Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Faisal Eudes Sam | -- | 1380 | 2022-11-10 12:24:36 | | | |
| 2 | Catherine Yang | -1 word(s) | 1379 | 2022-11-21 03:46:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jiang, Y.; Sam, F.E.; Li, J.; Bi, Y.; Ma, T.; Zhang, B. Effect of Benzothiadiazole on Phenols Metabolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/35297 (accessed on 08 February 2026).
Jiang Y, Sam FE, Li J, Bi Y, Ma T, Zhang B. Effect of Benzothiadiazole on Phenols Metabolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/35297. Accessed February 08, 2026.
Jiang, Yumei, Faisal Eudes Sam, Jixin Li, Yang Bi, Tengzhen Ma, Bo Zhang. "Effect of Benzothiadiazole on Phenols Metabolism" Encyclopedia, https://encyclopedia.pub/entry/35297 (accessed February 08, 2026).
Jiang, Y., Sam, F.E., Li, J., Bi, Y., Ma, T., & Zhang, B. (2022, November 18). Effect of Benzothiadiazole on Phenols Metabolism. In Encyclopedia. https://encyclopedia.pub/entry/35297
Jiang, Yumei, et al. "Effect of Benzothiadiazole on Phenols Metabolism." Encyclopedia. Web. 18 November, 2022.
Copy Citation
Benzothiadiazole (BTH) is a commercial chemical elicitor that can induce an innate immune response in grapevines and improve the phenolic components and color quality of grapes and corresponding products.
benzothiadiazole
phenolic compounds
elicitor
grape
quality
1. Synthesis of Plant Phenols
The soluble form of phenolics is mainly located in the vacuoles of plant cells, which may be in free or conjugated form, while the insoluble phenolics are mainly found in the cell wall matrix [1][2]. The veraison is the critical period in grapes during which phenols develop [3]. Phenolic compounds are usually synthesized from phenylalanine or tyrosine through the shikimic acid pathway in plant intracellular organs during the growth of plants [1][4][5]. They consist of an aromatic ring with one or more hydroxyl substituents and range from monomeric molecules to highly polymerized compounds [5][6]. The hydroxyl substituents on the aromatic ring are responsible for the antioxidant properties of the phenolic compound [5].
The synthesis of phenolic compounds originates from the branching of phenylpropanoids [7][8][9]. The first step of synthesis begins with the deamination of phenylalanine catalyzed by phenylalanine ammonia-lyase (PAL) [10]. Phenylalanine is a product of the shikimate pathway, which links carbohydrate metabolism with the production of aromatic amino acids and secondary metabolites [8]. In the second step, phenol skeletons are derived from malonyl-CoA and p-coumaroyl-CoA, which are biogenetically derived from phenylpropanoids and acetate pathways [8]. Subsequently, malonyl-CoA and p-coumaryl-CoA are transformed into phenols by stilbene synthase (STS) and chalcone synthase (CHS) through the formation of an aromatic ring (by adding three more carbon groups consisting of two C atoms) [3][7]. Through a bifurcation of this pathway, two major classes of phenolic compounds, flavonoids (by CHS) and stilbenes (by STS), can be synthesized. In addition, the flavonoid pathway leads to the synthesis of flavan-3-ols, flavonols, anthocyanins, and proanthocyanidins [8][11][12].
2. Influence of BTH
Presently, there are more studies on pre-harvest BTH treatment to enhance phenolic accumulation in grapes but fewer studies on the effects of BTH treatment on the phenolic metabolism of grapes. Based on the mechanism of SAR pathway, BTH (functional analog of SA) can stimulate the expression of protein defense genes (PR1, PR2, and PR5) associated with pathogenesis via the protein non-expressor gene 1 (NPR1), which is also associated with pathogenesis [13][14][15]. Expression of the protein defense genes then activates the SA signaling pathway leading to SAR establishment [10][14] and encoding protein defense and key enzymes of secondary metabolism (Figure 1) [3][16][17]. This is also accountable for the increase of phytoalexins, synthesis of protein defense genes, and reinforcement of cell walls, among others [18]. It has been reported that BTH inhibits ethylene and malondialdehyde (MDA) [19] but enhances the catalytic activity of various enzymes, particularly PAL, thereby influencing the synthesis of secondary metabolites in plants [20][21][22][23][24][25]. Paladines-Quezada et al. [26] suggested that grapevine cells treated with elicitors such as BTH activate defense responses that produce high amounts of superoxide radicals, H2O2, and other reactive oxygen species (ROS) in the cell wall. ROS can cause rapid cross-linking or interlacing of cell wall phenolic compounds. Wang et al. [21] also found that BTH can activate a SAR defense response in grape suspension cells and enhance the accumulation of stilbene phytoalexins, VvNPR1.1 and PR1 genes expression, and the cellular burst of H2O2. Cellular hypersensitive defense responses, including activation of defense genes and induction of defense compounds (e.g., phenols) in plants during oxidative burst could be attributed to the accumulation of H2O2 in plant cells [27]. Using elicitors such as BTH can induce early and rapid H2O2 production in cultured V. vinifera cells, triggering the expression of defense-related genes or phytoalexins (e.g., phenols) synthesis [21][28][29].
