable compounds, and hard plant material, among others. Decoction is only adapted for the extraction of thermostable compounds, and hard plant materials. Decoction usually contains more fat-soluble compounds than maceration and infusion. A unique piece of equipment called a percolator is used in percolation, another extraction method with a similar basic principle [68]. Dry powdered samples are placed into the percolator, added to boiling water, and macerated for 2 h. The percolation process is usually performed at a moderate rate until the extraction is completed before evaporation. It is recommended that the extraction is completed before evaporation to obtain a concentrated extract.
-
Hydro distillation
Hydro distillation is a conventional method of extracting bioactive compounds, principally essential oils from plants [69][70]. Hydrodistillation includes three main physicochemical processes namely hydrodiffusion, hydrolysis, and thermal decomposition [71]. At high extraction temperatures, some volatile constituents can be lost. This limits its use for the extraction of thermolabile substances. There are three kinds of hydrodistillation [72] called water-steam distillation, water distillation, and steam distillation. Regarding hydrodistillation, the vegetable material is first put into a compartment of the still, then sufficient water is added and then boiled. As an alternative, steam is injected directly into the plant material [73]. Although, as positive points of this kind of extraction method; it can be performed without using organic solvents and can be carried out before dehydration of the matrices used for extraction [71]. The main drawbacks of this method are the long extraction time, possible chemical changes in terpenes’ structures, and the loss of some polar molecules owing to the applied heat [71][74].
2.1.2. Non-Conventional Extraction Methods
-
Ultrasound-assisted extraction (UAE)
UAE has been commonly adopted in the last three decades as an important extraction efficient in pharmaceutical and food industries [75]. The mechanism is founded on the phenomenon of cavitation. The propagation of ultrasound in liquid systems is through a series of compressional and rarefaction waves, which can induce the production of cavitation bubbles within fluids [76][77]. The diameters of such bubbles expand over a few cycles until reaching a critical threshold, at which time they collapse and release a tremendous amount of energy, resulting in extraordinary temperatures (5000 K) and pressure (1000 atmospheres) at ambient temperature. During UAE, high temperature and pressure would destroy the cell walls of plant material, which facilitates the release of bioactive compounds from the plant cell walls and improve mass transport. The frequency, intensity, temperature, and duration of the ultrasound have a direct impact on the extraction frequency, and yields. In addition, solvent type and volume as well as sample characteristics such as sample particle size and moisture content are also important factors for an efficient extraction [78]. Compared to conventional methods, ultrasonic extraction has shown several advantages in terms of extraction yields and time [79].
-
Microwave assisted extraction (MAE)
MAE involves three phases [80]: the detachment of solutes from the active sites from the solid matrix under elevated temperature and pressure; diffusion of the solvent through the solid matrix; and release of the solutes from the matrix into the solvent. Microwave frequency is set between 300 MHz and 300 GHz. In order to warm up quickly under microwave radiation, the solvent has to be of a high dielectric constant (which measures the efficiency at which absorbed microwave energy can be transformed into heat within a material when an electric field is applied) [81]. The advantage of this technique is the reduction in extraction time and solvent volume compared to the conventional method (maceration and Soxhlet extraction). By using appropriate conditions, in order to avoid thermal degradation, better recoveries have been observed in the MAE method [82].
This approach, however, is restricted to small phenolics such as phenolic acids (gallic acid and ellagic acid), quercetin, isoflavin, and trans-resveratrol thanks to their stability at microwave heating conditions of up to 100 °C for 20 min. Additional cycles of MAE resulted in a drastic decrease in the yield of phenolics and flavonoids. The yield of phenolics and flavanones decreased, mainly owing to the oxidation of the compounds [83]. Tannins and anthocyanins may not be suitable for MAE, as they are potentially subject to high-temperature degradation [84].
-
Supercritical fluid extraction (SFE)
SFE, as an environmentally sustainable technique, has been widely used recently [85]. Over the critical pressure and temperature, the solvent may enter the supercritical state, which has both liquid-like (solvent power, negligible surface tension) and gas-like (high diffusivity and low viscosity) characteristics [85][86]. SFE uses solvents at temperatures and pressures beyond their critical points. Compared to normal liquids, supercritical liquid fluids can achieve improved transport qualities, which diffuse rapidly via solid materials, and thus achieve quicker extraction rates [73]. The strength of supercritical solvents can be easily modified by changing the pressure, temperature, or by adding modifiers to reduce the extraction [87].
-
Pressurized liquid extraction (PLE)
PLE is based on the use of solvents at elevated pressure and temperature to extract the desired component from the different matrices [46][88]. By increasing pressure, the temperature of the solvent in the liquid state may be higher than its boiling point at normal temperature, which could increase mass transfer and improves the solubility of analytes. By elevating pressure, the temperature of the solvent in the liquid state may be higher than its boiling point at normal temperature, which can increase mass transfer and improve the solubility of analytes. This extraction method may be performed over a temperature range of 21 to 200 °C and a pressure range of 35 to 200 bars [46]. If water is used as a solvent, PLE is also known as subcritical water extraction (SWE) [89]. As the water temperature is increased to 200–250 °C in SWE, it may be kept in a liquid state, whilst the dielectric constant (ε) of water is reduced from 80 to 25, which is similar to the dielectric constant of some organic solvents like methanol or ethanol [46][90].
