Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masaki Arata | -- | 2192 | 2022-11-17 12:42:39 | | | |
| 2 | Conner Chen | Meta information modification | 2192 | 2022-11-21 09:57:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Arata, M.; Usami, F.M.; Fujimori, T. Core PCP Proteins in Coordinating Cilia Orientation. Encyclopedia. Available online: https://encyclopedia.pub/entry/35112 (accessed on 07 February 2026).
Arata M, Usami FM, Fujimori T. Core PCP Proteins in Coordinating Cilia Orientation. Encyclopedia. Available at: https://encyclopedia.pub/entry/35112. Accessed February 07, 2026.
Arata, Masaki, Fumiko Matsukawa Usami, Toshihiko Fujimori. "Core PCP Proteins in Coordinating Cilia Orientation" Encyclopedia, https://encyclopedia.pub/entry/35112 (accessed February 07, 2026).
Arata, M., Usami, F.M., & Fujimori, T. (2022, November 17). Core PCP Proteins in Coordinating Cilia Orientation. In Encyclopedia. https://encyclopedia.pub/entry/35112
Arata, Masaki, et al. "Core PCP Proteins in Coordinating Cilia Orientation." Encyclopedia. Web. 17 November, 2022.
Copy Citation
As exemplified by the unidirectionally beating cilia of multi-ciliated cells, various epithelial cells polarize not only along the apical-basal axis (inside–outside axis) of epithelial tissues, but also on the plane of epithelial tissues. The latter cell polarity, which is perpendicular to the apical–basal axis, is referred to as planar cell polarity (PCP). Pioneering research using the wings of Drosophila melanogaster identified a group of proteins, core PCP proteins, that orchestrate the establishment of PCP.
cytoskeleton
motile cilia
multi-ciliated cells
planar cell polarity
1. Introduction
Multi-ciliated cells line the surface of the oviduct, the trachea, and the ventricle of the brain (Figure 1A,B show oviduct multi-ciliated cells). At the apical surface of multi-ciliated cells, tens to hundreds of motile cilia are formed. The directions of cilia movements are unidirectionally aligned within each cell (rotational polarity), and they are consistent with the orientation of the tissue axis (tissue-level polarity). In addition, cilia exhibit a metachronal wave, a wave-like propagation of cilia movements in the plane of the tissue, which is produced by a temporal coordination of cilia movements between neighboring cilia (Figure 1C–D’) [1][2][3][4][5]. The coordination of cilia movements is essential for the functions of organs. In the mammalian oviduct, cilia pick up ovulated oocytes at the ovary end of the oviduct and carry them to the uterus [6][7]; in the trachea, cilia transport mucus and eliminate debris and pathogens [8][9]; and in the ventricle, cilia generate a flow of cerebrospinal fluid that is required for homeostasis of the organ [10].
