1. Introduction
The interpretation of the cell’s environmental signals depends upon transducing and encoding these stimuli into a comprehensible cellular language. This selective process is facilitated by several ion channels located in the cell’s plasma membrane, among which the family of transient receptor potential (TRP) cation channels plays an essential role.
The first evidence for the existence of these channels was found in the
Drosophila melanogaster fly. Cosens and Manning
[1] analyzed a spontaneous mutant that displayed transient rather than sustained responses in the presence of prolonged bright illumination in electroretinogram (ERG) measurements
[1]. This mutation rendered the flies blind and the investigators who isolated this mutant named it the “A-type” mutant, ascribing its phenotype to a failure of photopigment regeneration
[2]. Nonetheless, several concerns were raised at the time of its isolation, with the main one being that the results were based on a single spontaneously occurring mutant, for which there was no description of its genetic background. Hence, there was a lack of knowledge on what genetic alterations this strain embodied with the results possibly being due to the additive effects of alterations in several genes mapping to the same chromosome
[3]. Pak and collaborators proved that the observed phenotype was indeed due to mutation in a single gene by isolating multiple mutated alleles from a baseline stock of known genetic background
[3]. It was also important to establish the cellular origins of the measured ERG components because it had not been determined whether the lack of a sustained response seen in the ERG of this strain originated from the photoreceptors or from other retinal cells
[3]. In 1975, Minke and collaborators performed intracellular recordings from the mutant photoreceptors and were able to determine that the defect arose from the photoreceptors. Thus, they concluded that this mutant is defective in phototransduction
[4]. Several other studies were performed with this mutant, which helped to forge a representative name for this mutant: the “transient receptor potential” or TRP
[4][5][6].
Ten years later, the
trp gene was isolated
[7] and characterized as a genomic region that encodes for some RNA species missing in the mutant fly
[8]. Interestingly, transformation of the mutant germline with the region encoding for the
trp gene reestablished the normal phototransduction phenotype in the
trp mutant fly, confirming the identity of this gene
[7]. Finally, the cloning of the
trp gene suggested a transmembrane protein with eight α-helices containing highly hydrophilic N- and C-termini, which was consistent with already known receptor/transporter/channel proteins
[9][10].
The TRP protein was localized to the rhabdomere, and hence thought to participate in phototransduction. Nonetheless, the absence of homologous proteins to the TRP in available databases and other results suggested that in null
trp alleles, there was still a persistent sustained receptor potential under dim light stimulation
[4][11] and so it was concluded that the
trp gene does not encode for the light-sensitive channel [
9,
10].
Later, it was shown that La
3+ could mimic the
trp phenotype [
12], and this led to the proposal that the
trp gene encoded for an inositide-activated Ca
2+ channel/transporter required for Ca
2+ store refilling [
13]. Voltage-clamp recording in the whole-cell configuration showed that the primary defect in the
trp mutant was a drastic reduction in the Ca
2+ permeability of the light-sensitive channels themselves [
14].
Then, another protein similar to the
trp gene product was found, the TRP-like (TRPL) [
15]. The product encoded by the
trpl gene exhibited characteristics of a transmembrane protein that contains two putative calmodulin-binding sites and an ankyrin-like repeat domain [
15]. Sequence homology analyses demonstrated that the central transmembrane region of the
trp and
trpl products resemble the transmembrane segments (S1–S6) of calcium channels, suggesting that these TRP proteins could act as ion channels [
15]. The functional description of invertebrate TRP proteins demonstrated that these proteins function as light-sensitive ion channels that allow calcium entry in
Drosophila photoreceptors [
14], and the phenotype of the
trp mutation was reinterpreted and it was suggested that the light response of
Drosophila is mediated by channels composed from the
trp and
trpl gene products [
14,
15]. In summary, there are two distinct conductances that give rise to the normal light-sensitive current: one encoded by the
trp gene that is highly Ca
2+ selective, and a second channel that is responsible for the residual light-sensitive current in the
trp mutants encoded by the homologous gene
trpl.
