| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Athina Trakaki | + 4506 word(s) | 4506 | 2020-12-10 08:06:12 | | | |
| 2 | Bruce Ren | -21 word(s) | 4485 | 2020-12-15 06:53:25 | | |
Video Upload Options
From an evolutionary perspective, lipoproteins are not only lipid transporters, but they also have important functions in many aspects of immunity. High-density lipoprotein (HDL) particles are the most abundant lipoproteins and the most heterogeneous in terms of their composition, structure, and biological functions. Despite strong evidence that HDL potently influences the activity of several immune cells, the role of HDL in skin diseases is poorly understood. Alterations in HDL-cholesterol levels have been observed in atopic dermatitis (eczema), psoriasis, urticaria, and angioedema. HDL-associated apolipoprotein (apo) A-I, apoA-IV, and apoC-III, and lyso-phosphatidylcholines potently suppress immune cell effector responses. Interestingly, recent studies provided evidence that skin diseases significantly affect HDL composition, metabolism, and function, which, in turn, could have a significant impact on disease progression, but may also affect the risk of cardiovascular disease and infections. Interestingly, not only a loss in function, but also, sometimes, a gain in function of certain HDL properties is observed.
1. Introduction
The prevalence of allergic and inflammatory skin diseases has dramatically increased in recent decades, a fact that is linked to changes in environmental exposures and lifestyle practices [1][2]. Despite strong evidence that high-density lipoprotein (HDL) potently influences the activity of several immune cells, including monocytes, macrophages, eosinophils, and neutrophils [3][4], the role of HDL particles in skin diseases is still poorly understood [5]. HDL particles are regarded as cholesterol transporters, mainly mediating the reverse cholesterol transport from extrahepatic peripheral tissues back to the liver. Although their association with reduced cardiovascular risk is well established [6][7][8][9], HDL-cholesterol raising therapies failed to improve the cardiovascular outcome [10][11][12], and recent studies challenged the causal role of low HDL-cholesterol levels in cardiovascular diseases [13].
HDL is quantitatively the most important lipoprotein in most species and mechanistic evidence points towards a role of HDL in physiological immune function [14], while low HDL-cholesterol levels are associated with a high risk of autoimmune disease in individuals from the general population [15]. In this context, the potential role of HDL in other diseases, such as infections and allergies, but also skin diseases, has gained much attention.
Apolipoprotein (apo) A-I is the main structural and functional apoprotein of HDL [16], and it plays a key role in the induction of cholesterol efflux from cells [17]. The interaction of HDL with cells results in cholesterol depletion in specific membrane microdomains enriched in cholesterol and sphingolipids, named lipid rafts, a mechanism that is known to disrupt raft-dependent signaling [18][19]. Their main role is the compartmentalization of molecules to form functional platforms for biological processes, such as toll-like receptors (TLRs) [20]. The lipid composition of rafts determines their function; the modification of lipid raft composition can modulate raft-dependent signaling due to protein delocalization and alter immune cell biological functions [20]. HDL, along with apoA-I, have been shown to disrupt the plasma membrane of lipid rafts in antigen presenting cells, leading to the inhibition of their capacity to stimulate T cell activation [21]. On the other hand, lyso-phosphatidylcholine, which is one of the main phospholipid subtypes carried by HDL particles [22], has been shown to directly activate TLRs 1, 2, and 4 in the absence of classical TLR-ligands; however, in the presence of classical TLR-ligands, it induces an anti-inflammatory phenotype [23]. TLRs are expressed by a plethora of cells in the skin, including Langerhans cells, keratinocytes, and several immune cells [24][25]. Furthermore, TLRs are implicated in the pathogenesis of atopic dermatitis [24][26][27] and psoriasis [24][27].
The composition and particle distribution of HDL are significantly altered in allergic and skin diseases, which ultimately lead to altered HDL functionality and an altered ability of HDL to modulate immune cell effector responses [4][28][29][30][31][32][33][34][35].
2. HDL Metabolism, Composition and Function
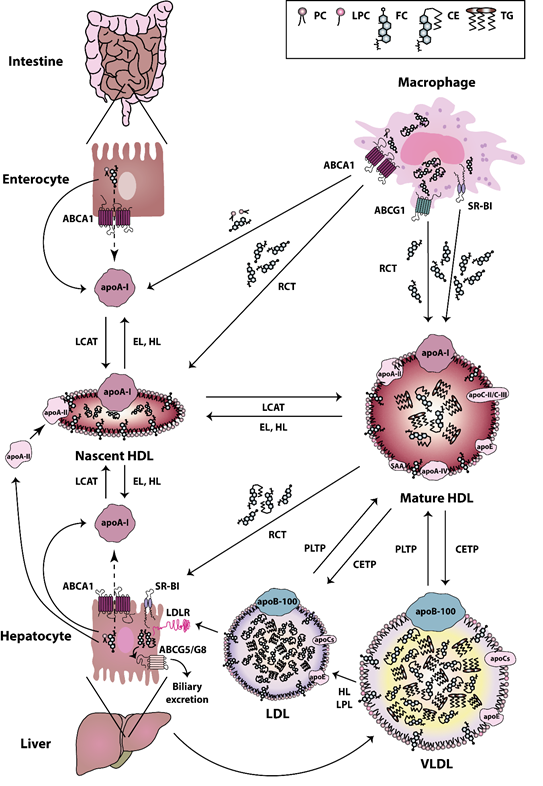
HDL particles are heterogeneous in terms of their composition, structure, and biological functions. The biogenesis of HDL is a complex process [36]. The first step in HDL formation is the secretion of apoA-I by the liver and intestine [37]. Secreted apoA-I interacts thereafter with ATP-binding cassette (ABC) transporter A1 (ABCA1), which leads to the rapid recruitment of cellular phospholipids and cholesterol to lipid-poor apoA-I. Afterwards, the lipidated apoA-I is gradually converted into discoidal HDL particles, containing unesterified cholesterol [38]. The acquisition of cholesterol and the esterification of free cholesterol to cholesteryl esters by the enzyme lecithin cholesterol acyltransferase (LCAT) [39] lead to the evolution of more mature, large-sized particles [40]. HDLs are extensively remodeled in the bloodstream via the action of lipid transfer proteins, such as cholesteryl-ester transfer protein (CETP), LCAT, and phospholipid transfer protein (PLTP). CETP is responsible for the bidirectional transfer of cholesteryl esters and triglycerides between plasma lipoproteins [41]. PLTP mediates the phospholipid transfer among lipoproteins [42], which converts HDL into larger and smaller particles [43][44]. In addition, certain lipases, such as endothelial and hepatic lipases, as well as lipid exchange with cellular transporters, such as ABCA1 and ABCG1, and scavenger receptor class B type I (SR-BI), affect HDL maturation and catabolism [45][46]. Plasma endothelial and hepatic lipases have specificity for phospholipids and triglycerides of large HDL and apoB-containing lipoproteins remnants [47][48]. The hydrolysis of triglycerides and phospholipids of HDL leads to the conversion of HDL2 into HDL3 and pre-beta HDL [44]. ABCA1 and ABCG1 both play a crucial role in the reverse cholesterol transport pathway. ABCA1 is responsible for the transfer of cellular phospholipids and cholesterol to lipid poor apoA-I, while ABCG1 promotes cholesterol efflux to more mature HDL particles [44]. SR-BI is primarily expressed by the liver, but it is also found in other tissues [49]. SR-BI absorbs cholesterol and cholesteryl ester of HDL without causing HDL degradation in the liver [50]. SR-BI also promotes cholesterol efflux from macrophages and other cell types to HDL particles, thus acting as a bidirectional cholesterol transporter [51] (Figure 1). HDL can be divided into the relatively cholesterol-rich, larger, spherical, and less dense HDL2 particles (1.063–1.125 g/mL), and the more protein-rich, smaller, and denser HDL3 particles (1.125–1.21 g/mL) [52]. The latter particles appear to display the most potent atheroprotective properties [53]. In addition to apoA-I and apoA-II, which are the main protein components, HDL particles contain other less abundant proteins, including apoA-IV, apoC-II, apoC-III, apoE, and serum amyloid A (SAA) [54]. Some studies reported that more than 100 different proteins are associated with HDL, which suggests a multiple functionality for the HDL particles [55]. Not all protein species are present on every single HDL particle, and most proteins are only carried by a small fraction of the HDL particles [56]. However, there is recent evidence that the HDL proteome of mature HDL3 and HDL2 subclasses may be less complex than expected and contains less than 20 proteins after extensive purification [57]. This seems to contradict other publications that assume a much more complex HDL proteome [56][58][59]. However, in these publications, not only HDL2 and HDL3 were isolated and investigated, but also pre-beta HDL. Therefore, the different number of identified proteins is due to the other purification strategies of the HDL subclasses. Moreover, more than 200 lipid species have been identified in HDL particles [60][61], including cholesterol (free or esterified), triglycerides, phospholipids, lyso-phospholipids, and sphingolipids [53]. The structure and dynamic properties of lipids significantly depend on their location in the particle (surface, intermediate region, core). Not only hydrophobicity, but also conformational entropy of the molecules, are the driving forces in the formation of the HDL structure [62]. For example, apoA-I has a strong preference for binding to HDL (d = 8–12 nm), as compared to larger, less curved low-density lipoproteins (LDL) (d = 20–24 nm) or very low-density lipoproteins (VLDL) (d = 40–100 nm). The high radius of curvature of HDL as compared to other lipoproteins causes packing defects of phospholipids, and this is the reason why other lipids and amphipathic proteins associate with HDL when compared to other lipoproteins [63]. In addition to the promotion of cellular cholesterol efflux, HDL particles display a number of anti-inflammatory activities, such as cytoprotective, vasodilatory, anti-oxidative, anti-thrombotic, and anti-infectious activities [53]. Among the HDL associated enzymes, paraoxonase (PON) is known to exert a protective effect against oxidative damage of circulating cells and lipoproteins and to modulate the susceptibility of HDL to atherogenic modifications, such as homocysteinylation and glycation, even exerting an anti- inflammatory role [64]. Other HDL-associated enzymes are LCAT, platelet-activating factor-acetyl hydrolase (PAF-AH) (also known as lipoprotein-associated phospholipase A2 (Lp-PLA2)), and PLTP. Among these, LCAT is responsible for the esterification of free cholesterol to cholesteryl esters [39]. PAF-AH is mainly associated with low-density lipoproteins, however about 30% is also associated with HDL [65]. PAF-AH is the major enzyme catabolizing platelet-activating factor (PAF) and PAF-like lipids, which are potent inflammatory mediators [66][67]. PLTP is a lipid transfer protein that is involved in the remodeling of HDL particles [43] and it has been reported to contribute to the anti-oxidative HDL activity [56].