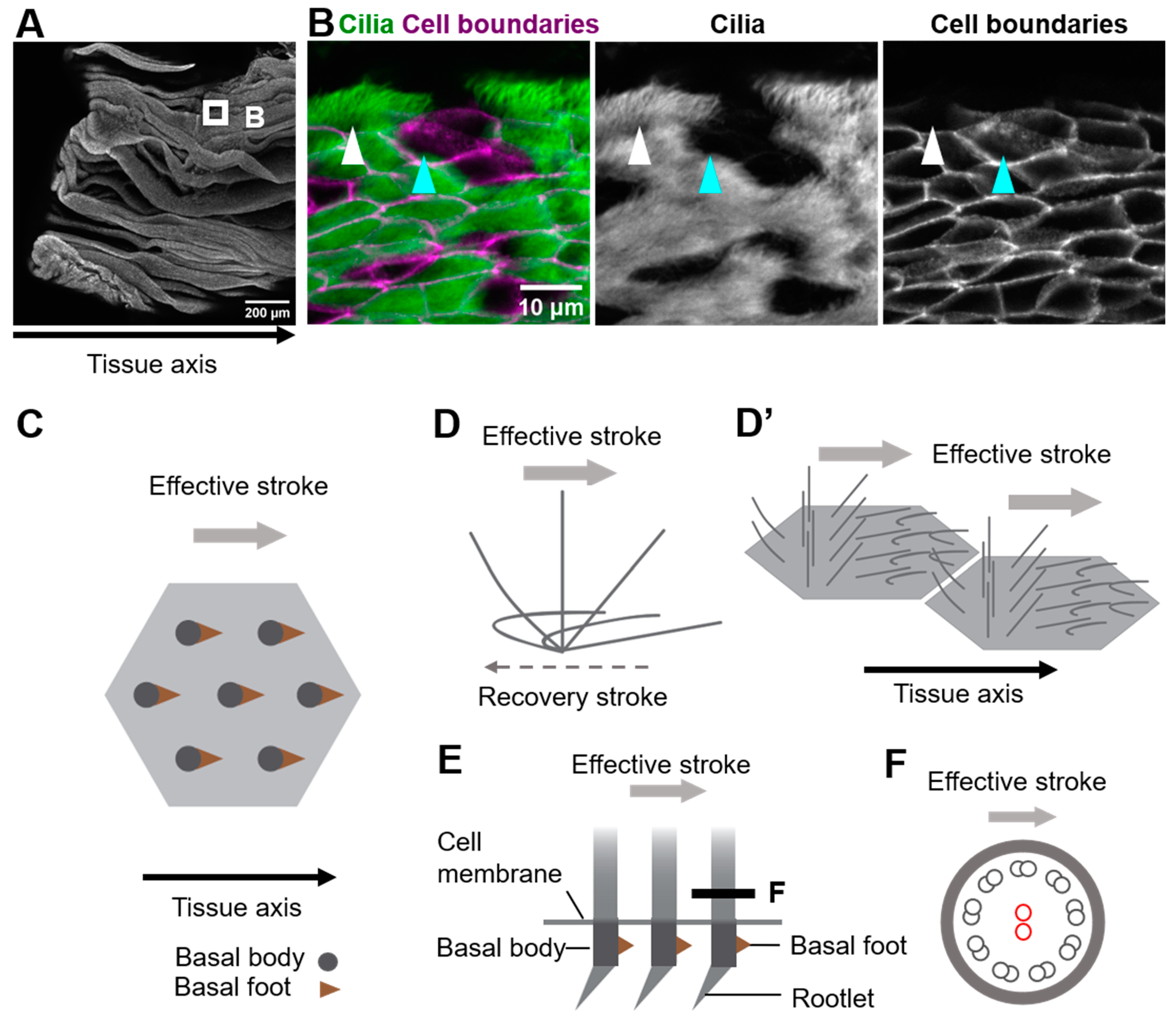
Figure 1. Coordination of cilia movements in multi-ciliated cells. (A) Ovary end of the oviduct was opened longitudinally and stained with E-cadherin. (B) Cilia (green; acetylated tubulin) and cell boundaries (magenta; E-cadherin) of the oviduct epithelium are visualized. The oviduct epithelium is composed of multi-ciliated cells (white arrowhead) and secretory cells (cyan arrowhead). At the apical surface of those multi-ciliated cells, about 150 cilia are formed on average. (C) Representative apical views of multi-ciliated cells. Gray circles and brown triangles indicate basal bodies and basal feet, respectively (also see Figure 1(E)). Note that the basal foot points in the same direction as the effective stroke (gray arrow). (D) Schematic representation of the movement of an individual cilium. Cilia repeat cyclic movements comprised of a fast effective stroke (gray arrow), and a slow recovery stroke (a backward motion; dotted arrow). (D’) A schematic of cilia movements in multi-ciliated cells. Apical surfaces of two multi-ciliated cells are shown. Note that the orientation of effective stroke (or recovery stroke) is consistent with that of the tissue axis. The phase of the beating cycles of cilia shifts between neighboring cilia and generates a metachronal wave, which is a wave-like propagation of cilia movements. (E) Lateral view of the basal region of cilia. The basal body has two appendages, the basal foot and the rootlet. (F) Cross-sectional view of the cilium along the gray line in (E). 9 + 2 microtubules are shown in small circles. The central pair of 9 + 2 microtubules (red circles) run perpendicular to the direction of the effective stroke (gray arrow).
Motile cilia of multi-ciliated cells are microtubule-based cell protrusions which show a biphasic movement comprised of a fast effective stroke, and a slow backward motion, the recovery stroke (Figure 1D). Inside the majority of motile cilia, 9 + 2 arrays of microtubules run along the longitudinal axis of the cilium, and a central pair of microtubules lie perpendicular to the beating orientation of the cilium (Figure 1E,F) [11]. The basal body lies at the base of cilium, and the basal foot and the rootlet are associated with the basal body (Figure 1E). A single basal foot protrudes from the lateral side of each basal body, and its direction is consistent with that of the effective stroke [12][13]. A rootlet is located at the proximal end of each basal body and extends to the center of the cell [14][15][16][17].