-
Enzyme-assisted extraction (EAE)
EAE is a potentially green extraction method due to the soft extraction conditions and its eco-friendship [91].
Enzymes are characterized by their high specificity and efficiency. They have the ability to degrade compositions and destroy the structural continuity of the plant cell wall, this latter promotes the liberation of bioactive constituents. Among the used enzymes, in this extraction method, are hemicellulase, cellulase, pectinase, and β-glucosidase. These enzymes could be extracted from different sources such as fungi, bacteria, fruit and vegetable extracts, or animal organs [55][91]. Several studies have demonstrated that EAEs improve the extraction performance of antioxidants, especially phenolics, flavonoids, and carotenoids [92][93][94].
-
High hydrostatic pressure extraction (HHPE)
HHPE is for very high cold isostatic hydraulic pressure ranging from 100 to 800 MPa and more [95]. HHPE is a new approach used for active constituents extracted from natural biomaterials. The advantage of this method is to improve mass exchange ratios, boosting cell permeability, as well as the diffusion of secondary metabolites in accordance with changes in phase transitions [96]. HHPE has been applied for the extraction of ginsenosides from Korean red ginseng [97], flavonoids from propolis [98], polyphenols from green tea leaves [59], and anthocyanins from grape by-products [96]. The use of HHPE has been shown to be very efficient, compared to conventional or other novel extraction methods by offering high extraction efficiencies and high extraction selectivity, as well as shorter time (1 min for most studies) and requiring less energy [59].
-
Pulsed electric field system (PEFS)
PEFS is a technique founded on the use of short-period pulses of high electrical field intensity (0.1–50 kV/cm) at ambient temperature [99]. The goal of PEFS applications is to make cell membranes permeable to improve the transfer of components from inside the cells [100]. Electrical fields of a few to hundred microseconds are able to intimate the formation of pores in the cell membrane, called also “electroporation”. On this basis, subsequent extraction of bioactive molecules can be performed [101]. Different investigations and advantages of pulsed electric field treatment have been found to enhance the extraction of bioactive compounds (antioxidants, tocopherols, polyphenols, and phytosterols) from various fruits, vegetables, and agricultural wastes [102][103]. Table 2 presents some examples of extraction methods for natural antioxidants.
Table 2. Examples of extraction methods of natural antioxidants.
| Extraction Method | Plant | Main Compounds | Main Results (Extract) | Reference |
|---|---|---|---|---|
| Soxhlet extraction | Spearmint (Mentha spicata L.) |
Flavonoids | Catechins = 0.144 mg/g | [60] |
| Maceration | Summer savory (Satureja hortensis L.) |
Phenols Flavonoids Anthocyanins |
TPC = 125.34 ± 0.13 mg GAE/g TFC = 16.27 ± 0.34 mg RU/g TAC = 115.21 ± 0.95 mg C3G/g |
[104] |
| Micro-waves assisted extraction | Pistacia leaves (Pistacia lentiscus L.) |
Phenols | TPC = 149.39 ± 8.11 mg GAE/g | [105] |
| Ultrasound assisted extraction | Rosemary leaves (Rosmarinus officinalis L.) |
Phenols | TPC = 2040 ± 40 ppm GAE TPC = 35.0 mg GAE/g |
[106][107] |
| Supercritical Fluid extraction | Rosemary (Rosmarinus officinalis L.) |
Carnosol Carnosic acid |
EC50 (DPPH) = 0.23 mg/mL | [108][109] |
| Pressurized liquid extraction | Spinach (Spinacia oleracea L.) |
Tocopherols Tocotrienols |
α-T = 284 ± 13 μg/kg β-T = 8 ± 0.1 μg/kg γ-T = 83 ± 3 μg/kg |
[109] |
| High hydrostatic pressure extraction | Green tea (Camellia sinensis L.) leaves |
Phenols | Yield of polyphenols at 4 min = 30.7 ± 0.8% | [110] |
| Pulsed electric field | Norway spruce (Picea abies L.) | Phenols | TPC = 8.52 g GAE/100 g | [111] |
| Enzyme-assisted extraction | Stevia (Stevia rebaudiana (Bert.) |
Flavonoids | Catechins = 89–102 g/100 g | [112] |
GAE = galic acid equivalent, EC = effective concentration, TPC = total phenolic content, TFC = total flavonoid content, TAC = total antioxidant capacity, DPPH = 2,2-diphenyl-1-picrylhydrazyl, α-T, β-T, and γ-T = α-, β-, and γ-tocopherols.