Figure 1. High-density lipoprotein (HDL) metabolism. HDL metabolism is a multistep process involving (i) the secretion of lipid-free apolipoproteins by the liver or intestine, (ii) the acquisition of cholesterol and phospholipids via ATP-binding cassette (ABC) transporter A1 (ABCA1), ABCG1, and scavenger receptor class B type I (SR-BI), (iii) the maturation by lecithin cholesterol acyltransferase (LCAT)-mediated cholesterol esterification and (iv) the final uptake of lipids by the liver. Cholesterol uptake is either mediated directly via SR-BI, or indirectly via cholesteryl-ester transfer protein (CETP)-mediated transfer of cholesteryl ester to very low-density lipoproteins (VLDL) and low-density lipoproteins (LDL) and uptake by the LDL-Receptor. The liver excretes then cholesterol into the bile, either directly via the action of ABCG5/G8 transporters, or indirectly following oxidation to bile acid and secretion via ABCB11 [68][69]. Abbreviations represent: ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette subfamily G member 1; ABCG5, ATP-binding cassette subfamily G member 5; ABCG8, ATP-binding cassette subfamily G member 8; apoA-I, apolipoprotein A-I; apoA-II, apolipoprotein A-II; apoA-IV, apolipoprotein A-IV; apoB-100, apolipoprotein B-100; apoC, apolipoprotein C; apoC-II, apolipoprotein C-II; apoC-III, apolipoprotein C-III; apoE, apolipoprotein E; CE, cholesteryl ester; CETP, cholesteryl ester transfer protein; EL, endothelial lipase; FC, free cholesterol; HDL, high-density lipoprotein; HL, hepatic lipase; LCAT, lecithin-cholesterol acyltransferase; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LPC, lyso-phosphatidylcholine; LPL, lipoprotein lipase; PC, phosphatidylcholine; PLTP, phospholipid transfer protein; RCT, reverse cholesterol transport; SAA, serum amyloid A; SR-BI, scavenger receptor class B type I; TG, triglyceride; VLDL, very low-density lipoprotein.
3. HDL in Inflammatory Skin Diseases
The skin is one of the largest immunologic organs, while it is often a target for allergic and immunologic responses [70]. Immune-mediated skin diseases, such as contact dermatitis, atopic dermatitis, psoriasis, urticaria, angioedema, and autoimmune blistering disorders are becoming all the more common nowadays, while most of them are chronic and inflammatory with both environmental and genetic factors contributing [70]. Many skin disorders are known to be associated with dyslipidemia, while some of the dermatological therapies are also known to predispose to lipid abnormalities [5].
3.1. Atopic Dermatitis is Associated with Complex Alterations in HDL Composition and Function
Atopic dermatitis (or eczema) is the most common atopic disease in young children and the most common skin disease in childhood [71]. Atopic dermatitis comprises a common chronic inflammatory skin disease with heterogeneous clinical phenotypes that are determined by both genetic and epigenetic dispositions [72]. In more than half of the patients the disease starts before the age of 6, while a less frequent onset is observed after the age of 20 [73]. Atopic dermatitis has different onset patterns and disease course is associated with distinct clinical features, food intolerance, risk of concomitant allergic diseases, and impact of psychic factors on symptoms [73]. In the last years, associations of atopic dermatitis with other inflammatory diseases have been reported, including systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease [74], and increased cardiovascular risk [75][76][77][78][79].
Although there is evidence that HDL is an important modulator of the immune response, few studies have investigated the role of HDL in human atopic dermatitis. A study conducted by Schäfer et al. reported increased HDL-cholesterol levels in patients in comparison to controls [80]; however, another study by Agón-Banzo et al. on a pediatric population, along with the study by Trieb et al., reported no difference [34][81].
A further study reported that apoA-I was highly expressed in the horny layer of the skin of atopic dermatitis patients in comparison to controls and it was associated with the severity of specific eruptions [82]. In a recent study by Trieb et al., the composition of HDL was evaluated in atopic dermatitis patients and control subjects [34]. Interestingly, the authors identified complex HDL compositional alterations. Specifically, the authors observed a significant enrichment of atopic dermatitis-HDL in apoA-II, the acute-phase protein SAA, and phosphatidylinositol, while a trend towards increased sphingomyelin content of atopic dermatitis-HDL was also observed [34]. Moreover, a significant reduction in atopic dermatitis-HDL content of apoC-III, apoE, cholesteryl ester, free cholesterol, lyso-phosphatidylcholine (especially 16:0 species), and phosphatidylethanolamine was observed when compared to the control subjects [34].
Eosinophils comprise a cell subset inducing tissue damage in the inflammatory infiltrate within the dermis of atopic dermatitis patients [83]. The effector responses of HDL isolated from patients suffering from atopic dermatitis and healthy controls were evaluated in a previous study while using freshly isolated human eosinophils [34]. Eosinophils were stimulated with eotaxin-2/CCL24 in the presence or absence of HDL (isolated from patients suffering from atopic dermatitis and healthy controls) and morphological changes (evaluated by the change in shape via flow cytometry) or chemotaxis was monitored. Of particular interest, the majority of HDL that was isolated from atopic dermatitis patients increased agonist induced eosinophil effector responses when compared to control-HDL. The authors demonstrated that the HDL-associated apoC-III and lyso-phosphatidylcholine species 16:0 and 18:0 effectively suppressed eosinophil shape change and migration [34]. Interestingly, the HDL content of apoC-III and lyso-phosphatidylcholine species 16:0 and 18:0 was much lower in HDL that was isolated from atopic dermatitis patients, and it was linked to an impaired ability of HDL to supress eosinophil effector responses. Moreover, by performing a detailed correlation analysis between function and composition of HDL isolated from atopic dermatitis patients, the authors demonstrated that the HDL-triglyceride content was negatively associated with the HDL activity towards agonist-induced eosinophil shape change and migration. In contrast, the HDL-associated SAA was associated with the ability of HDL to suppress agonist-induced eosinophil shape change [34]. In addition, the HDL-associated paraoxonase activity was decreased in atopic dermatitis-HDL; however, no change was observed in the capacity of atopic dermatitis-HDL to mobilize cholesterol from cells, when compared to the control-HDL [34].
In conclusion, there is increasing evidence that atopic dermatitis is associated with profound alterations in the HDL composition, linked to the formation of dysfunctional HDL. In contrast to the HDL that was isolated from allergic rhinitis patients [28], the ability of HDL to suppress eosinophil effector responses is suppressed in atopic dermatitis, which suggests disease specific links between HDL composition, dysfunction, and disease severity.
3.2. HDL in Psoriasis
Epidemiological and clinical studies have shown a consistent association of psoriasis with systemic metabolic disorders, including an increased prevalence of diabetes, obesity, and cardiovascular disease [84]. Psoriasis is a common chronic inflammatory skin disease, which affects approximately 2–3% of the population in Western countries [85], and it is equally prevalent in both sexes [86]. Psoriasis is characterized by the appearance of red scaly plaques, affecting any part of the body, but predominately appearing over elbows and knees, on the scalp, the perianal, and the umbilical region [85]. The pathogenesis of psoriasis is complex, involving the activation of plasmacytoid dendritic cells by epidermal antigens due to skin trauma as the initial step [87], followed by maturation of myeloid dendritic cells, which promote the differentiation of T cells into Th1 and Th17 cells, via the secretion of interleukin (IL)-6, IL-12, and IL-23 [88]. Pro-inflammatory cytokines and chemokines that are produced by activated keratinocytes are able to recruit a variety of inflammatory cells from the circulation, leading to a “vicious cycle” of excessive immune response [89].
Already in the 90s, studies reported alterations in plasma lipids [90][91] and HDL-apolipoprotein content [90] in psoriatic children. The results from studies evaluating, among others, HDL-cholesterol levels between psoriasis patients and controls, vary greatly, reporting either increased [92][93][94][95], decreased [29][30][32][33][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118] or unchanged [31][119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135] levels. Interestingly, Yu et al. demonstrated an increase in the small HDL subclass in psoriasis patients, which was associated with aortic inflammation [30], while Tom et al. reported a decrease in the large HDL subclass in paediatric psoriasis patients in comparison to controls, but no change in the small or medium HDL subclasses was observed [31].
Anti-inflammatory, anti-psoriatic therapies appear to induce complex changes in the HDL-cholesterol levels. The current treatment options include topicals, such as corticosteroids, as well as agents such as anthralin, synthetic vitamin D3 and vitamin A; phototherapy, including broad and narrowband-ultraviolet B (UVB), laser UVB, and psoralen and ultraviolet A (PUVA); systemics, such as methotrexate, cyclosporine, and retinoid receptor inhibitors (acitretin); and, biological therapeutics targeting tumor necrosis factor (TNF)-alpha, IL-23p40, or IL-17 [136]. Tofacitinib, an oral janus kinase (JAK) inhibitor [137][138][139], metformin, an anti-inflammatory agent activating adenosine monophosphate-activated protein kinase (AMPK) [140], and adalimumab [141], etanercept [142], or other TNF-alpha blockers [143] appear to increase HDL-cholesterol levels; whereas, topical [108] or systemic treatment with methotrexate [144] or acitretin [145] seem to decrease HDL-cholesterol levels. Etanercept [146], anti-IL17A antibodies, such as ixekizumab [147] and secukinumab [148], or other biologic treatments [149], appear not to affect HDL-cholesterol levels. In 2014, Holzer et al. demonstrated an increase in the large HDL subclass in psoriasis patients upon systemic and/or topical treatment in comparison to baseline [32]. In 2018 Mehta et al. reported an increase in the HDL-particle number at 12 weeks of phototherapy and a trend towards increase after adalimumab treatment, however at 52 weeks of adalimumab treatment a significant reduction of the HDL-particle number was observed in comparison to the baseline [150]; while, treatment with secukinumab induced no change in HDL particle number and size [151]. In 2017, Wolk et al. reported a striking increase in total HDL particles upon different dosages of tofacitinib for four or 16 weeks in comparison to baseline measurements; specifically the authors observed an increase in the small HDL subclass, while medium and large HDL subclasses remained unchanged [137]. Much like the effects of systemic or biological therapeutics on HDL-cholesterol levels, the distribution of HDL particles is also affected, since it appears to be dependent not only on the pharmacological agent, but also on the duration of treatment. In 2012, a study evaluated several aspects of HDL composition in HDL that was isolated by ultracentrifugation in a small cohort of psoriasis patients receiving mainly topical treatment [29]. Among the main HDL-associated proteins and lipids, the authors were able to demonstrate a reduction in the levels of apoA-I, total cholesterol, cholesteryl esters, free cholesterol, phosphatidylcholine and sphingomyelin, and an increase in the levels of apoA-II and acute-phase proteins, such as SAA and α-1-antitrypsin, in HDL that is derived from psoriasis patients in comparison to the controls [29]. However, previous studies reported increased [92][93], decreased [96][106][108], or unchanged [31][98][115][119][129][135] apoA-I levels in psoriasis patients compared to healthy controls.
