
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vivi Li | -- | 1205 | 2022-11-18 01:31:09 |
Video Upload Options
Bilayer graphene is a material consisting of two layers of graphene. One of the first reports of bilayer graphene was in the seminal 2004 Science paper by Geim and colleagues, in which they described devices "which contained just one, two, or three atomic layers"
1. Structure
Bilayer graphene can exist in the AB, or Bernal-stacked form,[1] where half of the atoms lie directly over the center of a hexagon in the lower graphene sheet, and half of the atoms lie over an atom, or, less commonly, in the AA form, in which the layers are exactly aligned.[2] In Bernal stacked graphene, twin boundaries are common; transitioning from AB to BA stacking.[3] Twisted layers, where one layer is rotated relative to the other, have also been observed.[3]
Quantum Monte Carlo methods have been used to calculate the binding energies of AA- and AB-stacked bilayer graphene, which are 11.5(9) and 17.7(9) meV per atom, respectively.[4] This is consistent with the observation that the AB-stacked structure is more stable than the AA-stacked structure.
2. Synthesis
Bilayer graphene can be made by exfoliation from graphite [5] or by chemical vapor deposition (CVD).[6] In 2016, Rodney S. Ruoff and colleagues showed that large single-crystal bilayer graphene could be produced by oxygen-activated chemical vapour deposition.[7] Later in the same year a Korean group reported the synthesis of wafer-scale single-crystal AB-stacked bilayer graphene [8]
3. Tunable Bandgap
Like monolayer graphene, bilayer graphene has a zero bandgap and thus behaves like a semimetal. In 2007, researchers predicted that a bandgap could be introduced if an electric displacement field were applied to the two layers: a so-called tunable band gap.[9] An experimental demonstration of a tunable bandgap in bilayer graphene came in 2009.[10] In 2015 researchers observed 1D ballistic electron conducting channels at bilayer graphene domain walls.[11] Another group showed that the band gap of bilayer films on silicon carbide could be controlled by selectively adjusting the carrier concentration.[12]
4. Emergent Complex States
In 2014 researchers described the emergence of complex electronic states in bilayer graphene, notably the fractional quantum Hall effect and showed that this could be tuned by an electric field.[13][14][15] In 2017 the observation of an even-denominator fractional quantum Hall state was reported in bilayer graphene.[16]
5. Excitonic Condensation
Bilayer graphene showed the potential to realize a Bose–Einstein condensate of excitons.[17] Electrons and holes are fermions, but when they form an exciton, they become bosons, allowing Bose-Einstein condensation to occur. Exciton condensates in bilayer systems have been shown theoretically to carry a large current.[18]
6. Superconductivity in Twisted Bilayer Graphene
Pablo Jarillo-Herrero of MIT and colleagues from Harvard and the National Institute for Materials Science, Tsukuba, Japan, have reported the discovery of superconductivity in bilayer graphene with a twist angle of 1.1° between the two layers. The discovery was announced in two papers published in Nature in March 2018.[19][20] The bilayer graphene was prepared from exfoliated monolayer graphene, with the second layer being manually rotated to a set angle with respect to the first layer. A Tc value of 1.7 K was observed with such specimens. Jarillo-Herrero has suggested that it may be possible to “...... imagine making a superconducting transistor out of graphene, which you can switch on and off, from superconducting to insulating. That opens many possibilities for quantum devices.”[21]
7. Field Effect Transistors
Bilayer graphene can be used to construct field effect transistors[22][23] or tunneling field effect transistors,[24] exploiting the small energy gap. However, the energy gap is smaller than 250 meV and therefore requires the use of low operating voltage (< 250 mV), which is too small to obtain reasonable performance for a field effect transistor,[22] but is very suited to the operation of tunnel field effect transistors, which according to theory from a paper in 2009 can operate with an operating voltage of only 100 mV.[24]
In 2016 researchers proposed the use of bilayer graphene to increase the output voltage of tunnel transistors (TT). They operate at a lower operating voltage range (150 mV) than silicon transistors (500 mV). Bilayer graphene's energy band is unlike that of most semiconductors in that the electrons around the edges form a (high density) van Hove singularity. This supplies sufficient electrons to increase current flow across the energy barrier. Bilayer graphene transistors use "electrical" rather than "chemical" doping.[25]
8. Ultrafast Lithium Diffusion
In 2017 an international group of researchers showed that bilayer graphene could act as a single-phase mixed conductor which exhibited Li diffusion faster than in graphite by an order of magnitude.[26] In combination with the fast electronic conduction of graphene sheets, this system offers both ionic and electronic conductivity within the same single-phase solid material. This has important implications for energy storage devices such as lithium ion batteries.