2.2. Enrichment Methods for Vegetable Oils with Medicinal and Aromatic Plants
The enrichment of edible VOs with antioxidant substances can be achieved in different ways [77][113].
-
Enrichment by natural maceration
One of the methods that can be carried out is enrichment by maceration is an old and easy-to-carry-out principle [114]. It allows extraction of liposoluble active ingredients by simple pressing, by mixing plant extracts in a fatty substance that acts as a natural solvent [115]. Valerija et al. have used it to enrich refined rapeseed oil with phenols and chlorophylls from olive leaves. Healthy leaves were sampled from the olive branches and washed in distilled water four times, three forms (whole, cut, and crushed) of fresh or dried olive leaves were prepared for maceration in oil ovens. The maximum total phenolics (220.4 ± 5.3 mg/kg) was achieved in VOs with fresh whole leaves after seven days of maceration, but the conversion of chlorophylls to oils was most effective when crushed and steam-bleached leaves were macerated for 28 days (79.10 ± 1.14 mg/kg) [116].
-
Enrichment by ultrasound-assisted maceration
Recently, new techniques have been developed for more efficiency regarding oil enrichment [24]. Namely, the enrichment of oils using ultrasounds; this method has shown good extraction results since it allows penetration and mass transfer [113]. Thanks to the cavitation principle that fosters the formation of tiny bubbles subjected to rapid adiabatic compression and expansion [63]. Achat et al. [63] adopted the ultrasonic maceration method to enrich olive oil with phenolic compounds from olive leaves under the following conditions: temperature of 16 °C, ultrasonic power of 60 W, and sonication time of 45 min.
-
Enrichment during oil extraction
In the same context, the study of Sanmartin et al. proposed a green, efficient, and innovative enrichment procedure. In the experimental conditions adopted, citrus and olive leaves are crushed and cryo-macerated with the olives during the extraction of oil. A higher antioxidant content was calculated in the enriched olive oils compared to the control sample, and a high concentration of oleuropein was detected in the olive oil extracted in the presence of the olive leaf (+50% in the olive oil). The organoleptic profiles of the enriched olive oils were also profitably improved in terms of overall pleasantness and odor complexity, compared to the control [117].
-
Enrichment with essential oil
Another technique aims at enriching VOs with an essential oil obtained from plants, as was done by Asensio et al. [25]. To this end, olive oil was flavored with oregano essential oils (OEO). Olive oil samples were spiked with 0.05% OEO and stored under dark and light conditions for 126 days. Samples with OEO showed low values of lipid oxidation indicators (UV absorption coefficients: K232, K269, peroxide value, and anisidine value), especially in the dark. Olive oil with OEO in dark displayed a low peroxide value (18.71 mEqO2 kg−1) [25].
-
Other techniques
Meanwhile, Medina et al. [14] have enriched various refined oils with phenolic extracts of olive leaves and olive pomace, by applying an alternative enrichment technique consisting of first preparing ethanolic extracts of olive leaves and pomace, adding them to refined oils, and finally evaporating the ethanol from the two-phase system. A significant improvement in the quality and stability parameters of the enriched oils was recorded [14]. Comparable results were found by Kozłowska and Gruczyńska [37] who evaluated the oxidative stability of sunflower and soybean oils enriched with plant extracts (marjoram, thyme, and oregano) using the same procedure.
On the other hand, Şahin et al. investigated the enrichment of corn oil with polyphenols by adding olive and lemon balm leaves extracts. After evaporation of the solvent in the extraction step, the extracts were dried and then partially dissolved in corn oil by a solid-liquid extraction method. The total phenolic content has been improved by 9.5 and 2.5 times compared to pure corn oil, and the antioxidant activity of the oil enriched with olive and lemon balm leaves extracts was found to be almost 14 and 6 times higher, respectively, than those of the untreated oil, and therefore the improved oil stability (18%) [30].
References
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Fanizzi, F.P.; Maffia, M. A Comparative Study of Phenols in Apulian Italian Wines. Foods 2017, 6, 24.
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93.
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical Evaluation, Antimicrobial Activity, and Determination of Bioactive Components from Leaves of Aegle Marmelos. BioMed Res. Int. 2014, 2014, e497606.
- Nieto, G. How Are Medicinal Plants Useful When Added to Foods? Medicines 2020, 7, 58.
- Saoudi, S.; Chammem, N.; Sifaoui, I.; Bouassida-Beji, M.; Jiménez, I.A.; Bazzocchi, I.L.; Silva, S.D.; Hamdi, M.; Bronze, M.R. Influence of Tunisian Aromatic Plants on the Prevention of Oxidation in Soybean Oil under Heating and Frying Conditions. Food Chem. 2016, 212, 503–511.
- Salta, F.N.; Mylona, A.; Chiou, A.; Boskou, G.; Andrikopoulos, N.K. Oxidative Stability of Edible Vegetable Oils Enriched in Polyphenols with Olive Leaf Extract. Food Sci. Technol. Int. 2007, 13, 413–421.