Due to these contradictory data, no direct and clear correlation between psoriasis and HDL quantity, particle size distribution, or composition has been demonstrated so far. Further studies are necessary in order to understand the observed effects.
However, the effects of anti-psoriatic therapy on some metrics of HDL function are more evident. During the last decade, studies coming from several groups have demonstrated significantly impaired HDL-cholesterol efflux capacity in psoriasis patients in comparison to controls [29][31][32][33], which appeared to recover upon systemic and/or topical treatment [32]. HDL-mediated cholesterol efflux capacity was negatively associated with psoriasis area severity index score [29][32], being significantly impaired in patients with higher psoriasis area severity index score [31], while it was positively associated with impaired levels of apoA-I, phosphatidylcholine, sphingomyelin [29], and total phospholipid HDL content [32]. A recent study conducted by Mehta et al. indicated reduced cholesterol efflux capacity at 52 weeks of adalimumab treatment [150]. The JAK inhibitor tofacitinib showed no change in cholesterol efflux capacity upon a 16-week treatment, as was recently reported by Wolk et al. [137], while secukinumab treatment for 12 or 52 weeks also induced no change [151].
Furthermore, the anti-inflammatory potential of HDL was markedly impaired in psoriasis patients when compared to controls [115]. Of particular interest, a study identified apoA-I, HDL-cholesterol, and HDL-cholesterol efflux capacity to be predictors of noncalcified coronary burden in psoriasis [152]. Moreover, an improved HDL-associated Lp-PLA2 activity in patients in comparison to controls was observed [29][32], which was positively correlated with the psoriasis area severity index score [29]. Upon systemic and/or topical treatment or biologic treatment, patients showed improved LCAT activity in comparison to the baseline [32][137].
In conclusion, recent studies provided clear evidence that psoriasis affects HDL composition that is linked to a significantly impaired capability to mobilize cholesterol from macrophages, a crucial step in reverse cholesterol transport. HDL quantity and other functionalities assessed in psoriasis patients, including paraoxonase activity and anti-oxidative properties of HDL, are contradictory. Interestingly, as demonstrated by Asefi et al., PON 55 methionine allele is a risk factor for psoriasis [153]. However, in psoriasis patients, unchanged [29], improved [134][135], or impaired [32][115][116][133][154][155][156] paraoxonase activity was observed in comparison to healthy controls. Rocha-Pereira et al. showed a significantly reduced total anti-oxidant potential in patients in comparison to controls [113], while others observed no difference in the anti-oxidant HDL capacity [29][32].
All of these data only suggest a loss of cholesterol efflux capacity of HDL in patients with psoriasis, corresponding to the increased cardiovascular risk of these patients, while other metrics of HDL quantity and quality are inconclusive. This also suggests that studying the influence of anti-psoriatic agents on HDL-cholesterol efflux capacity may help to identify treatment strategies with beneficial effects on long-term cardiovascular outcome.
3.3. HDL in Urticaria
Urticaria is a common chronic clinical condition that presents with angioedema, wheals (hives), or both [157], occurring in 15–25% of individuals at some point of life [158], and it is one of the 10 most common dermatoses [159]. Urticaria presents a high burden for the patient due to its chronic course and the difficulties in diagnosis and treatment, ultimately reducing performance and quality of life [160]. Urticaria is characterized by a recurrent, pruritic, wheals of pale, central swelling, and surrounding epidermal erythema, with the potential of appearance over any part of the body and with lesions ranging in size from a few millimeters to several centimeters [158]. Mast cells are the primary effector cells in urticaria, and their degranulation leads to a rapid release of a plethora of inflammatory mediators, such as leukotrienes, prostaglandins, and histamine, which, in turn, cause vasodilation and leakage of plasma below and in the skin. A more delayed secretion of inflammatory cytokines follows, including IL-4, IL-5, and TNF-alpha, potentially leading to further inflammatory responses and longer lasting lesions [161]. The pathogenesis, classification, diagnosis, and treatment options of urticaria have been extensively reviewed elsewhere [157][158][162], and they are not in the focus of the current review.
A study conducted by Amin and Rushdy has recently demonstrated significantly decreased serum levels of HDL-cholesterol in chronic spontaneous urticaria patients in comparison to control subjects, which were negatively associated with TNF-alpha [163]. Similarly, another study also demonstrated a reduction of serum HDL-cholesterol levels in chronic spontaneous urticaria patients in comparison to the controls, with HDL-cholesterol levels being negatively associated with right and left carotid intima media thickness, discussing the likelihood of a potentially increased atherosclerosis risk in those patients [164]. Further studies are warranted in order to confirm a potential link of HDL and urticaria.
3.4. HDL in Angioedema
Angioedema, in the absence of urticaria, is a rare condition that manifests itself by sudden, localized, non-pitting, erythematous, or skin-colored swelling of certain body parts, including the skin, mucous membranes, or both, the upper respiratory and intestinal epithelial linings [165]. Heat and pain comprise additional symptoms of the skin, although they are hardly accompanied by itching, desquamation, or staining of the skin [165]. When present, angioedema should be diagnosed with caution, since alternative diagnoses, including acquired angioedema, hereditary angioedema, or angioedema that is associated with angiotensin-converting enzyme inhibitors, all comprising life-threatening conditions, might also be true [158]. It can be further classified to idiopathic, histaminergic, hereditary type I, hereditary type II, and hereditary with normal C1 inhibitor, acquired and angiotensin-converting enzyme (ACE) inhibitor-induced [158]. Angioedema results from the release of vasoactive mediators, which increase the vascular permeability in the skin and submucosa, leading to plasma vascular leakage and a resulting edema, which can be attributed either to bradykinin- or to histamine-mediated mechanisms [158]. The exact pathophysiology, diagnosis, and treatment options have been described elsewhere [158][165], and they are not in the scope of this review.
Several different studies have determined the serum levels of HDL-cholesterol in angioedema patients, however currently no literature on potential HDL-associated compositional alterations in angioedema is available. A study conducted by Sloane et al. evaluating the potential side effects of long-term stanozolol therapy in hereditary angioedema patients has revealed reduced HDL-cholesterol levels after stanozolol treatment is some of the patients [166]. Other studies, evaluating possible adverse effects of danazol treatment, revealed significantly lower levels of serum HDL-cholesterol [167][168] and apoA-I in danazol treated patients when compared to control groups (either untreated patients or patients without long-term danazol treatment), as well as a higher risk of abnormally low HDL-cholesterol levels in danazol treated patients, indicating that long-term use of this drug is associated with increased early atherosclerosis risk [167]. A similar study by Birjmohun et al., which evaluated the effects of short- and long-term danazol treatment, revealed decreased apoA-I and HDL-cholesterol levels in short-term treated patients in comparison to the baseline measures, while long-term treatment did not adversely affect HDL-cholesterol concentration and apolipoproteins between patients and controls [35]. A more recent study by Nebenführer et al. revealed that danazol treated patients suffering from hereditary angioedema with C1 inhibitor deficiency had higher cardiovascular risk, as evaluated by the high body mass index and LDL/HDL ratio, in comparison to healthy controls [169].
Currently, information on functionality of HDL-associated enzymes in angioedema patients is only available by the study of Birjmohun et al., which evaluated the effects of short- and long-term danazol treatment in hereditary angioedema patients. This study revealed no adverse effects of short- and long-term danazol treatment on PON-1, PLTP, and CETP activities along with CETP mass between patients and controls. However, a trend towards decreased LCAT activity was observed in the long-term, although unaltered in the short-term danazol treated patients [35]. Further studies in larger cohorts are necessary in order to confirm the observed effects and understand the possible pathophysiological role of HDL in angioedema.
4. Conclusions
From an evolutionary point of view, lipoproteins display important functions in many aspects of immunity. Of all lipoproteins, HDL has the highest affinity for binding and neutralizing pathogen-associated lipids (e.g., LPS and lipoteichoic acid) [40][170], which mediate excessive immune activation in bacterial infections [40][171][172]. Research into the composition, distribution, and functionality of HDL particles in skin diseases has begun to attract attention, with several groups demonstrating changes in the composition and function of HDL.
A major weakness in HDL research is that HDL-cholesterol levels vary widely between different studies of the same disease background, which is possibly due to different study design, disease duration, or the presence of concomitant diseases, making it difficult to draw firm conclusions. However, the results of several studies provide compelling evidence that skin diseases significantly affect the composition and metabolism of HDL, which, in turn, could have a significant impact on disease progression and the risk of infection and cardiovascular disease.
HDL particles in inflammatory skin diseases have an altered composition, which results in an altered functionality; however, these changes are not consistent for different pathological backgrounds. Currently, there are no tests available for measuring the composition, function, and inflammatory properties of HDL in clinical practice. It is not clear to what extent inflammatory-HDL alterations are a driving force or only a biomarker of the disease. Future studies are needed in order to demonstrate causality.
References
- Chante Karimkhani; Robert P. Dellavalle; Luc E. Coffeng; Carsten Flohr; Roderick J. Hay; Sinéad M. Langan; Elaine O. Nsoesie; Alize J. Ferrari; Holly E. Erskine; Jonathan I. Silverberg; et al.Theo VosMorteza Naghavi Global Skin Disease Morbidity and Mortality. JAMA Dermatology 2017, 153, 406-412, 10.1001/jamadermatol.2016.5538.
- Ruby Pawankar; Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organization Journal 2014, 7, 1-12, 10.1186/1939-4551-7-12.
- Bi‐Lian Yu; Shu‐Hui Wang; Daoquan Peng; Shui‐Ping Zhao; HDL and immunomodulation: an emerging role of HDL against atherosclerosis. Immunology & Cell Biology 2010, 88, 285-290, 10.1038/icb.2009.112.
- A. L. Catapano; Angela Pirillo; Fabrizia Bonacina; Giuseppe Danilo Norata; HDL in innate and adaptive immunity. Cardiovascular Research 2014, 103, 372-383, 10.1093/cvr/cvu150.
- Manjunath M Shenoy; Chetana Shenoy; Gururaja K. Rao; Dyslipidemia in dermatological disorders. North American Journal of Medical Sciences 2015, 7, 421-8, 10.4103/1947-2714.168657.
- The Emerging Risk Factors Collaboration* The Emerging Risk Factors Collaboration*; Major Lipids, Apolipoproteins, and Risk of Vascular Disease. JAMA 2009, 302, 1993-2000, 10.1001/jama.2009.1619.
- Gordon, T.; Castelli, W.; Hjortland, M.C.; Kannel, W.B; Dawber T.R.; High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. The American Journal of Medicine 1977, 62, 707-714, 10.1016/0002-9343(77)90899-3.