Remarkably, the trp/trpl gene products lack the presence of several positively-charged amino acids in their S4, the region that typically constitutes the voltage sensor in Ca2+ channels, suggesting that these invertebrate TRP proteins lack this domain. These features were the first clues about the structure of invertebrate proteins belonging to the TRP family of ion channels.
Later, these structural characteristics were identified in the first mammalian homolog of the
TRP gene isolated from human fetal brain cDNA libraries. It was named the transient receptor potential channel-related 1 (TRPC1), a protein with similar structural organization to that of TRP proteins from invertebrates [
16]. This, in turn, led to the isolation and characterization of several TRP channels, resulting in discoveries pertaining to their pivotal roles in mammalian physiology.
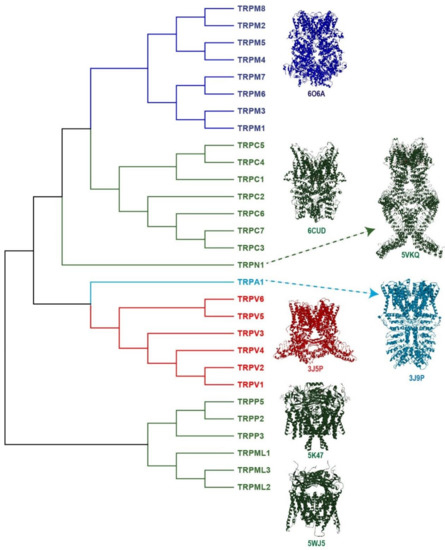
To date, TRP channels represent an extended and essential family of ion channels composed of 28 members divided according to homology in their primary sequences. This family is classified into seven subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), TRPA (ankyrin) and TRPN (no mechanoreceptor), and the latter is exclusively found in invertebrates and fish [
17] (). Additionally, another subfamily called TRPY (yeast) has been identified in vacuoles from
Saccharomyces cerevisiae [
18], although the details of its physiological roles remain unknown.
Figure 1. The Transient Receptor Potential (TRP) superfamily of ion channels. TRP ion channels are classified into seven subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), TRPA1 (ankyrin) and TRPN (no mechanoreceptor). The figure shows the phylogenetic analysis between the human TRP protein sequences; the alignment was obtained using ClustalW2 at the EMBL-EBI server. For TRPC2 (pseudogene) and TRPN (not expressed in humans), the sequences used were from mice and fish, respectively. The channels colored in dark-, light-blue and red refer to some of the channels known as the thermo-TRP, since there are activated by cool, cold and hot temperatures (TRPM8, TRPA1 and TRPV1, respectively). The figure also shows the tetrameric 3D-structure of a representative member of each subfamily. TRPM8 (6O6A), TRPC3 (6CUD), TRPN (5VKQ), TRPA1 (3J9P), TRPV1 (3J5P) TRPP2 (5K47) and TRPML1 (5WJ5). The symbols beneath each 3D-structure are the number access in the Protein Data Bank (PDB).
Interestingly, TRP channel localization and function are not limited to the cell surface. Some of these channels are intracellularly located in the membranes of organelles, regulating ion flux between the cytosol and these intracellular compartments [
19,
20]; furthermore, cell surface-located channels can also regulate calcium homeostasis in some organelles, such as the mitochondria. In this review, we will describe how TRPV1 activation modifies mitochondrial function. Here, we will discuss the data showing that the channel’s activation can induce mitochondrial dysfunction in neuronal and non-neuronal cells.
2. The TRPV1 Channel: A Key Transducer of Nociception
The discovery of the TRP channel, known as the “capsaicin” receptor or TRPV1, highlighted its essential role in mammalian nociception [
21,
22,
23]. Its isolation and cloning represented a significant step in the advancement of the pain research field, providing initial evidence of the relationship between the structure, the activity, and the physiological role of this channel [
21,
22,
23].