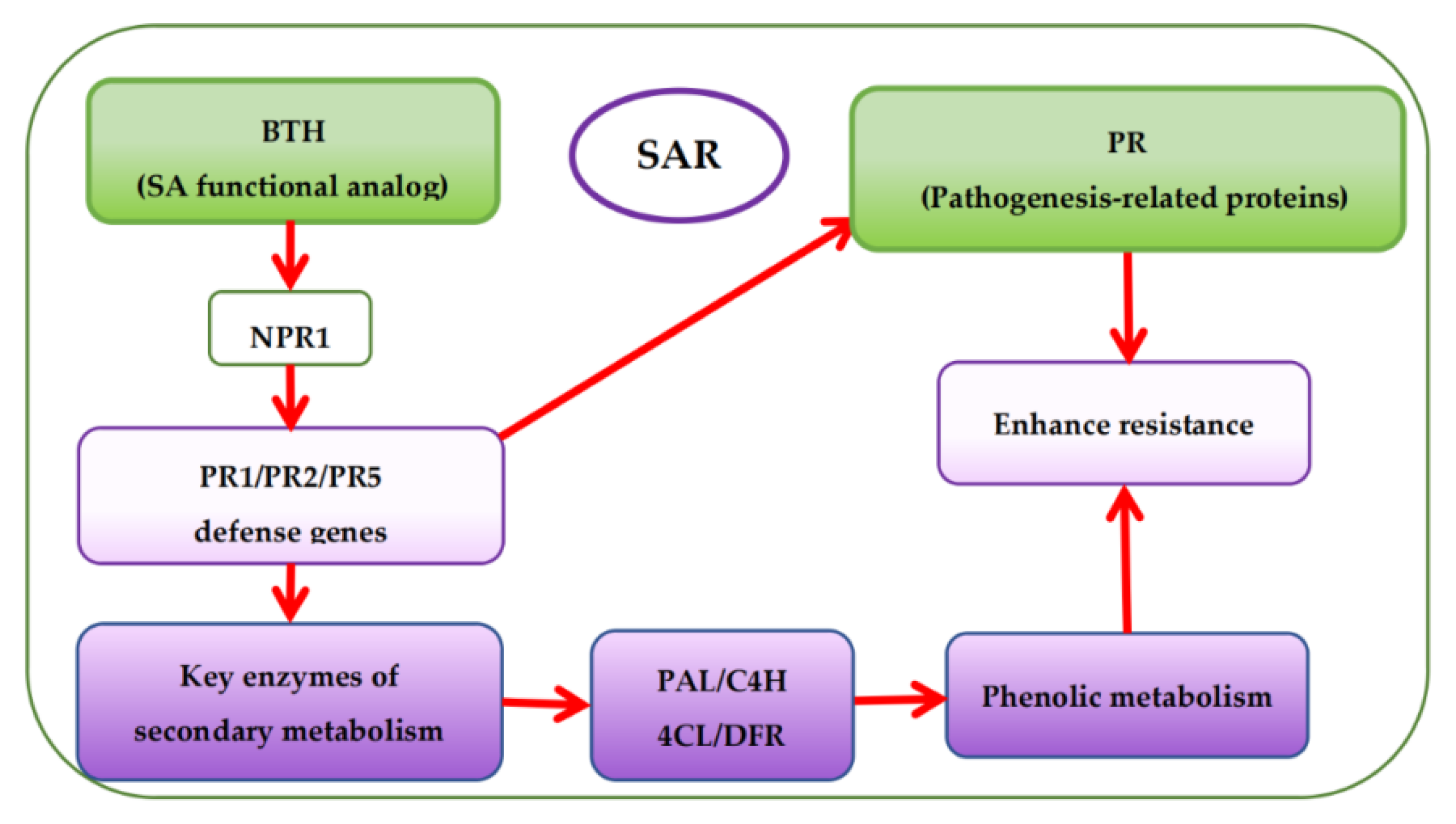
Figure 1. Overview of systemic acquired resistance (SAR) and phenolic accumulation mechanism with BTH treatment.