- P W Wilson; R D Abbott; W P Castelli; High density lipoprotein cholesterol and mortality. The Framingham Heart Study.. Arteriosclerosis: An Official Journal of the American Heart Association, Inc. 1988, 8, 737-741, 10.1161/01.atv.8.6.737.
- D J Gordon; J L Probstfield; R J Garrison; J D Neaton; W P Castelli; J D Knoke; D R Jacobs; S Bangdiwala; H A Tyroler; High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies.. Circulation 1989, 79, 8-15, 10.1161/01.cir.79.1.8.
- I. Karalis; J. Wouter Jukema; HDL Mimetics Infusion and Regression of Atherosclerosis: Is It Still Considered a Valid Therapeutic Option?. Current Cardiology Reports 2018, 20, 1-8, 10.1007/s11886-018-1004-9.
- Stephen J. Nicholls; Jordan Andrews; John J.P. Kastelein; Bela Merkely; Steven E. Nissen; Kausik K. Ray; Gregory G. Schwartz; Stephen G. Worthley; Connie Keyserling; Jean-Louis Dasseux; et al.Liddy GriffithSusan W. KimAlex JanssanGiuseppe Di GiovanniAnthony D. PisanielloDaniel J SchererPeter J PsaltisJulie Butters Effect of Serial Infusions of CER-001, a Pre-β High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial. JAMA Cardiology 2018, 3, 815-822, 10.1001/jamacardio.2018.2121.
- Jane Armitage; Michael V. Holmes; David Preiss; Cholesteryl Ester Transfer Protein Inhibition for Preventing Cardiovascular Events. Journal of the American College of Cardiology 2019, 73, 477-487, 10.1016/j.jacc.2018.10.072.
- Michael V. Holmes; Folkert W. Asselbergs; Tom M. Palmer; Fotios Drenos; Matthew B. Lanktree; Christopher P. Nelson; Caroline E. Dale; Sandosh Padmanabhan; Chris Finan; Daniel I. Swerdlow; et al.Vinicius TraganteErik P.A. Van IperenSuthesh SivapalaratnamSonia ShahClara C. ElbersTina ShahJorgen EngmannClaudia GiambartolomeiJon WhiteDelilah ZabanehReecha SofatStela McLachlanPieter A. DoevendansAnthony J. BalmforthAlistair S. HallKari E. NorthBerta AlmogueraRon C. HoogeveenMary CushmanMyriam FornageSanjay R. PatelSusan RedlineDavid S. SiscovickMichael Y. TsaiKonrad J. KarczewskiMarten H. HofkerW. Monique VerschurenMichiel L. BotsYvonne T. Van Der SchouwOlle MelanderAnna F. DominiczakRichard MorrisYoav Ben-ShlomoJackie PriceMeena KumariJens BaumertAnnette PetersBarbara ThorandWolfgang KoenigTom R. GauntSteve E. HumphriesRobert ClarkeHugh WatkinsMartin FarrallJames G. WilsonStephen S. RichPaul I.W. De BakkerLeslie A. LangeGeorge Davey SmithAlex P. ReinerPhilippa J. TalmudMika KivimäkiDebbie A. LawlorFrank DudbridgeNilesh J. SamaniBrendan J. KeatingAroon D. HingoraniJuan P. Casason behalf of the UCLEB consortium Mendelian randomization of blood lipids for coronary heart disease. European Heart Journal 2015, 36, 539-550, 10.1093/eurheartj/eht571.
- Giuseppe Danilo Norata; Angela Pirillo; Enrico Ammirati; Alberico L Catapano; Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 2012, 220, 11-21, 10.1016/j.atherosclerosis.2011.06.045.
- Christian M. Madsen; Anette Varbo; Børge G. Nordestgaard; Low HDL Cholesterol and High Risk of Autoimmune Disease: Two Population-Based Cohort Studies Including 117341 Individuals. Clinical Chemistry 2019, 65, 644-652, 10.1373/clinchem.2018.299636.
- W. Sean Davidson; Thomas B. Thompson; The Structure of Apolipoprotein A-I in High Density Lipoproteins. Journal of Biological Chemistry 2007, 282, 22249-22253, 10.1074/jbc.r700014200.
- Javaheri, A.; Rader, D.J.; Apolipoprotein A-I and Cholesterol Efflux The Good, the Bad, and the Modified. Circ. Res. 2014, 114, 1681–1683, https://doi.org/10.1161/CIRCRESAHA.114.303974.
- Mary Sorci-Thomas; Michael J. Thomas; High Density Lipoprotein Biogenesis, Cholesterol Efflux, and Immune Cell Function. Arteriosclerosis, Thrombosis, and Vascular Biology 2012, 32, 2561-2565, 10.1161/atvbaha.112.300135.
- Michael B. Fessler; John S. Parks; Intracellular Lipid Flux and Membrane Microdomains as Organizing Principles in Inflammatory Cell Signaling. The Journal of Immunology 2011, 187, 1529-1535, 10.4049/jimmunol.1100253.
- Pallavi Varshney; Vikas Yadav; N Saini; Lipid rafts in immune signalling: current progress and future perspective. Immunology 2016, 149, 13-24, 10.1111/imm.12617.
- Shu-Hui Wang; Shuguang Yuan; Dao-Quan Peng; Shuiping Zhao; HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis 2012, 225, 105-114, 10.1016/j.atherosclerosis.2012.07.029.
- Shi-Hui Law; Mei-Lin Chan; Gopal Kedihithlu Marathe; Farzana Parveen; Chu-Huang Chen; Liang-Yin Ke; An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. International Journal of Molecular Sciences 2019, 20, 1149, 10.3390/ijms20051149.
- Alan Brito Carneiro; Bruna Maria Ferreira Iaciura; Lilian Lie Nohara; Carla Duque Lopes; Esteban Mauricio Cordero Veas; Vania Sammartino Mariano; Patricia Torres Bozza; Ulisses Gazos Lopes; Georgia Correa Atella; Igor C. Almeida; et al.Mário Alberto Cardoso Silva-Neto Lysophosphatidylcholine Triggers TLR2- and TLR4-Mediated Signaling Pathways but Counteracts LPS-Induced NO Synthesis in Peritoneal Macrophages by Inhibiting NF-κB Translocation and MAPK/ERK Phosphorylation. PLoS ONE 2013, 8, e76233, 10.1371/journal.pone.0076233.
- Lloyd S. Miller; Robert L. Modlin; Toll-like receptors in the skin.. Seminars in Immunopathology 2007, 29, 15-26, 10.1007/s00281-007-0061-8.
- Kawasaki, T.; Kawai, T.; Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461, https://doi.org/10.3389/fimmu.2014.00461.
- Sammy S.W. Kang; Lynda S. Kauls; Anthony A. Gaspari; Toll-like receptors: Applications to dermatologic disease. Journal of the American Academy of Dermatology 2006, 54, 951-983, 10.1016/j.jaad.2005.05.004.
- Jamie E. McInturff; Robert L. Modlin; Jenny Kim; The Role of Toll-like Receptors in the Pathogenesis and Treatment of Dermatological Disease. Journal of Investigative Dermatology 2005, 125, 1-8, 10.1111/j.0022-202x.2004.23459.x.
- Athina Trakaki; Gunter J. Sturm; Gudrun Pregartner; Hubert Scharnagl; Thomas O. Eichmann; Markus Trieb; Eva Knuplez; Michael Holzer; Julia T. Stadler; Akos Heinemann; et al.Eva M. SturmGunther Marsche Allergic rhinitis is associated with complex alterations in high-density lipoprotein composition and function. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2019, 1864, 1280-1292, 10.1016/j.bbalip.2019.06.007.
- Michael Holzer; Peter Wolf; Sanja Curcic; Ruth Birner-Gruenberger; Wolfgang Weger; Martin Inzinger; Dalia El-Gamal; Christian Wadsack; Akos Heinemann; Gunther Marsche; et al. Psoriasis alters HDL composition and cholesterol efflux capacity. Journal of Lipid Research 2012, 53, 1618-1624, 10.1194/jlr.m027367.
- Yiding Yu; Nikhil Sheth; Parasuram Krishnamoorthy; Babak Saboury; Anna Raper; Amanda Baer; Rachel Ochotony; Julia Doveikis; Stephanie DerOhannessian; Abby S Van Voorhees; et al.Drew A TorigianAbass AlaviJoel M GelfandNehal N. Mehta Aortic vascular inflammation in psoriasis is associated with HDL particle size and concentration: a pilot study. American journal of cardiovascular disease 2012, 2, 285-292.
- Wynnis L. Tom; Martin P. Playford; Shehla Admani; Balaji Natarajan; Aditya A. Joshi; Lawrence F. Eichenfield; Nehal N. Mehta; Characterization of Lipoprotein Composition and Function in Pediatric Psoriasis Reveals a More Atherogenic Profile. Journal of Investigative Dermatology 2016, 136, 67-73, 10.1038/jid.2015.385.
- Michael Holzer; Peter Wolf; Martin Inzinger; Markus Trieb; Sanja Curcic; Lisa Pasterk; Wolfgang Weger; Akos Heinemann; Gunther Marsche; Anti-Psoriatic Therapy Recovers High-Density Lipoprotein Composition and Function. Journal of Investigative Dermatology 2014, 134, 635-642, 10.1038/jid.2013.359.
- Nehal N. Mehta; Ron Li; Parasuram Krishnamoorthy; Yiding Yu; William Farver; Amrith Rodrigues; Anna Raper; Mackenzie Wilcox; Amanda Baer; Stephanie DerOhannesian; et al.Megan WolfeMuredach P. ReillyDaniel J. RaderAbby VanVoorheesJoel M. Gelfand Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012, 224, 218-221, 10.1016/j.atherosclerosis.2012.06.068.
- Markus Trieb; Peter Wolf; Eva Knuplez; Wolfgang Weger; Christian Schuster; Miriam Peinhaupt; Michael Holzer; Athina Trakaki; Thomas Eichmann; Achim Lass; et al.Christian WadsackRufina SchuligoiAkos HeinemannGunther Marsche Abnormal composition and function of high-density lipoproteins in atopic dermatitis patients. Allergy 2019, 74, 398-402, 10.1111/all.13620.
- Birjmohun, R.S.; Kees Hovingh, G.; Stroes, E.S.G.; Hofstra, J.J.; Dallinga-Thie, G.M.; Meijers, J.C.M.; Kastelein, J.J.P.; Levi, M.; Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: Two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin. Ther. 2008, 30, 2314–2323, https://doi.org/10.1016/j.clinthera.2008.12.021.
- Vassilis I Zannis; Angeliki Chroni; Kyriakos E Kypreos; Horng-Yuan Kan; Thais Borges Cesar; Eleni E Zanni; Dimitris Kardassis; Probing the pathways of chylomicron and HDL metabolism using adenovirus-mediated gene transfer. Current Opinion in Lipidology 2004, 15, 151-166, 10.1097/00041433-200404000-00008.