9. Ultrahard Carbon from Epitaxial Bilayer Graphene
Researchers from the City University of New York have shown that sheets of bilayer graphene on silicon carbide temporarily become harder than diamond upon impact with the tip of an atomic force microscope.[27] This was attributed to a graphite-diamond transition, and the behavior appeared to be unique to bilayer graphene. This could have applications in personal armor.
10. Porous Nanoflakes
Hybridization processes change the intrinsic properties of graphene and/or induce poor interfaces. In 2014 a general route to obtain unstacked graphene via facile, templated, catalytic growth was announced. The resulting material has a specific surface area of 1628 m2 g-1, is electrically conductive and has a mesoporous structure.[28]
The material is made with a mesoporous nanoflake template. Graphene layers are deposited onto the template. The carbon atoms accumulate in the mesopores, forming protuberances that act as spacers to prevent stacking. The protuberance density is approximately 5.8×1014 m−2. Graphene is deposited on both sides of the flakes.[28]
During CVD synthesis the protuberances produce intrinsically unstacked double-layer graphene after the removal of the nanoflakes. The presence of such protuberances on the surface can weaken the π-π interactions between graphene layers and thus reduce stacking. The bilayer graphene shows a specific surface area of 1628 m2/g, a pore size ranging from 2 to 7 nm and a total pore volume of 2.0 cm3/g.[28]
Using bilary graphene as cathode material for a lithium sulfur battery yielded reversible capacities of 1034 and 734 mA h/g at discharge rates of 5 and 10 C, respectively. After 1000 cycles reversible capacities of some 530 and 380 mA h/g were retained at 5 and 10 C, with coulombic efficiency constants at 96 and 98%, respectively.[28]
Electrical conductivity of 438 S/cm was obtained. Even after the infiltration of sulfur, electrical conductivity of 107 S cm/1 was retained. The graphene's unique porous structure allowed the effective storage of sulfur in the interlayer space, which gives rise to an efficient connection between the sulfur and graphene and prevents the diffusion of polysulfides into the electrolyte.[28]
11. Characterisation
Hyperspectral global Raman imaging[29] is an accurate and rapid technique to spatially characterize product quality. The vibrational modes of a system characterize it, providing information on stoichiometry, composition, morphology, stress and number of layers. Monitoring graphene's G and D peaks (around 1580 and 1360 cm−1)[30][31] intensity gives direct information on the number of layers of the sample.
12. Three Dimensional Configurations
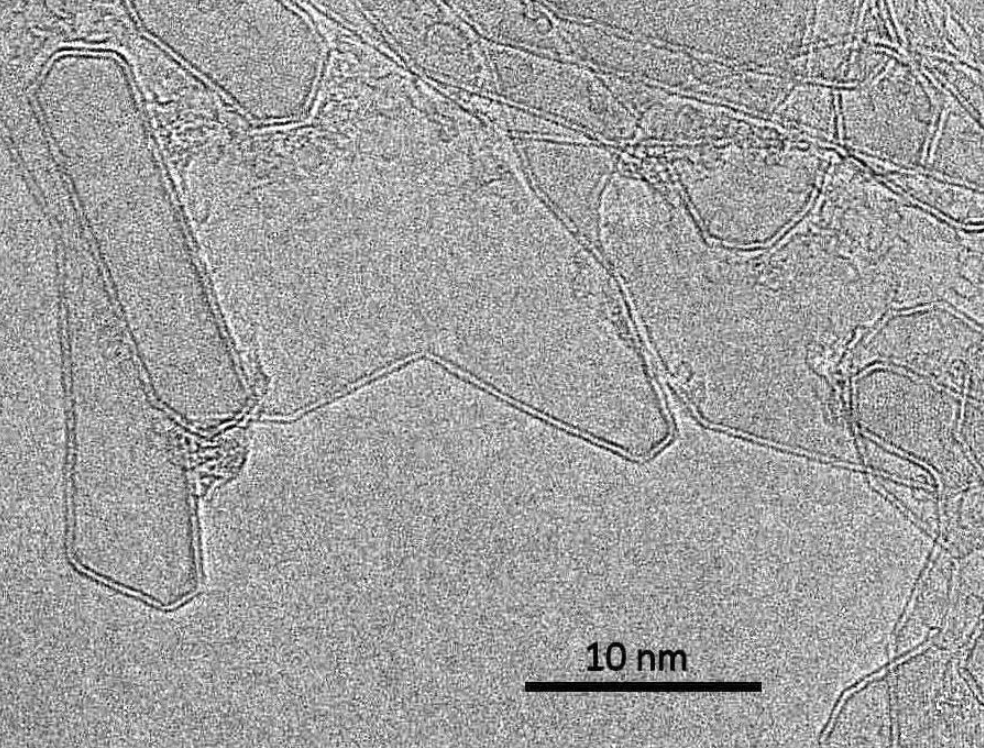