However, BTH can reduce the levels of primary metabolites such as amino acids and soluble sugars in treated grapes [21][30]. In particular, total soluble sugar and soluble sugar composition may vary significantly in BTH-treated berries [21]. Soluble sugars are not only responsible for fruit organoleptic quality but also act as key signaling molecules that can modulate the transcription of genes involved in defense responses and metabolic processes, thus affecting the biosynthesis of secondary metabolites [21][31]. Hence, there are soluble sugars associated with BTH effect on the phenolic compounds in grapes and the defense mechanisms induced by BTH. BTH treatment can stimulate lower sucrose-synthesizing enzymes (SS-synthesis, SPS, and SPP and higher sucrose-hydrolyzing enzyme (SS-cleavage), triggering a slow increase in sucrose breakdown, a decrease in glucose level and the buildup of fructose in grapes [21]. In addition, sugar accumulation in grape pulp and skin can also enhance the synthesis of anthocyanins [3][32][33]. Therefore, soluble sugars are associated with the impact of BTH on the phenolic contents of grapes and the defense mechanisms induced by BTH. Some authors [21] suggest that the phenylpropanoid pathway and the common precursor of sucrose metabolism (UDP-glucose) may be directed toward the biosynthesis of phenolic compounds by BTH treatment, whereas soluble sugar accumulation might reduce.
The increase of anthocyanins and stilbenes content in BTH-treated grapes could also be due to BTH induction of PAL [34]. Iriti et al. [3] found that the buildup of stilbenes in the berry skin decreased during the ripening period of untreated berries while the production of anthocyanins increased, possibly due to competition between the two branches of the phenylpropanoid pathway and the different regulation of the key enzymes STS and CHS. Meanwhile, it appears that BTH treatment reverses the opposite association between anthocyanin and resveratrol pathways to some extent. Hence, BTH may reduce competition between STS and CHS, enabling substrate binding and increasing anthocyanin and resveratrol synthesis. In BTH-treated berries, anthocyanin accumulation does not necessarily affect resveratrol synthesis during ripening. In other words, the usual metabolic switch between the two branches of the same metabolic pathway seems to be avoided by BTH treatment [3].
Anthocyanins can be affected by BTH treatments [35][36]. Gómez-Plaza et al. [35] demonstrated that BTH could activate enzymes related to anthocyanin metabolism. Repka et al. [37] and Gozzo [38] also reported that BTH treatment promoted the activities of enzymes (i.e., chalcone isomerase and PAL) in the phenylpropanoid pathway. Furthermore, BTH treatment can contribute to stilbenes and flavan-3-ols synthesis due to SAR and BTH induction of the expression of phenylpropanoid genes in grapevine [8]. In addition, in light of the effect of BTH treatment on grape polyphenols and the findings of earlier studies, Fumagalli et al. [16] suggested that BTH could enhance the activity of CHS during polyphenol or anthocyanin biosynthesis.
Studies on pre- or post-harvest BTH treatment have been conducted not so much in terms of secondary metabolic mechanisms in grapes as in various plants. In one study, post-harvest BTH treatment resulted in a higher anthocyanin content in strawberries and increased the activities of metabolic enzymes such as PAL, 4-coumarate/coenzyme A ligase (4-CL), cinnamate-4-hydroxylase (C4H), dihydroflavonol 4-reductase (DFR), glucose-6-phosphate dehydrogenase (G6PDH), tyrosine ammonia-lyase (TAL), and shikimate dehydrogenase (SKDH) [36]. This suggests that the increase in anthocyanin content by BTH may be due to the activation of associated metabolic enzymes. In addition, post-harvest BTH treatment resulted in the activation of PAL in peaches and mangoes [39][40], while the activity of peroxidases (POD) and polyphenol oxidase (PPO) increased in mangoes [40]. Similarly, post-harvest BTH treatments also induced PPO and POD activity in bananas [41] and loquats [42]. Interestingly, this approach has been reported to increase the total phenolic compounds in mangoes and bananas [40][41]. Furthermore, the exogenous application of BTH increases the expression of proanthocyanidin-related MBW complex (MYB-bHLH-WD40) and the content of flavan-3-ol in general [43], which promotes the accumulation of proanthocyanidins. Similar results were also reported by Felicijan et al. [44]. However, the activity and gene expression of phospholipase (phospholipase A2PLA2, phospholipase CPLC, and phospholipase DPLD) were inhibited by post-harvest BTH treatment in melons [45].