- Vassilis I. Zannis; F. Session Cole; Cynthia L. Jackson; David M. Kurnit; Sotirios K. Karathanasis; Distribution of apolipoprotein A-I, C-II, C-III, and E mRNA in fetal human tissues. Time-dependent induction of apolipoprotein E mRNA by cultures of human monocyte-macrophages. Biochemistry 1985, 24, 4450-4455, 10.1021/bi00337a028.
- Vassilis I. Zannis; Panagiotis Fotakis; Georgios Koukos; Dimitris Kardassis; Christian Ehnholm; Matti Jauhiainen; Angeliki Chroni; HDL Biogenesis, Remodeling, and Catabolism. Handbook of Experimental Pharmacology 2014, 224, 53-111, 10.1007/978-3-319-09665-0_2.
- Michael I. Mackness; Paul N. Durrington; HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis 1995, 115, 243-253, 10.1016/0021-9150(94)05524-m.
- Olivier Meilhac; Sébastien Tanaka; David Couret; High-Density Lipoproteins Are Bug Scavengers. Biomolecules 2020, 10, 598, 10.3390/biom10040598.
- Barter, P.J.; Brewer, H.B.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R.; Cholesteryl Ester Transfer Protein A Novel Target for Raising HDL and Inhibiting Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167, https://doi.org/10.1161/01.ATV.0000054658.91146.64.
- Meng Zhang; Xiaobo Zhai; Jinping Li; John J. Albers; Simona Vuletic; Gang Ren; Structural basis of the lipid transfer mechanism of phospholipid transfer protein (PLTP). Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2018, 1863, 1082-1094, 10.1016/j.bbalip.2018.06.001.
- John J Albers; Marian C Cheung; Emerging roles for phospholipid transfer protein in lipid and lipoprotein metabolism. Current Opinion in Lipidology 2004, 15, 255-260, 10.1097/00041433-200406000-00004.
- Von Eckardstein, A.; Kardassis, D.; High Density Lipoproteins. Handbook of Experimental Pharmacology 2015, 224, 13, 10.1007/978-3-319-09665-0.
- Gary F. Lewis; Daniel J. Rader; New Insights Into the Regulation of HDL Metabolism and Reverse Cholesterol Transport. Circulation Research 2005, 96, 1221-1232, 10.1161/01.res.0000170946.56981.5c.
- Laurent Yvan-Charvet; Nan Wang; Alan R. Tall; Role of HDL, ABCA1, and ABCG1 Transporters in Cholesterol Efflux and Immune Responses. Arteriosclerosis, Thrombosis, and Vascular Biology 2010, 30, 139-143, 10.1161/atvbaha.108.179283.
- Cyrille Maugeais; Uwe J. F. Tietge; Uli C. Broedl; Dawn Marchadier; William Cain; Mary G. McCoy; Sissel Lund-Katz; Jane M. Glick; Daniel J. Rader; Dose-Dependent Acceleration of High-Density Lipoprotein Catabolism by Endothelial Lipase. Circulation 2003, 108, 2121-2126, 10.1161/01.cir.0000092889.24713.dc.
- Silvia Santamarina-Fojo; Herminia González-Navarro; Lita Freeman; Elke Wagner; Zengxuan Nong; Hepatic Lipase, Lipoprotein Metabolism, and Atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology 2004, 24, 1750-1754, 10.1161/01.atv.0000140818.00570.2d.
- Susan Acton; Attilio Rigotti; Katherine T. Landschulz; Shangzhe Xu; Helen H. Hobbs; Monty Krieger; Identification of Scavenger Receptor SR-BI as a High Density Lipoprotein Receptor. Science 1996, 271, 518-520, 10.1126/science.271.5248.518.
- Monty Krieger; Charting the Fate of the “Good Cholesterol”: Identification and Characterization of the High-Density Lipoprotein Receptor SR-BI. Annual Review of Biochemistry 1999, 68, 523-558, 10.1146/annurev.biochem.68.1.523.
- Tamara A. Pagler; Sebastian Rhode; Angelika Neuhofer; Hildegard Laggner; Wolfgang Strobl; Claudia Hinterndorfer; Ivo Volf; Margit Pavelka; Erik R. M. Eckhardt; Deneys R. Van Der Westhuyzen; et al.Gerhard J. SchützHerbert Stangl SR-BI-mediated High Density Lipoprotein (HDL) Endocytosis Leads to HDL Resecretion Facilitating Cholesterol Efflux. Journal of Biological Chemistry 2006, 281, 11193-11204, 10.1074/jbc.m510261200.
- Anatol Kontush; Mats Lindahl; Marie Lhomme; Laura Calabresi; M. John Chapman; W. Sean Davidson; Structure of HDL: Particle Subclasses and Molecular Components. Handbook of Experimental Pharmacology 2014, 224, 3-51, 10.1007/978-3-319-09665-0_1.
- Laurent Camont; M. John Chapman; Anatol Kontush; Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends in Molecular Medicine 2011, 17, 594-603, 10.1016/j.molmed.2011.05.013.
- Tomas Vaisar; Proteomics investigations of HDL: challenges and promise.. Curr Vasc Pharmacol 2012, 10, 410-21, 10.2174/157016112800812755.
- John T Wilkins; Henrique S Seckler; HDL modification: recent developments and their relevance to atherosclerotic cardiovascular disease. Current Opinion in Lipidology 2019, 30, 24-29, 10.1097/mol.0000000000000571.
- Davidson, W.S.; Silva, R.A.G.D.; Chantepie, S.; Lagor, W.R.; Chapman, M.J.; Kontush, A.; Proteomic Analysis of Defined HDL Subpopulations Reveals Particle-Specific Protein Clusters. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 870–876, https://doi.org/10.1161/ATVBAHA.109.186031.
- Michael Holzer; Sabine Kern; Ruth Birner-Grünberger; Sanja Curcic; Akos Heinemann; Gunther Marsche; Refined purification strategy for reliable proteomic profiling of HDL2/3: Impact on proteomic complexity. Scientific Reports 2016, 6, 1–10, 10.1038/srep38533.
- Baohai Shao; Jay W. Heinecke; Quantifying HDL proteins by mass spectrometry: how many proteins are there and what are their functions?. Expert Review of Proteomics 2017, 15, 31-40, 10.1080/14789450.2018.1402680.
- HDL Proteome Watch. The Davidson & Shah Lab Websitehttps://homepages.uc.edu/~davidswm/HDLproteome.html
- Philipp Wiesner; Katharina Leidl; Alfred Boettcher; Gerd Schmitz; Gerhard Liebisch; Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. Journal of Lipid Research 2009, 50, 574-585, 10.1194/jlr.d800028-jlr200.
- Max Scherer; Alfred Böttcher; Gerhard Liebisch; Lipid profiling of lipoproteins by electrospray ionization tandem mass spectrometry. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2011, 1811, 918-924, 10.1016/j.bbalip.2011.06.016.
- Timo Vuorela; Andrea Catte; Perttu S. Niemelä; Anette Hall; Marja T. Hyvönen; Siewert-Jan Marrink; Mikko Karttunen; Ilpo Vattulainen; Role of Lipids in Spheroidal High Density Lipoproteins. PLOS Computational Biology 2010, 6, e1000964, 10.1371/journal.pcbi.1000964.
- Nicholas M. Frame; Olga Gursky; Structure of serum amyloid A suggests a mechanism for selective lipoprotein binding and functions: SAA as a hub in macromolecular interaction networks. FEBS Letters 2016, 590, 866-879, 10.1002/1873-3468.12116.
- Gianna Ferretti; Tiziana Bacchetti; C. Moroni; S. Savino; A. Liuzzi; F. Balzola; V. Bicchiega; Paraoxonase Activity in High-Density Lipoproteins: A Comparison between Healthy and Obese Females. The Journal of Clinical Endocrinology & Metabolism 2005, 90, 1728-1733, 10.1210/jc.2004-0486.
- D M Stafforini; T M McIntyre; M E Carter; Stephen M. Prescott; Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor.. Journal of Biological Chemistry 1987, 262, 4215–4222.
- Snyder, F.; Platelet-activating factor and its analogs: Metabolic pathways and related intracellular processes. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 1995, 1254, 231–249.
- D M Stafforini; T M McIntyre; G A Zimmerman; Stephen M. Prescott; Platelet-activating Factor Acetylhydrolases. Journal of Biological Chemistry 1997, 272, 17895-17898, 10.1074/jbc.272.29.17895.
- Zbyszko Chowaniec; Anna Skoczyńska; Plasma lipid transfer proteins: The role of PLTP and CETP in atherogenesis. Advances in Clinical and Experimental Medicine 2018, 27, 429-436, 10.17219/acem/67968.
- Arnold Von Eckardstein; Tachometer for Reverse Cholesterol Transport?. Journal of the American Heart Association 2012, 1, e003723, 10.1161/jaha.112.003723.
- Fonacier, L.S.; Dreskin, S.C.; Leung, D.Y.M.; Mineola, C.; Denver, A.; Allergic Skin Diseases. J Allergy Clin Immunol. 2010, 125, S138–S149.
- Hywel Williams; Colin Robertson; Alistair Stewart; Nadia Aït-Khaled; Gabriel Anabwani; Ross Anderson; Innes Asher; Richard Beasley; Bengt Björkstén; Michael Burr; et al.Tadd ClaytonJulian CranePhilippa EllwoodUlrich KeilChris LaiJavier MallolFernando MartinezEdwin MitchellStephen MontefortNeil PearceJayant ShahBonnie SibbaldDavid StrachanErika Von MutiusStephan K. Weiland Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. Journal of Allergy and Clinical Immunology 1999, 103, 125-138, 10.1016/s0091-6749(99)70536-1.
- Th. Bieber; Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy 2012, 67, 1475-1482, 10.1111/all.12049.
- Garmhausen, D.; Hagemann, T.; Bieber, T.; Dimitriou, I.; Fimmers, R.; Diepgen, T.; Novak, N.; Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 498–506.
- Lung-Chi Wu; Chian-Yaw Hwang; Pei-I Chung; Tuan-Chun Hua; Yen-Da Chen; Szu-Yin Chu; Din-Dar Lee; Y.T. Chang; Wen-Jen Wang; H.N. Liu; et al.Chih-Chiang Chen Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case-control study in Taiwan. Pediatric Allergy and Immunology 2014, 25, 586–592, 10.1111/pai.12274.
- Jonathan I. Silverberg; Philip Greenland; Eczema and cardiovascular risk factors in 2 US adult population studies. Journal of Allergy and Clinical Immunology 2015, 135, 721-728.e6, 10.1016/j.jaci.2014.11.023.