- Şahin, S.; Sayım, E.; Bilgin, M. Effect of Olive Leaf Extract Rich in Oleuropein on the Quality of Virgin Olive Oil. J. Food Sci. Technol. 2017, 54, 1721–1728.
- Paiva-Martins, F.; Correia, R.; Félix, S.; Ferreira, P.; Gordon, M.H. Effects of Enrichment of Refined Olive Oil with Phenolic Compounds from Olive Leaves. J. Agric. Food Chem. 2007, 55, 4139–4143.
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. Folium): Potential Effects on Glycemia and Lipidemia. ANM 2020, 76, 10–15.
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462.
- Sun, W.; Frost, B.; Liu, J. Oleuropein, Unexpected Benefits! Oncotarget 2017, 8, 17409.
- Jaber, H.; Ayadi, M.; Makni, J.; Rigane, G.; Sayadi, S.; Bouaziz, M. Stabilization of Refined Olive Oil by Enrichment with Chlorophyll Pigments Extracted from Chemlali Olive Leaves. Eur. J. Lipid Sci. Technol. 2012, 114, 1274–1283.
- Japón-Luján, R.; Janeiro, P.; Luque de Castro, M.D. Solid−Liquid Transfer of Biophenols from Olive Leaves for the Enrichment of Edible Oils by a Dynamic Ultrasound-Assisted Approach. J. Agric. Food Chem. 2008, 56, 7231–7235.
- Sánchez de Medina, V.; Priego-Capote, F.; Jiménez-Ot, C.; Luque de Castro, M.D. Quality and Stability of Edible Oils Enriched with Hydrophilic Antioxidants from the Olive Tree: The Role of Enrichment Extracts and Lipid Composition. J. Agric. Food Chem. 2011, 59, 11432–11441.
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98.
- Lalas, S.; Dourtoglou, V. Use of Rosemary Extract in Preventing Oxidation during Deep-Fat Frying of Potato Chips. J. Amer. Oil Chem. Soc. 2003, 80, 579–583.
- Che Man, Y.B.; Jaswir, I. Effect of Rosemary and Sage Extracts on Frying Performance of Refined, Bleached and Deodorized (RBD) Palm Olein during Deep-Fat Frying. Food Chem. 2000, 69, 301–307.
- Chammem, N.; Saoudi, S.; Sifaoui, I.; Sifi, S.; de Person, M.; Abderraba, M.; Moussa, F.; Hamdi, M. Improvement of Vegetable Oils Quality in Frying Conditions by Adding Rosemary Extract. Ind. Crops Prod. 2015, 74, 592–599.
- Hailemariam, G.; Emire, S. Antioxidant Activity and Preservative Effect of Thyme (Thymus schimperi R.). Br. J. Appl. Sci. Technol. 2013, 3, 1311–1326.
- Jabri Karoui, I.; Msaada, K.; Abderrabba, M.; Marzouk, B. Bioactive Compounds and Antioxidant Activities of Thyme-Enriched Refined Corn Oil. J. Agric. Sci. Technol. 2015, 18, 79–91.
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and Other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. Des. 2019, 25, 1809–1816.
- Baranauskaite, J.; Kubiliene, A.; Marksa, M.; Petrikaite, V.; Vitkevičius, K.; Baranauskas, A.; Bernatoniene, J. The Influence of Different Oregano Species on the Antioxidant Activity Determined Using HPLC Postcolumn DPPH Method and Anticancer Activity of Carvacrol and Rosmarinic Acid. BioMed Res. Int. 2017, 2017, e1681392.
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. subsp. vulgare L. under Different Growth Conditions. Molecules 2013, 18, 14948–14960.
- Peñalvo, G.C.; Robledo, V.R.; Callado, C.S.-C.; Santander-Ortega, M.J.; Castro-Vázquez, L.; Victoria Lozano, M.; Arroyo-Jiménez, M.M. Improving Green Enrichment of Virgin Olive Oil by Oregano. Effects on Antioxidants. Food Chem. 2016, 197, 509–515.
- Asensio, C.M.; Nepote, V.; Grosso, N.R. Consumers’ Acceptance and Quality Stability of Olive Oil Flavoured with Essential Oils of Different Oregano Species. Int. J. Food Sci. Technol. 2013, 48, 2417–2428.
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative Study on the Chemical Composition of Laurel (Laurus nobilis L.) Leaves from Greece and Georgia and the Antibacterial Activity of Their Essential Oil. Heliyon 2020, 6, e05491.
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares-Cerda, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-Assisted Extraction of Phenolic Compounds from Laurus nobilis L. and Their Antioxidant Activity. Ultrason. Sonochem. 2013, 20, 1149–1154.
- Taoudiat, A.; Djenane, D.; Ferhat, Z.; Spigno, G. The Effect of Laurus nobilis L. Essential Oil and Different Packaging Systems on the Photo-Oxidative Stability of Chemlal Extra-Virgin Olive Oil. J. Food Sci. Technol. 2018, 55, 4212–4222.