Three dimensional bilayer graphene was reported in 2009.[32][33][34] This can be produced by passing an electric current through graphite. It consists of hollow, three-dimensional shells bounded by bilayer graphene. Bilayer nanotubes and nanoparticles appear on the graphene shells, along with nanotubes seamlessly joined to the shells.
References
- K Yan; H Peng; Y Zhou; H Li; Z Liu (2011). "Formation of bilayer Bernal graphene: layer-by-layer epitaxy via chemical vapor deposition". Nano Lett. 11: 1106–10. doi:10.1021/nl104000b. Bibcode: 2011NanoL..11.1106Y. https://dx.doi.org/10.1021%2Fnl104000b
- Z Liu; K Suenaga PJF Harris; S Iijima (2009). "Open and closed edges of graphene layers". Phys. Rev. Lett. 102: 015501. doi:10.1103/physrevlett.102.015501. Bibcode: 2009PhRvL.102a5501L. https://dx.doi.org/10.1103%2Fphysrevlett.102.015501
- Min, Lola; Hovden, Robert; Huang, Pinshane; Wojcik, Michal; Muller, David A.; Park, Jiwoong (2012). "Twinning and Twisting of Tri- and Bilayer Graphene". Nano Letters 12 (3): 1609–1615. doi:10.1021/nl204547v. PMID 22329410. Bibcode: 2012NanoL..12.1609B. https://dx.doi.org/10.1021%2Fnl204547v
- E. Mostaani, N. D. Drummond and V. I. Fal'ko (2015). "Quantum Monte Carlo Calculation of the Binding Energy of Bilayer Graphene". Phys. Rev. Lett. 115: 115501. doi:10.1103/PhysRevLett.115.115501. PMID 26406840. Bibcode: 2015PhRvL.115k5501M. https://dx.doi.org/10.1103%2FPhysRevLett.115.115501
- Y Zhang; T Tang; C Girit; Z Hao; MC Martin; A Zettl; MF Crommie; YR Shen et al. (2009). "Direct observation of a widely tunable bandgap in bilayer graphene". Nature 459: 820–23. doi:10.1038/nature08105. PMID 19516337. Bibcode: 2009Natur.459..820Z. https://dx.doi.org/10.1038%2Fnature08105
- W Liu (2014). "Controllable and rapid synthesis of high-quality and large-area Bernal stacked bilayer graphene using chemical vapor deposition". Chem. Mater. 26: 907–15. doi:10.1021/cm4021854. https://dx.doi.org/10.1021%2Fcm4021854
- Y Hao (2016). "Oxygen-activated growth and bandgap tunability of large single-crystal bilayer graphene". Nature Nanotechnology 11: 820–23. doi:10.1038/nnano.2015.322. Bibcode: 2016NatNa..11..426H. https://dx.doi.org/10.1038%2Fnnano.2015.322
- VL Nguyen (2016). "Wafer-Scale Single-Crystalline AB-Stacked Bilayer Graphene". Adv. Mater.. doi:10.1002/adma.201601760. https://dx.doi.org/10.1002%2Fadma.201601760
- Min, Hongki; Sahu, Bhagawan; Banerjee, Sanjay; MacDonald, A. (2007). "Ab initio theory of gate induced gaps in graphene bilayers". Physical Review B 75 (15): 155115. doi:10.1103/PhysRevB.75.155115. Bibcode: 2007PhRvB..75o5115M. https://dx.doi.org/10.1103%2FPhysRevB.75.155115
- Y Zhang; T Tang; C Girit; Z Hao; MC Martin; A Zettl; MF Crommie; YR Shen et al. (2009). "Direct observation of a widely tunable bandgap in bilayer graphene.". Nature 459: 820–23. doi:10.1038/nature08105. PMID 19516337. Bibcode: 2009Natur.459..820Z. https://dx.doi.org/10.1038%2Fnature08105