- Yuki M.F. Andersen; Alexander Egeberg Md; Gunnar H. Gislason; Peter R. Hansen; Lone Skov; Jacob P. Thyssen; Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. Journal of Allergy and Clinical Immunology 2016, 138, 310-312.e3, 10.1016/j.jaci.2016.01.015.
- Vincent Yi-Fong Su; Tzeng-Ji Chen; Chiu-Mei Yeh; Kun-Ta Chou; Man-Hsin Hung; Szu-Ying Chu; Kang-Cheng Su; Yu-Sheng Chang; Ya-Hsuan Lin; Chia-Jen Liu; et al. Atopic dermatitis and risk of ischemic stroke: A nationwide population-based study. Annals of Medicine 2014, 46, 84-89, 10.3109/07853890.2013.870018.
- Jonathan I. Silverberg; Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy 2015, 70, 1300-1308, 10.1111/all.12685.
- Marie Standl; Falko Tesch; Hansjörg Baurecht; E. Rodríguez; Martina Müller-Nurasyid; Christian Gieger; Annette Peters; Rui Wang-Sattler; Cornelia Prehn; Jerzy Adamski; et al.Florian KronenbergHolger SchulzSibylle KoletzkoTamara SchikowskiAndrea Von BergI. LehmannDietrich BerdelJoachim HeinrichJochen SchmittS. Weidinger Association of Atopic Dermatitis with Cardiovascular Risk Factors and Diseases. Journal of Investigative Dermatology 2017, 137, 1074-1081, 10.1016/j.jid.2016.11.031.
- Schäfer, T.; Ruhdorfer, S.; Weigl, L.; Wessner, D.; Heinrich, J.; Döring, A.; Wichmann, H.E.; Ring, J.; Intake of unsaturated fatty acids and HDL cholesterol levels are associated with manifestations of atopy in adults. Clin. Exp. Allergy 2003, 33, 1360–1367.
- Pedro Jesús Agón‐Banzo; Rosalía Sanmartin; A.J. García-Malinis; Ángela Hernández‐Martín; José Puzo; Divina Doste; Carlos Pardos; Yolanda Gilaberte; Body mass index and serum lipid profile: Association with atopic dermatitis in a paediatric population. Australasian Journal of Dermatology 2019, 61, e60-e64, 10.1111/ajd.13154.
- Yumiko Yamane; Kayano Moriyama; Chie Yasuda; Satoshi Miyata; Michiko Aihara; Zenro Ikezawa; Kaoru Miyazaki; New Horny Layer Marker Proteins for Evaluating Skin Condition in Atopic Dermatitis. International Archives of Allergy and Immunology 2009, 150, 89-101, 10.1159/000210385.
- Fu-Tong Liu; Heidi Goodarzi; Huan-Yuan Chen; IgE, Mast Cells, and Eosinophils in Atopic Dermatitis. Clinical Reviews in Allergy & Immunology 2011, 41, 298-310, 10.1007/s12016-011-8252-4.
- Rahat S. Azfar; Joel M. Gelfand; Psoriasis and metabolic disease: epidemiology and pathophysiology. Current Opinion in Rheumatology 2008, 20, 416-422, 10.1097/bor.0b013e3283031c99.
- Wolf-Henning Boehncke; Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Frontiers in Immunology 2018, 9, 579, 10.3389/fimmu.2018.00579.
- Boehncke, W.H.; Schön, M.P.; Psoriasis. Lancet 2015, 386, 983–994.
- Roberto Lande; Josh Gregorio; Valeria Facchinetti; Bithi Chatterjee; Yi-Hong Wang; Bernhard Homey; Wei Cao; Yui-Hsi Wang; Bing Su; Frank O. Nestle; et al.Tomasz ZalIra MellmanJens-Michael SchröderYong-Jun LiuMichel Gilliet Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564-569, 10.1038/nature06116.
- Lisa C. Zaba; Judilyn Fuentes-Duculan; Narat John Eungdamrong; Maria Veronica Abello; Inna Novitskaya; Katherine C. Pierson; Juana Gonzalez; James G. Krueger; Michelle A. Lowes; Psoriasis Is Characterized by Accumulation of Immunostimulatory and Th1/Th17 Cell-Polarizing Myeloid Dendritic Cells. Journal of Investigative Dermatology 2009, 129, 79-88, 10.1038/jid.2008.194.
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G.; The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181.
- Ferretti, G.; Alleva, R.; Taus, M.; Simonetti, O.; Cinti, B.; Offidani, A.M.; Bossi, G.; Curatola, G.; Abnormalities of plasma lipoprotein composition and fluidity in psoriasis. Acta Derm. Venereol. 1994, 74, 171–175.
- G. Ferretti; Oriana Simonetti; A M Offidani; L Messini; B Cinti; I Marshiseppe; G Bossi; G Curatola; Changes of Plasma Lipids and Erythrocyte Membrane Fluidity in Psoriatic Children. Pediatric Research 1993, 33, 506-509, 10.1203/00006450-199305000-00017.
- Lai-Shan Tam; B. Tomlinson; T. T.-W. Chu; M. Li; Y.-Y. Leung; L.-W. Kwok; T. K. Li; T. Yu; Y.-E. Zhu; K.-C. Wong; et al.E. W.-L. KunE. K. Li Cardiovascular risk profile of patients with psoriatic arthritis compared to controls--the role of inflammation. Rheumatology 2008, 47, 718-723, 10.1093/rheumatology/ken090.
- Lotus Mallbris; Fredrik Granath; Anders Hamsten; Mona Ståhle; Psoriasis is associated with lipid abnormalities at the onset of skin disease. Journal of the American Academy of Dermatology 2006, 54, 614-621, 10.1016/j.jaad.2005.11.1079.
- Lenka Borska; Jan Kremláček; Ctirad Andrys; Jan Krejsek; Kvetoslava Hamakova; Pavel Borsky; Vladimir Palicka; Vit Rehacek; Andrea Malkova; Zdenek Fiala; et al. Systemic Inflammation, Oxidative Damage to Nucleic Acids, and Metabolic Syndrome in the Pathogenesis of Psoriasis. International Journal of Molecular Sciences 2017, 18, 2238, 10.3390/ijms18112238.
- Y. C. Nakhwa; R. Rashmi; K. H. Basavaraj; Dyslipidemia in Psoriasis: A Case Controlled Study. International Scholarly Research Notices 2014, 2014, 1-5, 10.1155/2014/729157.
- Chaoyang Miao; Jing Li; Ying Li; Xiaoyan Zhang; Obesity and dyslipidemia in patients with psoriasis. Medicine 2019, 98, e16323, 10.1097/md.0000000000016323.
- Lana Bassi Ferdinando; Paula Kaori Fukumoto; Sarah Sanches; Lincoln Helder Zambaldi Fabricio; Thelma L. Skare; Metabolic syndrome and psoriasis: a study in 97 patients. Revista da Associação Médica Brasileira 2018, 64, 368-373, 10.1590/1806-9282.64.04.368.
- Kinga Pietrzak-Franciszkiewicz; Paweł Chabros; Ewelina Grywalska; Paweł Kiciński; Kinga Franciszkiewicz-Pietrzak; Dorota Krasowska; Grzegorz Kandzierski; Serum lipid metabolism in psoriasis and psoriatic arthritis – an update. Archives of Medical Science 2019, 15, 369-375, 10.5114/aoms.2018.74021.
- Pietrzak, A.; Kadzielewski, J.; Janowski, K.; Roliński, J.; Krasowska, D.; Chodorowska, G.; Paszkowski, T.; Kapeć, E.; Jastrzbska, I.; Tabarkiewicz, J.; et al.Lotti, Τ. Lipoprotein (a) in patients with psoriasis: Associations with lipid profiles and disease severity. Int. J. Dermatol. 2009, 48, 379–387.
- Aldona Pietrzak; E. Grywalska; M. Walankiewicz; T. Lotti; J. Roliński; W. Myśliński; P. Chabros; D. Piekarska-Myślińska; K. Reich; Psoriasis and metabolic syndrome in children: current data. Clinical and Experimental Dermatology 2017, 42, 131-136, 10.1111/ced.13014.
- Mehdi Taheri Sarvtin; Mohammad Taghi Hedayati; Tahereh Shokohi; Zohreh Hajheydari; Serum lipids and lipoproteins in patients with psoriasis.. Archives of Iranian Medicine 2014, 17, 343–346.
- Seraj Ahmed Khan; Sudha Agrawal; Nirmal Baral; Madhab Lamsal; Evaluation of ADA activity as a potential marker of disease severity in psoriasis patients. Psoriasis: Targets and Therapy 2018, 8, 59-63, 10.2147/ptt.s174119.
- Hanan Hassan Sabry; Jehan Hassan Sabry; Aliaa El Husseiny Daifalla; Essam Mohamed Akl; Ahmed Mohamed Hamed; Ahmed Abd Allah Torky; Serum markers for asymptomatic atherosclerosis in Egyptian psoriatic patients: study controlled by Doppler estimation of carotid intima-media thickness. Vascular Health and Risk Management 2018, 14, 145-152, 10.2147/vhrm.s164274.
- Olivia Komorowska; Michal Bohdan; Aneta Szczerkowska-Dobosz; Dorota Rawicz-Zegrzda; Maria Dudziak; Tomasz Zdrojewski; Marcin Gruchala; Dorota Purzycka-Bohdan; Roman Nowicki; Assessment of Cardiovascular Risk Factors in Patients with Psoriasis.. Acta dermatovenerologica Croatica : ADC 2016, 24, 261-267.
- Monia Asmi; Wiem Zidi; Amel Mebazaa; Yosra Zayani; Imen Ayadi; Moncef Feki; Amel Osman; Naziha Kaabachi; Serum Lipid Level in Tunisian Patients with Psoriasis. Clinical Laboratory 2014, 60, 1043-7, 10.7754/clin.lab.2013.130535.
- Xiao-Wen Pang; Kai Lin; Wen Liu; Ping Zhang; Sainan Zhu; Characterization of the abnormal lipid profile in Chinese patients with psoriasis. International journal of clinical and experimental pathology 2015, 8, 15280-15284.
- Bharath Manu Akkara Veetil; Eric L. Matteson; Hilal Maradit Kremers; Marian T McEvoy; Cynthia S. Crowson; Trends in lipid profiles in patients with psoriasis: a population-based analysis. BMC Dermatology 2012, 12, 20-20, 10.1186/1471-5945-12-20.
- S. Coimbra; Hugo Oliveira; Flávio Reis; Luís Belo; Susana Rocha; Alexandre Quintanilha; Américo Figueiredo; Frederico Teixeira; Elisabeth Castro; Petronila Rocha-Pereira; et al.A. Santos-Silva Psoriasis therapy and cardiovascular risk factors: A 12-week follow-up study. American Journal of Clinical Dermatology 2010, 11, 423-432, 10.2165/11319310-000000000-00000.