- Farag, R.S.; Mahmoud, E.A.; Basuny, A.M. Use Crude Olive Leaf Juice as a Natural Antioxidant for the Stability of Sunflower Oil during Heating. Int. J. Food Sci. Technol. 2007, 42, 107–115.
- Şahin, S.; Bilgin, M.; Sayım, E.; Güvenilir, B. Effects of Natural Antioxidants in the Improvement of Corn Oil Quality: Olive Leaf vs. Lemon Balm. Int. J. Food Sci. Technol. 2017, 52, 374–380.
- Kiritsakis, K.; Rodríguez-Pérez, C.; Gerasopoulos, D.; Segura-Carretero, A. Olive Oil Enrichment in Phenolic Compounds during Malaxation in the Presence of Olive Leaves or Olive Mill Wastewater Extracts. Eur. J. Lipid Sci. Technol. 2017, 119, 1600425.
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive Leaf Addition Increases Olive Oil Nutraceutical Properties. Molecules 2019, 24, 545.
- Jung, H.; Kim, I.; Jung, S.; Lee, J. Oxidative Stability of Chia Seed Oil and Flax Seed Oil and Impact of Rosemary (Rosmarinus officinalis L.) and Garlic (Allium cepa L.) Extracts on the Prevention of Lipid Oxidation. Appl. Biol. Chem. 2021, 64, 6.
- Moczkowska, M.; Karp, S.; Horbanczuk, O.K.; Hanula, M.; Wyrwisz, J.; Kurek, M.A. Effect of Rosemary Extract Addition on Oxidative Stability and Quality of Hemp Seed Oil. Food Bioprod. Process. 2020, 124, 33–47.
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary Extract Can Be Used as a Synthetic Antioxidant to Improve Vegetable Oil Oxidative Stability. Ind. Crops Prod. 2016, 80, 141–147.
- Kasimoglu, Z.; Tontul, I.; Soylu, A.; Gulen, K.; Topuz, A. The Oxidative Stability of Flavoured Virgin Olive Oil: The Effect of the Water Activity of Rosemary. Food Meas. 2018, 12, 2080–2086.
- Kozłowska, M.; Gruczyńska, E. Comparison of the Oxidative Stability of Soybean and Sunflower Oils Enriched with Herbal Plant Extracts. Chem. Pap. 2018, 72, 2607–2615.
- Kozłowska, M.; Zawada, K. Evaluation of Oxidative Stability of Vegetable Oils Enriched with Herb Extracts by EPR Spectroscopy. Chem. Pap. 2015, 69, 950–957.
- Gambacorta, G.; Faccia, M.; Pati, S.; Lamacchia, C.; Baiano, A.; La Notte, E. Changes in the Chemical and Sensorial Profile of Extra Virgin Olive Oils Flavored with Herbs and Spices During Storage. J. Food Lipids 2007, 14, 202–215.
- Jorge, N.; Veronezi, C.M.; Del Ré, P.V. Antioxidant Effect of Thyme (Thymus vulgaris L.) and Oregano (Origanum vulgare L.) Extracts in Soybean Oil Under Thermoxidation. J. Food Process. Preserv. 2015, 39, 1399–1406.
- Veillet, S.; Tomao, V.; Chemat, F. Ultrasound Assisted Maceration: An Original Procedure for Direct Aromatisation of Olive Oil with Basil. Food Chem. 2010, 123, 905–911.
- Veronezi, C.M.; Costa, T.; Jorge, N. Basil (Ocimum basilicum L.) as a Natural Antioxidant. J. Food Process. Preserv. 2014, 38, 255–261.
- Yekdane, N.; Goli, S. Effect of Pomegranate Juice on Characteristics and Oxidative Stability of Microencapsulated Pomegranate Seed Oil Using Spray Drying. Food Bioprocess Technol. 2019, 12, 1614–1625.
- Fregapane, G.; Guisantes-Batan, E.; Ojeda-Amador, R.M.; Salvador, M.D. Development of Functional Edible Oils Enriched with Pistachio and Walnut Phenolic Extracts. Food Chem. 2020, 310, 125917.
- Abdalla, A.E.; Roozen, J.P. Effect of Plant Extracts on the Oxidative Stability of Sunflower Oil and Emulsion. Food Chem. 1999, 64, 323–329.
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18.
- Metzner, C.-R.; Poiana, M.-A. Fruit-Based Natural Antioxidants in Edible Oils: A Review. J. Agroaliment. Process. Technol. 2018, 24, 110–117.
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of Solvent and Temperature on Extraction of Phenolic Compounds from Grape Seed, Antioxidant Activity and Colour of Extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401.
- Chew, K.K.; Ng, S.Y.; Thoo, Y.Y.; Khoo, M.Z.; Wan mustapha, W.; Chun Wai, H. Effect of Ethanol Concentration, Extraction Time and Extraction Temperature on the Recovery of Phenolic Compounds and Antioxidant Capacity of Centella Asiatica Extracts. Int. Food Res. J. 2011, 18, 571–578.