Overall, the influence of BTH on grapes has been reported in the literature, particularly on grape color, phenolics, variety, and metabolism (Table 1). Based on the existing literature, many studies have been conducted on the effects of BTH on grape phenolics in relation to variety, but less on the effects on grape metabolism and sensory characteristic such as color and mouthfeel (which are associated with phenolic compounds). Hence, future studies can be conducted in these areas.
Table 1. Summary of literature on BTH impact on grape variety, phenols, color, and metabolism.
| Research Area | Subclasses | Reference |
|---|---|---|
| Variety | Monastrell | [7][10][26][35][46][47][48][49][50][51] |
| Merlot | [3][10][20][35][48][51] | |
| Syrah | [10][35][51][52][53] | |
| Cabernet Sauvignon | [48][49] | |
| Groppello | [54] | |
| Beauty Seedless | [55] | |
| Cabernet Gernischt | [30] | |
| Phenols | Anthocyanin | [3][7][10][20][26][35][46][47] |
| Flavonols | [7][35][46][47][50][51] | |
| Proanthocyanidins (Tannins) | [10][20][22][35][51] | |
| Stilbenes | [3][21][50][53] | |
| Metabolism | Enzymes | [3][20][21][22][35] |
| Hydrogen dioxide and ROS | [26][35] | |
| Color | Color intensity | [7][10][30][35][48][51] |
References
- Machado, T.; Portugal, I.; Padilha, C.; Padilha, F.F.; Li Ma, M. New Trends in the Use of Enzymes for the Recovery of Polyphenols in Grape Byproducts. J. Food Biochem. 2021, 45, e13712.
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216.
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole Enhances Resveratrol and Anthocyanin Biosynthesis in Grapevine, Meanwhile Improving Resistance to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413.
- Myrtsi, E.D.; Koulocheri, S.D.; Iliopoulos, V.; Haroutounian, S.A. High-Throughput Quantification of 32 Bioactive Antioxidant Phenolic Compounds in Grapes, Wines and Vinification Byproducts by LC–MS/MS. Antioxidants 2021, 10, 1174.
- Zeb, A. Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis, 1st ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; ISBN 3030747670.
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; Silva-james, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594.
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the Phenolic Compound Content of Grapes by Preharvest Application of Abscisic Acid and a Combination of Methyl Jasmonate and Benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983.
- Flamini, R.; Mattivi, F.; Rosso, M.D.; Arapitsas, P. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669.
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid Metabolism in Ripening Fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416.
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y. Improving Phenolic and Chromatic Characteristics of Monastrell, Merlot and Syrah Wines by Using Methyl Jasmonate and Benzothiadiazole. Oeno One 2017, 51, 17–27.
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483.
- Yu, D.; Huang, T.; Tian, B. Advances in Biosynthesis and Biological Functions of Proanthocyanidins in Horticultural Plants. Foods 2020, 9, 1774.
- Zehra, A.; Anant, N.; Meena, M.; Swapnil, P. Current Research in Microbial Sciences Efficiency of Microbial Bio-Agents as Elicitors in Plant Defense Mechanism under Biotic Stress: A Review. Curr. Res. Microb. Sci. 2021, 2, 100054.
- Ali, S.; Mir, Z.A.; Tyagi, A.; Mehari, H.; Meena, R.P.; Bhat, J.A.; Yadav, P.; Papalou, P.; Rawat, S.; Grover, A. Overexpression of NPR1 in Brassica juncea Confers Broad Spectrum Resistance to Fungal Pathogens. Front. Plant Sci. 2017, 8, 1693.
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287.
- Fumagalli, F.; Rossoni, M.; Iriti, M.; Gennaro, A.D.; Faoro, F.; Borroni, E.; Borgo, M.; Scienza, A.; Sala, A.; Folco, G. From Field to Health: A Simple Way to Increase the Nutraceutical Content of Grape as Shown by NO-Dependent Vascular Relaxation. J. Agric. Food Chem. 2006, 54, 5344–5349.