2. Roles of Core PCP Proteins in Coordinating Cilia Orientation
As exemplified by the unidirectionally beating cilia of multi-ciliated cells, various epithelial cells polarize not only along the apical-basal axis (inside–outside axis) of epithelial tissues, but also on the plane of epithelial tissues. The latter cell polarity, which is perpendicular to the apical–basal axis, is referred to as planar cell polarity (PCP) [18][19][20][21][22][23][24][25][26][27][28]. Pioneering research using the wings of Drosophila melanogaster identified a group of proteins, core PCP proteins, that orchestrate the establishment of PCP (Figure 2). Core PCP proteins are an evolutionally conserved group of proteins comprised of transmembrane proteins, Flamingo/Starry night (Fmi/Stan), Van Gogh (Vang) and Frizzled (Fz), as well as cytoplasmic proteins Prickle (Pk), Dishevelled (Dvl) and Diego (Dgo) [21][26][29][30]. Each Drosophila wing epithelial cell forms an actin-rich cell protrusion, a wing hair, at the apical cortex and each wing hair points to the distal end of the wing, which is a hallmark of PCP in the wing [21]. Just before the onset of wing hair formation, cell boundary localization of core PCP proteins is strongly biased along the tissue axis (Figure 2A). Fz- and Vang-containing complexes (referred to as the Fz- and Vang-complex in Drosophila, respectively, or the FZD- and VANGL-complex in vertebrates, as used hereafter) localize at the distal and proximal side of the cell, respectively (Figure 2A,A’) [28][31][32][33][34][35]. When each member of core PCP proteins is lacking, the orientations of wing hairs are not coordinated along the tissue axis [21]. In addition to the Drosophila wing, the asymmetric distribution of core PCP proteins was observed in various organs and animals, including multi-ciliated cells of the mouse oviduct, trachea, and ventricle (Figure 2B) [20][36][37][38]. The establishment of the polarized distribution of core PCP proteins precedes the formation of multi-cilia in the developing oviduct [20][39]. Furthermore, the loss of core PCP proteins abrogates the orientation of cilia, suggesting that the asymmetric distribution of core PCP proteins provides a cue to orient cilia. In contrast to Drosophila genes encoding core PCP proteins, those in vertebrates are often duplicated and might have divergent functions (Figure 2A”). For example, there are three homologs of Drosophila Flamingo, CELSR1, 2, and 3 in mice, and these have different expression patterns and show different phenotypes when their functions are lost [20][40][41][42].