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit. Sep. Purif. Technol. 2016, 162, 68–76.
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-Assisted Extraction of Phenolics from Winemaking by-Products: Antioxidant Potential and Inhibition of Alpha-Glucosidase and Lipase Activities. Food Chem. 2016, 212, 395–402.
- Nguyen, V.T.; Pham, H.N.T.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Influence of Solvents and Novel Extraction Methods on Bioactive Compounds and Antioxidant Capacity of Phyllanthus Amarus. Chem. Pap. 2016, 70, 556–566.
- Faidi, K.; Baaka, N.; Hammami, S.; Mokni, R.E.; Mighri, Z.; Mhenni, M.F. Extraction of Carotenoids from Lycium Ferocissimum Fruits for Cotton Dyeing: Optimization Survey Based on a Central Composite Design Method. Fibers Polym. 2016, 17, 36–43.
- Strati, I.F.; Oreopoulou, V. Effect of Extraction Parameters on the Carotenoid Recovery from Tomato Waste. Int. J. Food Sci. Technol. 2011, 46, 23–29.
- Li, H.-B.; Jiang, Y.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Evaluation of Two Methods for the Extraction of Antioxidants from Medicinal Plants. Anal. Bioanal. Chem. 2007, 388, 483–488.
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of Bioactive Compounds and Essential Oils from Mediterranean Herbs by Conventional and Green Innovative Techniques: A Review. Food Res. Int. 2018, 113, 245–262.
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196.
- Rocchetti, G.; Pagnossa, J.P.; Blasi, F.; Cossignani, L.; Hilsdorf Piccoli, R.; Zengin, G.; Montesano, D.; Cocconcelli, P.S.; Lucini, L. Phenolic Profiling and in Vitro Bioactivity of Moringa Oleifera Leaves as Affected by Different Extraction Solvents. Food Res. Int. 2020, 127, 108712.
- Xu, D.-P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.-N.; Li, H.-B. Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha Integerrima by Response Surface Methodology. Molecules 2016, 21, 18.
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of Different Extraction Methods for the Extraction of Major Bioactive Flavonoid Compounds from Spearmint (Mentha spicata L.) Leaves. Food Bioprod. Process. 2011, 89, 67–72.
- Sukri, N.A. Effect of Different Types of Solvent on Extraction of Phenolic Compounds from Cosmos caudatus. Doctoral Dissertation, UMP, New York, NY, USA, 2012.
- Redfern, J.; Kinninmonth, M.; Burdass, D.; Verran, J. Using Soxhlet Ethanol Extraction to Produce and Test Plant Material (Essential Oils) for Their Antimicrobial Properties. J. Microbiol. Biol. Educ. 2014, 15, 45–46.
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet Extraction: Past and Present Panacea. J. Chromatogr. A 2010, 1217, 2383–2389.
- Naudé, Y.; Beer, W.H.J.; Jooste, S.; Merwe, L.; Rensburg, S. Comparison of Supercritical Fluid Extraction and Soxhlet Extraction for the Determination of DDT, DDD and DDE in Sediment. Water SA 1998, 24, 205–214.
- Amid, A.; Salim, R.J.M.; Adenan, M.I. The Factors Affecting the Extraction Condition for Neuroprotective Activity of Centella Asiatica Evaluated by Metal Chelating Activity Assay. J. Appl. Sci. 2010, 10, 837–842.
- Extraction Technologies for Medicinal and Aromatic Plants—PDF Free Download. Available online: https://docplayer.net/21093504-Extraction-technologies-for-medicinal-and-aromatic-plants.html (accessed on 6 September 2022).
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the Extraction Process of Polyphenols from Thymus serpyllum L. Herb Using Maceration, Heat- and Ultrasound-Assisted Techniques. Sep. Purif. Technol. 2017, 179, 369–380.
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and Purification of Anthocyanins: A Review. J. Agric. Food Res. 2022, 8, 100306.
- Milos, M.; Radonic, A.; Mastelic, J. Seasonal Variation in Essential Oil Compositions of Cupressus sempervirens L. J. Essent. Oil Res. 2002, 14, 222–223.
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the Antioxidant Properties of De-Odourised Aqueous Extracts from Selected Lamiaceae Herbs. Food Chem. 2003, 83, 255–262.
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436.
- Vankar, P.S. Essential Oils and Fragrances from Natural Sources. Reson 2004, 9, 30–41.
- Silva, L.V.; Nelson, D.L.; Drummond, M.F.B.; Dufossé, L.; Glória, M.B.A. Comparison of Hydrodistillation Methods for the Deodorization of Turmeric. Food Res. Int. 2005, 38, 1087–1096.
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243.
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108.
- Soria, A.C.; Villamiel, M. Effect of Ultrasound on the Technological Properties and Bioactivity of Food: A Review. Trends Food Sci. Technol. 2010, 21, 323–331.