- Yuksel, E.P.; Yuksel, S.; Yenercag, M.; Soylu, K.; Aydin, F.; Senturk, N.; Yucel, H.; Canturk, T.; Turanli, A.Y.; Impaired heart rate recovery indices in psoriasis patients. Med. Sci. Monit. 2014, 20, 350–354.
- Amel Mebazaa; M El Asmi; W Zidi; Y Zayani; R Cheikh Rouhou; S El Ounifi; F Kanoun; M Mokni; A Ben Osman; M Feki; et al.H SlimaneN Kaabachi Metabolic syndrome in Tunisian psoriatic patients: prevalence and determinants. Journal of the European Academy of Dermatology and Venereology 2011, 25, 705-709, 10.1111/j.1468-3083.2010.03856.x.
- Sirin, M.C.; Korkmaz, S.; Erturan, I.; Filiz, B.; Aridogan, B.C.; Cetin, E.S.; Yildirim, M.; Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. An. Bras. Dermatol. 2020, 95, 575–582.
- Bajaj, S.; Mandal, S.; Singh, K.G.; Prajapati, R.; Metabolic Diseases and Associated Complications in Patients with Psoriasis. J. Assoc. Physicians India 2020, 68, 44–46.
- P. Rocha-Pereira; A. Santos-Silva; Irene Rebelo; Américo Figueiredo Md; Alexandre Quintanilha; Frederico Teixeira; Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clinica Chimica Acta 2001, 303, 33-39, 10.1016/s0009-8981(00)00358-2.
- Nilgun Solak Tekin; Ishak Özel Tekin; Figen Barut; Emine Yilmaz Sipahi; Accumulation of Oxidized Low-Density Lipoprotein in Psoriatic Skin and Changes of Plasma Lipid Levels in Psoriatic Patients. Mediators of Inflammation 2007, 2007, 78454, 10.1155/2007/78454.
- Lei He; Shucun Qin; Lin Dang; Guohua Song; Shutong Yao; Nana Yang; Yuzhen Li; Psoriasis decreases the anti-oxidation and anti-inflammation properties of high-density lipoprotein. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2014, 1841, 1709-1715, 10.1016/j.bbalip.2014.09.008.
- Murat Usta; Enver Turan; Hale Aral; Berrin Bercik Inal; Mehmet Salih Gurel; Guvenc Guvenen; Serum paraoxonase-1 activities and oxidative status in patients with plaque-type psoriasis with/without metabolic syndrome. Journal of Clinical Laboratory Analysis 2011, 25, 289-295, 10.1002/jcla.20471.
- Claudia Reynoso-Von Drateln; Esperanza Martnez-Abundis; Blanca Rebeca Balczar-Muoz; Rafael Bustos-Saldaa; Manuel Gonzlez-Ortiz; Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. Journal of the American Academy of Dermatology 2003, 48, 882-885, 10.1067/mjd.2003.446.
- Sudhakar Thungaturthi; Sabitha Vadakedath; Prathyusha Pavuluri; Jhansi Rani; Rajkumar Gundu; Jai Bheem; Venkataramana Kandi; Atherogenesis in Psoriasis: Evaluation of the Serum Activities of Non-high-density Lipoprotein Cholesterol and Other Lipids Among Newly Diagnosed Psoriasis Patients.. Cureus 2019, 11, e4203, 10.7759/cureus.4203.
- Bekir Sami Uyanik; Zeki Ari; Ece Onur; Kamer Gündüz; Sevcan Tanülkü; Kubra Durkan; Serum Lipids and Apolipoproteins in Patients with Psoriasis. Clinical Chemistry and Laboratory Medicine (CCLM) 2002, 40, 65–68, 10.1515/cclm.2002.013.
- Rony Shreberk-Hassidim; Eran Galili; Ayal Hassidim; Yuval Ramot; Ilan Merdler; Sharon Baum; Abraham Zlotogorski; Aviv Barzilai; Nadav Astman; Epidemiology and Comorbidities of Psoriasis among Israeli Adolescents: A Large Cross-Sectional Study. Dermatology 2019, 235, 488-494, 10.1159/000501032.
- Asha, K.; Singal, A.; Sharma, S.B.; Arora, V.K.; Aggarwal, A.; Dyslipidaemia & oxidative stress in patients of psoriasis: Emerging cardiovascular risk factors. Indian J. Med. Res. 2017, 146, 708–713.
- Shraddha Madanagobalane; Sankarasubramanian Anandan; Prevalence of Metabolic Syndrome In South Indian Patients with Psoriasis Vulgaris and the Relation Between Disease Severity and Metabolic Syndrome: A Hospital-Based Case-Control Study. Indian Journal of Dermatology 2012, 57, 353-357, 10.4103/0019-5154.100474.
- Sebastian Uczniak; Zofia A. Gerlicz; Magdalena Kozłowska; Andrzej Kaszuba; Presence of selected metabolic syndrome components in patients with psoriasis vulgaris. Advances in Dermatology and Allergology 2016, 33, 114-119, 10.5114/ada.2016.59153.
- Cenk Akcali; Burcin Buyukcelik; Necmettin Kirtak; Serhat Inaloz; Clinical and laboratory parameters associated with metabolic syndrome in Turkish patients with psoriasis. Journal of International Medical Research 2014, 42, 386-394, 10.1177/0300060513502891.
- Zindancı, I.; Albayrak, O.; Kavala, M.; Kocaturk, E.; Can, B.; Sudogan, S.; Koç, M.; Prevalence of Metabolic Syndrome in Patients with Psoriasis. Sci. World J. 2012, 2012, 312463.
- Love, T.J.; Qureshi, A.A.; Karlson, E.W.; Gelfand, J.M.; Choi, H.K.; Prevalence of the metabolic syndrome in psoriasis: Results from the national health and nutrition examination survey, 2003–2006. Arch. Dermatol. 2011, 147, 419–424.
- I.M. Miller; T. Skaaby; C. Ellervik; G.B.E. Jemec; Quantifying cardiovascular disease risk factors in patients with psoriasis: a meta-analysis. British Journal of Dermatology 2013, 169, 1180-1187, 10.1111/bjd.12490.
- Suleyman Piskin; Figen Gurkok; Galip Ekuklu; Mustafa Senol; Serum Lipid Levels in Psoriasis. Yonsei Medical Journal 2003, 44, 24-26, 10.3349/ymj.2003.44.1.24.
- Deniz Seçkin; Lale Tokgözoğlu; Sevinç Akkaya; Are lipoprotein profile and lipoprotein (a) levels altered in men with psoriasis?. Journal of the American Academy of Dermatology 1994, 31, 445-449, 10.1016/s0190-9622(94)70208-x.
- M Akhyani; Ah Ehsani; Reza Mahmoud Robati; Am Robati; The lipid profile in psoriasis: a controlled study. Journal of the European Academy of Dermatology and Venereology 2007, 21, 1330-1332, 10.1111/j.1468-3083.2007.02260.x.
- Seishima, M.; Seishima, M.; Mori, S.; Noma, A.; Serum lipid and apolipoprotein levels in patients with psoriasis. British Journal of Dermatology 1994, 130, 738-742, 10.1111/j.1365-2133.1994.tb03411.x.
- M Farshchian; A Zamanian; A-R Monsef; H Mahjub; Serum lipid level in Iranian patients with psoriasis. Journal of the European Academy of Dermatology and Venereology 2007, 21, 802-805, 10.1111/j.1468-3083.2006.02099.x.
- G. Ferretti; T. Bacchetti; Anna Campanati; Oriana Simonetti; G. Liberati; Annamaria Offidani; Correlation between lipoprotein(a) and lipid peroxidation in psoriasis: role of the enzyme paraoxonase-1. British Journal of Dermatology 2012, 166, 204-207, 10.1111/j.1365-2133.2011.10539.x.
- Aysun Toker; Melek Kadı; A. Kadir Yıldırım; Hulya Aksoy; Fatih Akçay; Serum lipid profile paraoxonase and arylesterase activities in psoriasis. Cell Biochemistry and Function 2009, 27, 176-180, 10.1002/cbf.1553.
- Sorokin, A.V.; Kotani, K.; Elnabawi, Y.A.; Dey, A.K.; Sajja, A.P.; Yamada, S.; Ueda, M.; Harrington, C.L.; Baumer, Y.; Rodante, J.A.; et al.Gelfand, J.M.Chen, M.Y.Joshi, A.A.Playford, M.P.Remaley, A.T.Mehta, N.N. Association between oxidation-modified lipoproteins and coronary plaque in psoriasis an observational cohort study. Circ. Res. 2018, 123, 1244–1254.
- Jackelyn B. Golden; Thomas S. McCormick; Nicole L. Ward; IL-17 in psoriasis: implications for therapy and cardiovascular co-morbidities.. Cytokine 2013, 62, 195-201, 10.1016/j.cyto.2013.03.013.
- R. Wolk; Ehrin J. Armstrong; Tine Willum Hansen; Bruce Thiers; Shuping Lan; Anna M. Tallman; Mandeep Kaur; Svitlana Tatulych; Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. Journal of Clinical Lipidology 2017, 11, 1243-1256, 10.1016/j.jacl.2017.06.012.
- Jashin J Wu; Bruce E. Strober; Peter R. Hansen; Ole Ahlehoff; Alexander Egeberg; Abrar A. Qureshi; Debbie Robertson; Hernan Valdez; Huaming Tan; Robert Wolk; et al. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. Journal of the American Academy of Dermatology 2016, 75, 897-905, 10.1016/j.jaad.2016.06.012.
- K. Papp; A. Menter; B. Strober; R.G. Langley; M. Buonanno; R. Wolk; P. Gupta; S. Krishnaswami; H. Tan; J.A. Harness; et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. British Journal of Dermatology 2012, 167, 668-677, 10.1111/j.1365-2133.2012.11168.x.
- Surjit Singh; Anil Bhansali; Randomized Placebo Control Study of Metformin in Psoriasis Patients with Metabolic Syndrome (Systemic Treatment Cohort). Indian Journal of Endocrinology and Metabolism 2017, 21, 581-587, 10.4103/ijem.IJEM_46_17.
- Arianna Zangrilli; Mauro Bavetta; Martina Scaramella; Luca Bianchi; Long-term treatment of psoriatic patients with adalimumab reduces disease severity and maintains a favorable lipid pattern and a low Atherogenic Index.. Giornale Italiano di Dermatologia e Venereologia 2018, 153, 146-154.
- Lluís Puig; Robert Strohal; Joanne Fuiman; Ronald Pedersen; Annette Szumski; Andrew S. Koenig; Deborah Robertson; Heinz Drexel; Cardiometabolic biomarkers in chronic plaque psoriasis before and after etanercept treatment. Journal of Dermatological Treatment 2014, 25, 470-481, 10.3109/09546634.2013.848260.