- Gacnik, S.; Veberic, R.; Marinovic, S.; Halbwirth, H.; Mikulic-, M. Scientia Horticulturae Effect of Pre-Harvest Treatments with Salicylic and Methyl Salicylic Acid on the Chemical Profile and Activity of Some Phenylpropanoid Pathway Related Enzymes in Apple Leaves. Sci. Hortic. 2021, 277, 109794.
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 13, 10.
- Xu, T.; Jiang, Y.; Li, J.; Zhang, N. Effect of BTH Treatment on Physiological Characteristics and Sensory Quality of ‘Yujinxiang ’ Muskmelon. Sci. Technol. Food Ind. 2014, 35, 315–318.
- Iriti, M.; Rossoni, M.; Borgo, M.; Ferrara, L.; Faoro, F. Induction of Resistance to Gray Mold with Benzothiadiazole Modifies Amino Acid Profile and Increases Proanthocyanidins in Grape: Primary versus Secondary Metabolism. J. Agric. Food Chem. 2005, 53, 9133–9139.
- Wang, K.; Liao, Y.; Cao, S.; Di, H.; Zheng, Y. Postharvest Biology and Technology Effects of Benzothiadiazole on Disease Resistance and Soluble Sugar Accumulation in Grape Berries and Its Possible Cellular Mechanisms Involved. Postharvest Biol. Technol. 2015, 102, 51–60.
- Miliordos, D.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L Var. Mouhtaro Plants 2021, 13, 5802.
- Bektas, Y.; Eulgem, T. Synthetic Plant Defense Elicitors. Front. Plant Sci. 2015, 5, 804.
- Ge, Y.; Tang, Q.; Li, C.; Duan, B.; Li, X.; Wei, M. LWT—Food Science and Technology Acibenzolar-S-Methyl Treatment Enhances Antioxidant Ability and Phenylpropanoid Pathway of Blueberries during Low Temperature Storage. LWT—Food Sci. Technol. 2019, 110, 48–53.
- Jiang, Y.; Li, X.; Bi, Y.; Zhou, X.; Zhou, W.; Ge, Y. Inhibition of postharvest volatile compounds release by preharvest acibenzolar-S-methyl (BTH) treatment on muskmelons (cv. Yindi). Trans. CSAE 2007, 23, 243–247.
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls. Chemistry 2022, 4, 98–111.
- Torres, M.A. ROS in Biotic Interactions. Physiol. Plant. 2010, 138, 414–429.
- Verhagen, B.W.M.; Trotel-Aziz, P.; Couderchet, M.; Höfte, M.; Aziz, A. Pseudomonas Spp.-Induced Systemic Resistance to Botrytis Cinerea Is Associated with Induction and Priming of Defence Responses in Grapevine. J. Exp. Bot. 2010, 61, 249–260.
- Belchí-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernández-Pérez, F.; Bru, R.; Pedreño, M.A. Early Signaling Events in Grapevine Cells Elicited with Cyclodextrins and Methyl Jasmonate. Plant Physiol. Biochem. PPB 2013, 62, 107–110.
- Salifu, R.; Jiang, Y.; Ba, L.; Zhang, Z.; Feng, L.; Li, J. Influence of Benzothiadiazole on the Amino Acids and Aroma Compositions of ‘Cabernet Gernischt’ Grapes (Vitis vinifera L.). Horticulturae 2022, 8, 812.
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709.
- Jeandet, P.; Bessis, R.A.; Gautheron, B. The Production of Resveratrol (3,5,4′-Trihydroxystilbene) by Grape Berries in Different Developmental Stages. Am. J. Enol. Vitic. 1991, 42, 41–46.
- Davies, C.; Robinson, S.P. Sugar Accumulation in Grape Berries. Cloning of Two Putative Vacuolar Invertase CDNAs and Their Expression in Grapevine Tissues. Plant Physiol. 1996, 111, 275–283.
- Iriti, M.; Faoro, F. Does Benzothiadiazole-Induced Resistance Increase Fitness Cost in Bean? J. Plant Pathol. 2003, 85, 265–270.
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; José, I.; Gil-Muñoz, R. Effect of Elicitors on the Evolution of Grape Phenolic Compounds during the Ripening Period. J. Sci. Food Agric. 2017, 97, 977–983.
- Cao, S.; Hu, Z.; Zheng, Y.; Yang, Z.; Lu, B. Effect of BTH on Antioxidant Enzymes, Radical-Scavenging Activity and Decay in Strawberry Fruit. Food Chem. 2011, 125, 145–149.