- Chemat, F.; Périno-Issartier, S.; Loucif, L.; Elmaataoui, M.; Mason, T.J. Enrichment of Edible Oil with Sea Buckthorn By-Products Using Ultrasound-Assisted Extraction. Eur. J. Lipid Sci. Technol. 2012, 114, 453–460.
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A Comparative Analysis of the ‘Green’ Techniques Applied for Polyphenols Extraction from Bioresources. Chem. Biodivers. 2015, 12, 1635–1651.
- Virot, M.; Tomao, V.; Le Bourvellec, C.; Renard, C.M.C.G.; Chemat, F. Towards the Industrial Production of Antioxidants from Food Processing By-Products with Ultrasound-Assisted Extraction. Ultrason Sonochem 2010, 17, 1066–1074.
- Marongiu, B.; Porcedda, S.; Piras, A.; Rosa, A.; Deiana, M.; Dessì, M.A. Antioxidant Activity of Supercritical Extract of Melissa officinalis subsp. Officinalis and Melissa officinalis subsp. inodora. Phytother. Res. 2004, 18, 789–792.
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2006, 1, 7–18.
- Kaufmann, B.; Christen, P. Recent Extraction Techniques for Natural Products: Microwave-Assisted Extraction and Pressurised Solvent Extraction. Phytochem. Anal. 2002, 13, 105–113.
- Trusheva, B.; Trunkova, D.; Bankova, V. Different Extraction Methods of Biologically Active Components from Propolis: A Preliminary Study. Chem. Cent. J. 2007, 1, 13.
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967.
- Yen, H.-W.; Yang, S.-C.; Chen, C.-H.; Jesisca; Chang, J.-S. Supercritical Fluid Extraction of Valuable Compounds from Microalgal Biomass. Bioresour. Technol. 2015, 184, 291–296.
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical Fluid Extraction of Plant Flavors and Fragrances. Molecules 2013, 18, 7194–7238.
- Patil, A.A.; Sachin, B.S.; Wakte, P.S.; Shinde, D.B. Optimization of Supercritical Fluid Extraction and HPLC Identification of Wedelolactone from Wedelia Calendulacea by Orthogonal Array Design. J. Adv. Res. 2014, 5, 629–635.
- Herrero, M.; Sánchez-Camargo, A.d.P.; Cifuentes, A.; Ibáñez, E. Plants, Seaweeds, Microalgae and Food by-Products as Natural Sources of Functional Ingredients Obtained Using Pressurized Liquid Extraction and Supercritical Fluid Extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38.
- Nakajima, H. Mass Transfer: Advances in Sustainable Energy and Environment Oriented Numerical Modeling; BoD—Books on Demand: Norderstedt, Germany, 2013; ISBN 978-953-51-1170-2.
- Anurukvorakun, O. Subcritical Water for the Extraction of Flavonoids. Res. J. Phranakhon Rajabhat Sci. Technol. 2012, 7, 1–9.
- Cheng, X.; Bi, L.; Zhao, Z.; Chen, Y. Advances in Enzyme Assisted Extraction of Natural Products.; Atlantis Press: Amsterdam, The Netherlands, 2015; pp. 371–375.
- Ranveer, R.C.; Patil, S.N.; Sahoo, A.K. Effect of Different Parameters on Enzyme-Assisted Extraction of Lycopene from Tomato Processing Waste. Food Bioprod. Process. 2013, 91, 370–375.
- Dinkova, R.; Heffels, P.; Shikov, V.; Weber, F.; Schieber, A.; Mihalev, K. Effect of Enzyme-Assisted Extraction on the Chilled Storage Stability of Bilberry (Vaccinium myrtillus L.) Anthocyanins in Skin Extracts and Freshly Pressed Juices. Food Res. Int. 2014, 65, 35–41.
- Tomaz, I.; Maslov, L.; Stupić, D.; Preiner, D.; Ašperger, D.; Kontić, J.K. Recovery of Flavonoids from Grape Skins by Enzyme-Assisted Extraction. Sep. Sci. Technol. 2016, 51, 255–268.
- Shouqin, Z.; Jun, X.; Changzheng, W. High Hydrostatic Pressure Extraction of Flavonoids from Propolis. J. Chem. Technol. Biotechnol. 2005, 80, 50–54.
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91.
- Ghafoor, K.; Kim, S.-O.; Lee, D.-U.; Seong, K.; Park, J. Effects of High Hydrostatic Pressure on Structure and Colour of Red Ginseng (Panax ginseng). J. Sci. Food Agric. 2012, 92, 2975–2982.
- Kim, S.-O.; Park, C.-W.; Moon, S.-Y.; Lee, H.-A.; Kim, B.-K.; Lee, D.-U.; Lee, J.-H.; Park, J.-Y. Effects of High-Hydrostatic Pressure on Ginsenoside Concentrations in Korean Red Ginseng. Food Sci. Biotechnol. 2007, 16, 848–853.