TRPV1 cDNA was isolated from a rodent dorsal root ganglion (DRG) library and expressed in oocytes to perform membrane recordings, confirming that capsaicin evokes currents through the activation of this channel [
22]. Full characterization of the activity and properties of TRPV1 showed that it is a nonselective cation channel which exhibits preference to Ca
2+ ions [
22].
The TRPV1 protein is abundantly expressed in small and medium diameter sensory neurons from trigeminal and dorsal root ganglia (TG and DRG, respectively), as well as in the nodose ganglia [
22,
23,
24]. Its expression is mainly associated with Substance P-producing DRG neurons, which are widely related to pain perception [
23]. Additionally, low TRPV1 expression has been reported in specific regions of the central nervous system, and in non-neuronal cells [
25,
26,
27], where elucidation of its roles in these areas has been extensively studied.
Additionally, it was demonstrated that natural compounds activate the polymodal TRPV1 channel. Examples of these compounds are capsaicin, resiniferatoxin and allicin (the pungent compound of chili peppers and chemical compounds found in
Euphorbia resinifera and garlic, respectively) [
22,
28]. Also, TRPV1 is activated by noxious heat (around 42 °C) and by extracellular acid and basic intracellular pH [
22,
23,
29]. Furthermore, TRPV1 is activated by some endogenous compounds, such as anandamide, lysophosphatidic acid (LPA), and by metabolites derived from arachidonic acid and linoleic acid byproducts, among others [
30,
31,
32,
33].
Transgenic mice lacking TRPV1 expression (TRPV1-KO) evidenced that this channel is a crucial transducer of several noxious signals that converge to integrate painful stimuli, since these mice display impaired nociception [
21].
Until now, several reports support the essential role of TRPV1 modulation through modifications such as phosphorylation, which is associated with lowering the activation threshold, usually referred as sensitization [
34]. In contrast, dephosphorylation or reduction of the channels available on the cell surface causes desensitization [
35,
36]. These phenomena are crucial in hyperalgesia and chronic pain events, where inflammatory molecules sensitize TRPV1 [
37].
2.1. TRPV1 Structural Features
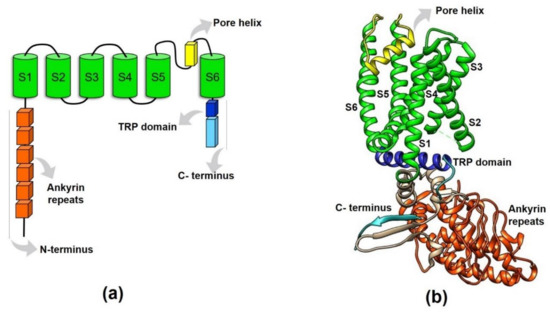
Prediction of the TRPV1 protein structure suggested it was a protein containing six transmembrane segments (S1–S6) joined by intracellular and extracellular loops [
22], where the re-entrant loop between S5 and S6 form the pore or ion conduction pathway when the channel is assembled as a homotetramer (a).
Figure 2. Structural features of the TRPV1 protein. (a) This figure schematizes the domains of a TRPV1 monomer (or subunit). The amino- and carboxy- termini are intracellularly located (N- and C- termini), the six ankyrin repeats and the TRP-box are contained in the N- and C-termini, respectively. The re-entrant loop between the S5–S6 forms the ionic conduction pore when the tetramer is formed. (b) 3D-representation of a TRPV1 subunit displaying the arrangement of the domains represented in (a). S1–S6: transmembrane segments.
The first 3D structural details of TRPV1 were obtained using X-ray crystallographic techniques [
38]. These assays confirmed the presence of a domain with six ankyrin repeats (ARD), each consisting of a pair of antiparallel α-helices linked by a finger loop. Functionally, these ankyrin repeats have an important role for the interaction between ATP and calmodulin (CaM) [
38]. Additionally, a CaM binding site located in the C-terminal region of TRPV1 was also described [
39,
40], suggesting that CaM plays a critical role in TRPV1 modulation.