- L Ju (2015). "Topological valley transport at bilayer graphene domain walls.". Nature 520: 650–55. doi:10.1038/nature14364. Bibcode: 2015Natur.520..650J. https://dx.doi.org/10.1038%2Fnature14364
- T Ohta (2006). "Controlling the Electronic Structure of Bilayer Graphene". Science 313: 951–954. doi:10.1126/science.1130681. Bibcode: 2006Sci...313..951O. https://dx.doi.org/10.1126%2Fscience.1130681
- A Kou (2014). "Electron-hole asymmetric integer and fractional quantum Hall effect in bilayer graphene". Science 345 (6192): 55–57. doi:10.1126/science.1250270. PMID 24994644. Bibcode: 2014Sci...345...55K. https://dx.doi.org/10.1126%2Fscience.1250270
- K Lee (2014). "Chemical potential and quantum Hall ferromagnetism in bilayer graphene". Science 345 (6192): 58–61. doi:10.1126/science.1251003. PMID 24994645. Bibcode: 2014Sci...345...58L. https://dx.doi.org/10.1126%2Fscience.1251003
- P Maher (2014). "Tunable fractional quantum Hall phases in bilayer graphene". Science 345 (6192): 61–64. doi:10.1126/science.1252875. Bibcode: 2014Sci...345...61M. https://dx.doi.org/10.1126%2Fscience.1252875
- Li J I A (2017). Even denominator fractional quantum Hall states in bilayer graphene. doi:10.1126/science.aao2521. Bibcode: 2017Sci...358..648L. https://dx.doi.org/10.1126%2Fscience.aao2521
- Barlas, Y.; Côté, R.; Lambert, J.; MacDonald, A. H. (2010). "Anomalous Exciton Condensation in Graphene Bilayers". Physical Review Letters 104 (9): 096802. doi:10.1103/PhysRevLett.104.096802. PMID 20367001. Bibcode: 2010PhRvL.104i6802B. https://dx.doi.org/10.1103%2FPhysRevLett.104.096802
- Su, J. J.; MacDonald, A. H. (2008). "How to make a bilayer exciton condensate flow". Nature Physics 4 (10): 799–802. doi:10.1038/nphys1055. Bibcode: 2008NatPh...4..799S. https://dx.doi.org/10.1038%2Fnphys1055
- Y Cao, V Fatemi, S Fang, K Watanabe, T Taniguchi, E Kaxiras, P Jarillo-Herrero (2018). "Direct observation of a widely tunable bandgap in bilayer graphene.". Nature. doi:10.1038/nature26160. Bibcode: 2018Natur.556...43C. https://dx.doi.org/10.1038%2Fnature26160
- Y Cao, V Fatemi, A Demir,, S Fang, SL Tomarken, JY Luo, J D Sanchez-Yamagishi, K Watanabe, T Taniguchi, E Kaxiras, R C Ashoori, P Jarillo-Herrero (2018). "Correlated insulator behaviour at half-filling in magic-angle graphene superlattices.". Nature. doi:10.1038/nature26154. Bibcode: 2018Natur.556...80C. https://dx.doi.org/10.1038%2Fnature26154
- "Graphene superlattices could be used for superconducting transistors". The Next Big Future. https://www.nextbigfuture.com/2018/03/graphene-superlattices-could-be-used-for-superconducting-transistors.html. Retrieved 10 April 2018.
- Fiori, Gianluca; Iannaccone, Giuseppe (March 2009). "On the Possibility of Tunable-Gap Bilayer Graphene FET". IEEE Electron Device Letters 30 (3): 261–264. doi:10.1109/led.2008.2010629. ISSN 0741-3106. Bibcode: 2009IEDL...30..261F. http://ieeexplore.ieee.org/document/4785198/.