- López, N.; Puértolas, E.; Condón, S.; Raso, J.; Alvarez, I. Enhancement of the Extraction of Betanine from Red Beetroot by Pulsed Electric Fields. J. Food Eng. 2009, 90, 60–66.
- Vorobiev, E.; Lebovka, N. Pulsed-Electric-Fields-Induced Effects in Plant Tissues: Fundamental Aspects and Perspectives of Applications. In Electrotechnologies for Extraction from Food Plants and Biomaterials; Food Engineering Series; Springer: New York, NY, USA, 2008; pp. 39–81. ISBN 978-0-387-79374-0.
- Bursać Kovačević, D.; Putnik, P.; Sandra, P.; Ježek, D.; Karlovic, S.; Verica, D.-U. High Hydrostatic Pressure Extraction of Flavonoids from Freeze-Dried Red Grape Skin as Winemaking By-Product. Ann. Nutr. Metab. 2015, 67, 521–522.
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A Comparative Study of Physical Pretreatments for the Extraction of Polyphenols and Proteins from Vine Shoots. Food Res. Int. 2014, 65, 462–468.
- Guderjan, M.; Elez-Martínez, P.; Knorr, D. Application of Pulsed Electric Fields at Oil Yield and Content of Functional Food Ingredients at the Production of Rapeseed Oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 55–62.
- Mašković, P.; Veličković, V.; Mitić, M.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J. Summer Savory Extracts Prepared by Novel Extraction Methods Resulted in Enhanced Biological Activity. Ind. Crops Prod. 2017, 109, 875–881.
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia Lentiscus Leaves as a Source of Phenolic Compounds: Microwave-Assisted Extraction Optimized and Compared with Ultrasound-Assisted and Conventional Solvent Extraction. Ind. Crops Prod. 2014, 61, 31–40.
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M.J. Assisted Extraction of Rosemary Antioxidants with Green Solvents. J. Food Eng. 2012, 109, 98–103.
- Bellumori, M.; Innocenti, M.; Binello, A.; Boffa, L.; Mulinacci, N.; Cravotto, G. Viñas. Comptes Rendus Chim. 2016, 19, 699–706.
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical Carbon Dioxide Extraction of Antioxidant Fractions from Selected Lamiaceae Herbs and Their Antioxidant Capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107.
- Viñas, P.; Bravo-Bravo, M.; López-García, I.; Pastor-Belda, M.; Hernández-Córdoba, M. Pressurized Liquid Extraction and Dispersive Liquid-Liquid Microextraction for Determination of Tocopherols and Tocotrienols in Plant Foods by Liquid Chromatography with Fluorescence and Atmospheric Pressure Chemical Ionization-Mass Spectrometry Detection. Talanta 2014, 119, 98–104.
- Xi, J.; Shen, D.; Zhao, S.; Lu, B.; Li, Y.; Zhang, R. Characterization of Polyphenols from Green Tea Leaves Using a High Hydrostatic Pressure Extraction. Int. J. Pharm. 2009, 382, 139–143.
- Bouras, M.; Grimi, N.; Bals, O.; Vorobiev, E. Impact of Pulsed Electric Fields on Polyphenols Extraction from Norway Spruce Bark. Ind. Crops Prod. 2016, 80, 50–58.
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-Assisted Extraction of Bioactives from Plants. Trends Biotechnol. 2012, 30, 37–44.
- Achat, S.; Tomao, V.; Madani, K.; Chibane, M.; Elmaataoui, M.; Dangles, O.; Chemat, F. Direct Enrichment of Olive Oil in Oleuropein by Ultrasound-Assisted Maceration at Laboratory and Pilot Plant Scale. Ultrason. Sonochemistry 2012, 19, 777–786.
- Ayadi, M.A.; Grati-Kamoun, N.; Attia, H. Physico-Chemical Change and Heat Stability of Extra Virgin Olive Oils Flavoured by Selected Tunisian Aromatic Plants. Food Chem. Toxicol. 2009, 47, 2613–2619.
- Turon, F. Recettes de macérâts huileux: Intérêt pour la cosmétique. OCL 2004, 11, 411–413.
- Germek, V.; Benček, M.; Lukić, B.; Bbroznić, D.; Koprivnjak, O. Natural Enrichment of Refined Rapeseed Oil with Phenols and Chlorophylls from Olive Leaves of Oblica Cultivar. Croat. J. Food Sci. Technol. 2019, 11, 202–209.
- Sanmartin, C.; Taglieri, I.; Macaluso, M.; Sgherri, C.; Ascrizzi, R.; Flamini, G.; Venturi, F.; Quartacci, M.F.; Luro, F.; Curk, F.; et al. Cold-Pressing Olive Oil in the Presence of Cryomacerated Leaves of Olea or Citrus: Nutraceutical and Sensorial Features. Molecules 2019, 24, 2625.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
3 times
(View History)
Update Date:
21 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