- Repka, V.; Fischerová, I.; Šilhárová, K. Methyl Jasmonate Is a Potent Elicitor of Multiple Defense Responses in Grapevine Leaves and Cell-Suspension Cultures. Biol. Plant 2004, 48, 273–283.
- Gozzo, F. Systemic Acquired Resistance in Crop Protection: From Nature to a Chemical Approach. J. Agric. Food Chem. 2003, 51, 4487–4503.
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH Treatment Induces Resistance of Peach (Prunus Persica L. Cv. Jiubao) Fruit to Infection by Penicillium Expansum and Enhances Activity of Fruit Defense Mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269.
- Zhu, X.; Cao, J.; Wang, Q.; Jiang, W. Postharvest Infiltration of BTH Reduces Infection of Mango Fruits (Mangifera Indica L. Cv. Tainong) by Colletotrichum gloeosporioides and Enhances Resistance Inducing Compounds. J. Phytopathol. 2008, 156, 68–74.
- Lin, J.; Gong, D.; Zhu, S.; Zhang, L.; Zhang, L. Expression of PPO and POD Genes and Contents of Polyphenolic Compounds in Harvested Mango Fruits in Relation to Benzothiadiazole-Induced Defense against Anthracnose. Sci. Hortic. 2011, 130, 85–89.
- Zhu, S.J.; Zhang, Z.W.; Xu, J.W.; Ma, L.Y.; Tang, W.L.; Liu, D.J. Effect of BTH treatment on storability and activity of related enzymes of harvested loquat fruit. Acta Hortic. 2007, 750, 445–450.
- Ullah, C.; Tsai, C.-J.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic Acid Activates Poplar Defense against the Biotrophic Rust Fungus Melampsora larici-populina via Increased Biosynthesis of Catechin and Proanthocyanidins. New Phytol. 2019, 221, 960–975.
- Felicijan, M.; Kristl, J.; Krajnc, A.U. Pre-Treatment with Salicylic Acid Induces Phenolic Responses of Norway Spruce (Picea Abies) Bark to Bark Beetle (Ips Typographus) Attack. Trees-Struct. Funct. 2016, 30, 2117–2129.
- Hu, Y.; Li, J.; Wang, Y.; Wang, B.; Zhang, G.; Zhang, R.; Jiang, Y. Effect of Postharvest Benzothiadiazole Treatment on Membrane Phospholipid Metabolism of Thick-Skinned Muskmelon. Food Sci. 2018, 39, 165–172.
- Paladines-Quezada, D.F.; Fern, I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.G.C.; Mart, A.; Gil-Muñoz, R. Application of Elicitors in Two Ripening Periods of Vitis vinifera L. cv Monastrell: Influence on Anthocyanin. Molecules 2021, 26, 1689.
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fern, I. Elicitors and Pre-Fermentative Cold Maceration: Effects on Polyphenol Concentration in Monastrell Grapes and Wines. Biomolecules 2019, 9, 671.
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697.
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Influence of the use of elicitors over the composition of cell wall grapes. In Proceedings of the In Vino Analytica Scientia, Salamanca, Spain, 17–20 July 2017; pp. 2–3.
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A. Increasing Bioactive Phenolic Compounds in Grapes: Response of Six Monastrell Grape Clones to Benzothiadiazole and Methyl Jasmonate Treatments. Am. J. Enol. Vitic. 2013, 64, 459–465.
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Ignacio, J. Improving Grape Phenolic Content and Wine Chromatic Characteristics through the Use of Two Different Elicitors: Methyl Jasmonate versus Benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290.
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of Benzothiadiazole and Methyl Jasmonate on the Volatile Compound Composition of Vitis vinifera L. Monastrell Grapes and Wines. Am. J. Enol. Vitic. 2012, 3, 394–401.
- Fernandez-Marin, M.I.; Guerrero, F.; Puertas, B. Impact of preharvest and postharvest treatment combinations on increase of stilbene content in grape. J. Int. Sci. Vigne Vin 2013, 47, 203–212.
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in vineyard (Vitis vinifera L. cv Groppello Gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205.
- Mhetre, V.; Patel, V.B.; Singh, S.K.; Verma, M.K. Effect of New Generation Bio-Regulators on Anthocyanins and Berry Quality of Grape Cv. Beauty Seedless Effect of New Generation Bio-Regulators on Anthocyanins and Berry Quality of Grape Cv. Beauty Seedless. Indian J. Agric. Sci. 2021, 91, 116–119.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
722
Revisions:
2 times
(View History)
Update Date:
22 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