A few years later, single-particle electron cryo-microscopy (cryo-EM) allowed for the solving of the first TRPV1 three-dimensional (3D) structure at a low resolution (19 Å) [
41]. This structure confirmed the tetrameric channel conformation with a large open basket-like domain corresponding to the intracellular N- and C-termini, and a compact transmembrane domain that resembles that of the transmembrane domain structure of voltage-gated potassium (Kv) and sodium (Nav) channels [
41]. TRP channels in general resemble voltage-gated ion channels. They display reminiscent structural features such as the presence of an antiparallel β-sheet from the linker region and a β-strand from the C-terminus region that make contact with two of the ankyrin repeats of an adjacent subunit (which presumably is important for channel assembly) [
42]. These features are similar to the T1 domain of Kv channels where this region plays an important role in subunit assembly [
43]. On the other hand, the wider outer pore and the shorter selectivity filter of TRPV1 also point to similarities with bacterial Nav channels [
42].
Then, a better resolution (3.4 Å) 3D structure for TRPV1 was obtained, evidencing amino acid side chains and β-sheets located in the cytosolic domain of the protein [
42] (b). This ultra-structural analysis also described the tetrameric TRPV1 structure with a central ion conduction pore conformed by two transmembrane α-helices (S5–S6) and a short pore helix (S5-P-S6), together with a voltage-sensor like domain consisting of a cluster of four transmembrane α-helices (S1–S4) [
42] (b). The structural details of TRPV1 were primarily confirmed by this new model, suggesting that the TRPV1 channel shares many structural features with voltage-gated channels and potassium channels. However, unlike Kv channels, TRPV1 is not strongly voltage-dependent due to the lack of positively-charged residues in S4 [
42].
Moreover, this approach also allowed for the resolution of the “TRP domain” within the C- terminus. It was revealed that this conserved 25 amino acid domain consists of an α-helical structure parallel to the membrane, that encompasses the first two-thirds of the TRP domain and interacts with the S4–S5 linker (b). Thus, the TRP helix seems to have a critical role in integrating allosteric modulation between the domains of the channel [
42].
2.2. Intracellular TRPV1 Localization
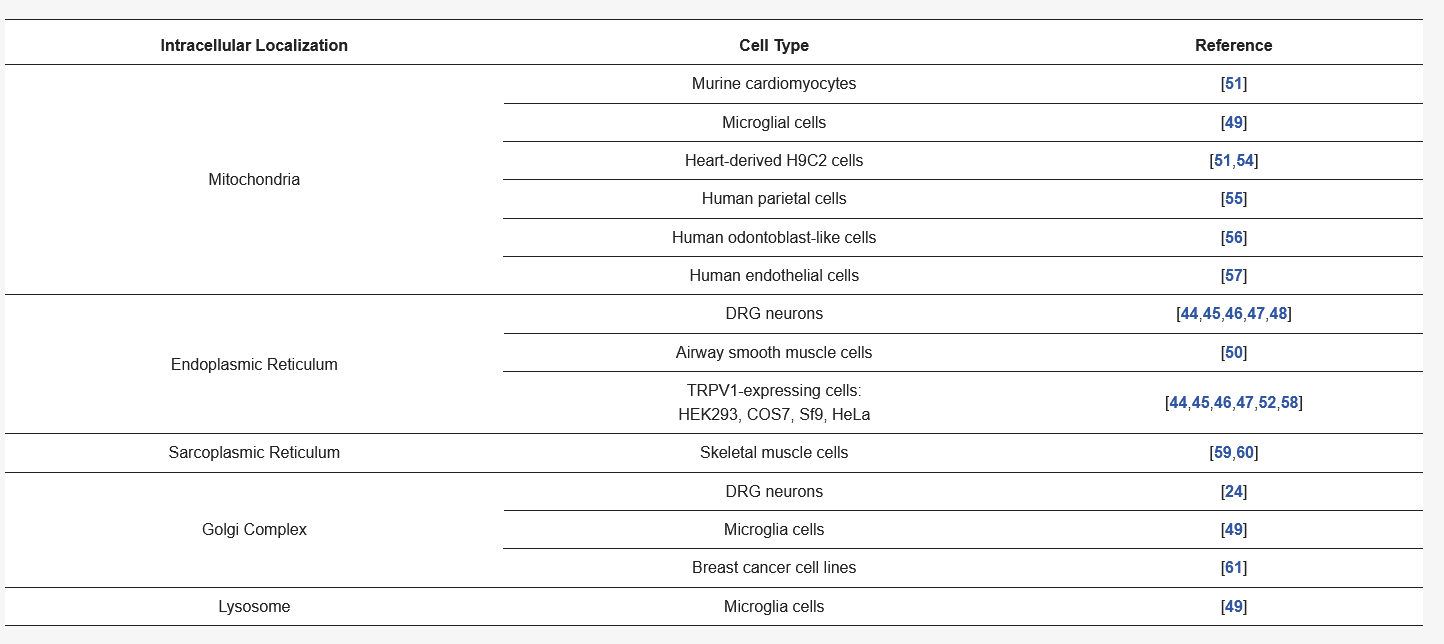
After the discovery of TRPV1 as a protein expressed in the plasma membrane of small and medium-sized DRG neurons [