- Schwierz, F. (2010). "Graphene transistors". Nature Nanotechnology 5 (7): 487–496. doi:10.1038/nnano.2010.89. PMID 20512128. Bibcode: 2010NatNa...5..487S. https://dx.doi.org/10.1038%2Fnnano.2010.89
- Fiori, Gianluca; Iannaccone, Giuseppe (October 2009). "Ultralow-Voltage Bilayer Graphene Tunnel FET". IEEE Electron Device Letters 30 (10): 1096–1098. doi:10.1109/led.2009.2028248. ISSN 0741-3106. Bibcode: 2009IEDL...30.1096F. http://ieeexplore.ieee.org/document/5233800/.
- Irving, Michael (May 24, 2016). "Ultra-low power graphene-based transistor could enable 100 GHz clock speeds" (in en). http://newatlas.com/graphene-transistor-clock-speeds-100-ghz/43499.
- Kühne, M (2017). "Ultrafast lithium diffusion in bilayer graphene". Nature Nanotechnology 12: 895–900. doi:10.1038/nnano.2017.108. Bibcode: 2017NatNa..12..895K. https://dx.doi.org/10.1038%2Fnnano.2017.108
- Gao, Y (2018). "Ultrahard carbon film from epitaxial two-layer graphene". Nature Nanotechnology 13: 133–138. doi:10.1038/s41565-017-0023-9. Bibcode: 2018NatNa..13..133G. https://dx.doi.org/10.1038%2Fs41565-017-0023-9
- Tue, 03/04/2014 - 3:35pm. "Researchers develop intrinsically unstacked double-layer graphene". Rdmag.com. doi:10.1038/ncomms4410. http://www.rdmag.com/news/2014/03/researchers-develop-intrinsically-unstacked-double-layer-graphene. Retrieved 2014-04-05.
- Gaufrès, E.; Tang, N. Y.-Wa; Lapointe, F.; Cabana, J.; Nadon, M.-A.; Cottenye, N.; Raymond, F.; Szkopek, T. et al. (24 November 2013). "Giant Raman scattering from J-aggregated dyes inside carbon nanotubes for multispectral imaging". Nature Photonics 8: 72–78. doi:10.1038/NPHOTON.2013.309. Bibcode: 2014NaPho...8...72G. https://dx.doi.org/10.1038%2FNPHOTON.2013.309
- Li, Q.-Q.; Zhang, X.; Han, W.-P.; Lu, Y.; Shi, W.; Wu, J.-B.; Tan, P.-H. (27 December 2014). "Raman spectroscopy at the edges of multilayer graphene". Carbon 85: 221–224. doi:10.1016/j.carbon.2014.12.096. https://dx.doi.org/10.1016%2Fj.carbon.2014.12.096
- Wu, Jiang-Bin; Zhang, Xin; Ijäs, Mari; Han, Wen-Peng; Qiao, Xiao-Fen; Li, Xiao-Li; Jiang, De-Sheng; Ferrari, Andrea C. et al. (10 November 2014). "Resonant Raman spectroscopy of twisted multilayer graphene". Nature Communications 5: 5309. doi:10.1038/ncomms6309. Bibcode: 2014NatCo...5E5309W. https://dx.doi.org/10.1038%2Fncomms6309
- Harris PJF (2009). "Ultrathin graphitic structures and carbon nanotubes in a purified synthetic graphite". J. Phys.: Condens. Matter 21: 355009. doi:10.1088/0953-8984/21/35/355009. Bibcode: 2009JPCM...21I5009H. https://dx.doi.org/10.1088%2F0953-8984%2F21%2F35%2F355009
- Harris PJF (2012). "Hollow structures with bilayer graphene walls". Carbon 50: 3195–3199. doi:10.1016/j.carbon.2011.10.050. https://dx.doi.org/10.1016%2Fj.carbon.2011.10.050
- "Bilayer graphene formed by passage of current through graphite: evidence for a three dimensional structure". Nanotechnology 25: 465601. 2014. doi:10.1088/0957-4484/25/46/465601. Bibcode: 2014Nanot..25.5601H. https://dx.doi.org/10.1088%2F0957-4484%2F25%2F46%2F465601