- Dey, A.K.; Joshi, A.A.; Chaturvedi, A.; Lerman, J.B.; Aberra, T.M.; Rodante, J.A.; Teague, H.L.; Harrington, C.L.; Rivers, J.P.; Chung, J.H.; et al.Kabbany, M.T.Natarajan B.Silverman,J.I.Ng, Q.Sanda, G.E.Sorokin, A.V.Baumer, Y.Gerson, E.Prussick, R.B.Ehrlich, A.Green, L.J.Lockshin, B.N.Ahlman, M.A.Playford, M.P.Gelfand, J.M.Mehta, N.N. Association between skin and aortic vascular inflammation in patients with psoriasis: A case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017, 2, 1013–1018.
- Sevgi Kılıc; Selma Emre; Ahmet Metin; Semra Isıkoglu; Ozcan Erel; Effect of the systemic use of methotrexate on the oxidative stress and paraoxonase enzyme in psoriasis patients. Archives of Dermatological Research 2013, 305, 495-500, 10.1007/s00403-013-1366-1.
- Sabrina Corbetta; R Angioni; A Cattaneo; P Beck-Peccoz; A Spada; Effects of retinoid therapy on insulin sensitivity, lipid profile and circulating adipocytokines. European Journal of Endocrinology 2006, 154, 83-86, 10.1530/eje.1.02057.
- T. Bacchetti; Anna Campanati; G. Ferretti; Oriana Simonetti; G. Liberati; Annamaria Offidani; Oxidative stress and psoriasis: the effect of antitumour necrosis factor-α inhibitor treatment. British Journal of Dermatology 2013, 168, 984-989, 10.1111/bjd.12144.
- Egeberg, A.; Wu, J.J.; Korman, N.; Solomon, J.A.; Goldblum, O.; Zhao, F.; Mallbris, L.; Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: Results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J. Am. Acad. Dermatol. 2018, 79, 104–109.e8.
- Joel M. Gelfand; Daniel B. Shin; Kristina Callis Duffin; April W. Armstrong; A. Blauvelt; Stephen K. Tyring; Alan M Menter; Scott Gottlieb; Benjamin N. Lockshin; Eric L Simpson; et al.Farid KianifardRajendra Prasad SarkarElisa MuscianisiJennifer SteadmanMark A. AhlmanMartin P. PlayfordAditya A. JoshiAmit Kumar DeyThomas J. WernerAbass AlaviNehal N. Mehta A Randomized Placebo-Controlled Trial of Secukinumab on Aortic Vascular Inflammation in Moderate-to-Severe Plaque Psoriasis (VIP-S). Journal of Investigative Dermatology 2020, 140, 1784-1793.e2, 10.1016/j.jid.2020.01.025.
- O Ahlehoff; Tine Willum Hansen; Gunnar Gislason; M. Frydland; L.E. Bryld; H. Elming; Gregor B. E. Jemec; Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study. Journal of the European Academy of Dermatology and Venereology 2016, 30, 819-823, 10.1111/jdv.13152.
- Nehal N. Mehta; Daniel B. Shin; Aditya A. Joshi; Amit Kumar Dey; April W. Armstrong; Kristina Callis Duffin; Zelma Chiesa Fuxench; Charlotte L. Harrington; Rebecca A. Hubbard; Robert E. Kalb; et al.Alan MenterDaniel J. RaderMuredach P. ReillyEric L. SimpsonJ. TakeshitaDrew A. TorigianThomas J. WernerAndrea B. TroxelStephen K. TyringSuzette Baez VanderBeekAbby S. Van VoorheesMartin P. PlayfordMark A. AhlmanAbass AlaviJoel M. Gelfand Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers. Circulation: Cardiovascular Imaging 2018, 11, 1-11, 10.1161/circimaging.117.007394.
- Joel M. Gelfand; Daniel B. Shin; Kristina Callis Duffin; April W. Armstrong; A. Blauvelt; Stephen K. Tyring; Alan M Menter; Scott Gottlieb; Benjamin N. Lockshin; Eric L Simpson; et al.Farid KianifardRajendra Prasad SarkarElisa MuscianisiJennifer SteadmanMark A. AhlmanMartin P. PlayfordAditya A. JoshiAmit Kumar DeyThomas J. WernerAbass AlaviNehal N. Mehta A Randomized Placebo-Controlled Trial of Secukinumab on Aortic Vascular Inflammation in Moderate-to-Severe Plaque Psoriasis (VIP-S). Journal of Investigative Dermatology 2020, 140, 1784-1793.e2, 10.1016/j.jid.2020.01.025.
- Henrique L. Staniak; Marcio Sommer Bittencourt; Itamar De Souza Santos; Rodolfo Sharovsky; Cid Sabbag; Alessandra C. Goulart; Paulo A. Lotufo; Isabela M. Benseñor; Association between psoriasis and coronary calcium score. Atherosclerosis 2014, 237, 847-852, 10.1016/j.atherosclerosis.2014.11.004.
- M. Asefi; Asad Vaisiraygani; Fariborz Bahrehmand; Amir Kiani; Zohreh Rahimi; H. Nomani; Ali Ebrahimi; Heidar Tayebinia; T. Pourmotabbed; Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. British Journal of Dermatology 2012, 167, 1279-1286, 10.1111/j.1365-2133.2012.11170.x.
- Nemati Houshang; Khodarahmi Reza; Sadeghi Masoud; Ebrahimi Ali; Rezaei Mansour; Asad Vaisi-Raygani; Antioxidant status in patients with psoriasis. Cell Biochemistry and Function 2014, 32, 268-273, 10.1002/cbf.3011.
- Tiziana Bacchetti; Oriana Simonetti; Francesca Ricotti; Annamaria Offidani; Gianna Ferretti; Plasma oxidation status and antioxidant capacity in psoriatic children. Archives of Dermatological Research 2020, 312, 33-39, 10.1007/s00403-019-01976-z.
- M. Elaine Husni; W. H. Wilson Tang; Michael Lucke; U. M. Chandrasekharan; Danielle M. Brennan Ms; Stanley L. Hazen; Correlation of High-Density Lipoprotein-Associated Paraoxonase 1 Activity With Systemic Inflammation, Disease Activity, and Cardiovascular Risk Factors in Psoriatic Disease. Arthritis & Rheumatology 2018, 70, 1240-1250, 10.1002/art.40499.
- T. Zuberbier; R. Asero; C. Bindslev-Jensen; G. Walter Canonica; M. K. Church; A. M. Giménez-Arnau; C. E. H. Grattan; A. Kapp; M. Maurer; H. F. Merk; et al.B. RogalaS. SainiM. Sánchez-BorgesP. Schmid-GrendelmeierH. SchünemannP. StaubachG. A. VenaB. Wedi EAACI/GA²LEN/EDF/WAO guideline: management of urticaria. Allergy 2009, 64, 1427-1443, 10.1111/j.1398-9995.2009.02178.x.
- Kanani, A.; Betschel, S.D.; Warrington, R.; Urticaria and angioedema. Allergy Asthma Clin. Immunol. 2018, 14, 59.
- Torsten Zuberbier; M. Balke; M. Worm; G. Edenharter; M. Maurer; Epidemiology of urticaria: a representative cross-sectional population survey. Clinical and Experimental Dermatology 2010, 35, 869-873, 10.1111/j.1365-2230.2010.03840.x.
- Marcus Maurer; J.-P. Ortonne; T. Zuberbier; Chronic urticaria: an internet survey of health behaviours, symptom patterns and treatment needs in European adult patients. British Journal of Dermatology 2009, 160, 633-641, 10.1111/j.1365-2133.2008.08920.x.
- Amar, S.M.; Dreskin, S.C.; Urticaria. Prim. Care Clin. Off. Pract. 2008, 35, 141–157.
- Camila Antia; Katherine Baquerizo; Abraham Korman; Jonathan A. Bernstein; Ali Alikhan; Urticaria: A comprehensive review. Journal of the American Academy of Dermatology 2018, 79, 599-614, 10.1016/j.jaad.2018.01.020.
- Mariam M. Amin; M. Rushdy; Hyperlipidemia in association with pro-inflammatory cytokines among chronic spon-taneous urticaria: case-control study. European Annals of Allergy and Clinical Immunology 2018, 50, 254-261, 10.23822/eurannaci.1764-1489.68.
- Mahizer Yaldiz; Kiyasettin Asil; Evaluation of carotid intima media thickness and hematologic inflammatory markers in patients with chronic spontaneous urticaria. Advances in Dermatology and Allergology 2020, 37, 214-220, 10.5114/ada.2018.79567.
- Kaplan, A.P.; Greaves, M.W.; Angioedema. J. Am. Acad. Dermatol. 2005, 53, 373–388.
- Sloane, D.E.; Lee, C.W.; Sheffer, A.L.; Hereditary angioedema: Safety of long-term stanozolol therapy. J. Allergy Clin. Immunol. 2007, 120, 654–658.
- Gábor Széplaki; Lilian Varga; Szilvia Valentin; Mónika Kleiber; István Karádi; László Romics; George Füst; Henriette Farkas; Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema. Journal of Allergy and Clinical Immunology 2006, 115, 864-869, 10.1016/j.jaci.2004.12.1130.
- Róbert Szegedi; Gábor Széplaki; Lilian Varga; Zoltán Prohászka; Zoltán Széplaki; István Karádi; George Füst; Henriette Farkas; Long-term danazol prophylaxis does not lead to increased carotid intima-media thickness in hereditary angioedema patients. Atherosclerosis 2008, 198, 184-191, 10.1016/j.atherosclerosis.2007.09.025.
- Zsuzsa Nebenführer; Erika Szabó; Erika Kajdácsi; Kinga Viktória Kőhalmi; I. Karadi; András Zsáry; Henriette Farkas; László Cervenak; Flow-mediated vasodilation assay indicates no endothelial dysfunction in hereditary angioedema patients with C1-inhibitor deficiency. Annals of Allergy, Asthma & Immunology 2019, 122, 86-92, 10.1016/j.anai.2018.10.004.
- Wurfel, M.M.; Kunitake, S.T.; Lichenstein, H.; Kane, J.P.; Wright, S.D.; Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994, 180, 1025–1035.
- Johannes H. M. Levels; Philip R. Abraham; Erik P. Van Barreveld; Joost C. M. Meijers; Sander J. H. Van Deventer; Distribution and Kinetics of Lipoprotein-Bound Lipoteichoic Acid. Infection and Immunity 2003, 71, 3280-3284, 10.1128/iai.71.6.3280-3284.2003.
- Johannes H. M. Levels; P. R. Abraham; A. Van Den Ende; S. J. H. Van Deventer; Distribution and Kinetics of Lipoprotein-Bound Endotoxin. Infection and Immunity 2001, 69, 2821-2828, 10.1128/iai.69.5.2821-2828.2001.