22,
23,
24], its intracellular location was also reported [
24,
44,
45,
46] (). Initially, TRPV1 localization in organelles was determined in DRG neurons and TRPV1-expressing COS7 cells by using confocal and electron microscopy, demonstrating for the first time that TRPV1 was found in intracellular compartments (such as the ER) of these cells [
44,
46,
47,
48]. Further reports also revealed internal TRPV1 localization in the mitochondria and ER of TRPV1-expressing HEK293 cells, cardiomyocytes, glial cells, and muscle cells, among others [
45,
49,
50,
51,
52] (). Intracellular localization in diverse types of TRPV1-expressing cells highlights its specificity and rules out effects of overexpression of the channel in transfected HEK293 cells [
53] ().
Table 1. Intracellular localization of the TRPV1 channels in different cell types.
TRPV1 expression has also been detected in rat skeletal muscle, where this channel is mainly located in the sarcoplasmic reticulum [
60], and it has been suggested to contribute to the regulation of excitation and contraction through Ca
2+ homeostasis [
59]. Particularly, TRPV1 has been isolated from membrane fractions located at longitudinal sarcoplasmic reticula [
59]. Besides, proximity ligation assays achieved in human airway smooth muscle (ASM) cells indicated that TRPV1 is expressed in a fashion where it is closely associated to sarco/endoplasmic reticulum Ca
2+-ATPase (SERCA) pumps [
50].
Furthermore, the co-localization of TRPV1 has been reported with the 58 K Golgin protein (a marker of the Golgi complex) in microglial cells, suggesting its localization in this internal cell compartment [
49]. Also, TRPV1 protein has been visualized in the Golgi compartment of small-diameter DRG neurons and forming aggregates in the Golgi of some breast cancer cell lines [
24,
61] (). These findings could suggest the presence of an available pool of functional channels located in the Golgi or of channels being transported to the plasma membrane, an observation that needs more detailed analysis.
Remarkably, several reports support the intracellular availability of TRPV1 channels in the form of functional pools, enhancing the versatility of this channel as a cell surface signal transducer and a regulator of Ca
2+ mobilization between the cytosol and some organelles [
45,
46,
47]. Additionally, several reports show that the activation of TRPV1 channels located in the cell surface also play an essential role in regulating Ca
2+ homeostasis of intracellular compartments, such as the mitochondria.