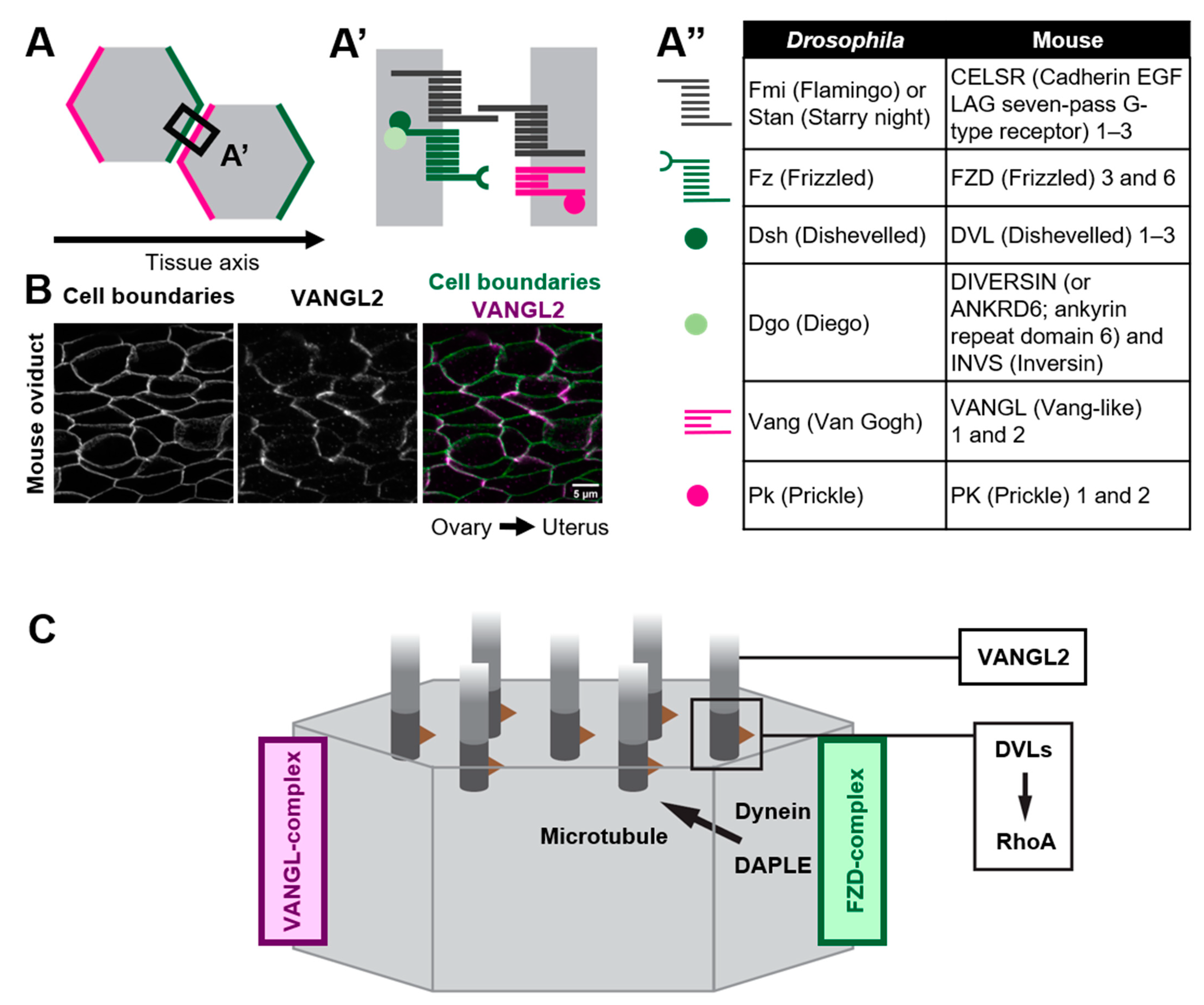
Figure 2. Polarized distribution of core PCP proteins along the tissue axis. (A–A”) Core PCP proteins form an asymmetric complex at cell boundaries. (A,A’) FZD-containing complex (FZD-complex; green) and VANGL-containing complex (VANGL-complex; magenta) are segregated to opposite cell boundaries (note that their distribution is polarized along the tissue axis). Extracellular domain of CELSRs provides intercellular bridges between the FZD-complex and the VANGL-complex, thus enabling the coupling of cell polarity at a multicellular level. (A”) A list of members of core PCP proteins in Drosophila and their counterparts in mice. (B) Mouse oviduct epithelium was stained for E-cadherin and a core PCP protein, VANGL2. E-cadherin labels cell boundaries. Note the zigzag pattern of VANGL2 signals which highlights polarity in the cell-boundary distribution of VANGL2 along the ovary–uterus axis. (C) Mechanisms by which core PCP proteins control the coordinated movements of cilia.
2.1. Multicellular and Tissue-Level Coordination of Cilia Orientation
An important role of core PCP proteins in PCP establishment is their non-cell-autonomous effect on the orientation of adjacent cells [23]. CELSRs are atypical cadherins and their homophilic interactions through their extracellular domains enable the intercellular coupling of FZD- and VANGL-complexes (Figure 2A,A’) [23]. In Xenopus skin, the transplantation of VANGL2-overexpressing tissues showed non-cell autonomous effects on adjacent wild type cells. In these wild type cells, cilia pointed away from the transplant. VANGL2-knocked down transplants showed opposite effects to the overexpressing transplants. In other words, cilia always pointed to cells with lower VANGL2-level [43]. In the oviduct of CELSR1 knockout mice, cilia orientation is still coordinated in each cell, but the mean angle of cilia orientations in each cell varies among adjacent cells [39]. These observations suggest that core PCP proteins are required for the intercellular coordination of cilia orientation [41].
To align the orientation of cells along the tissue axis, factors that transmit information regarding tissue orientation are necessary. Such factors are referred to as global factors, which include a gradient of extracellular concentration of WNT molecules [44][45][46], differences in the level of expression of atypical cadherins Dachsous/Fat and their modulator Four-jointed [23][25][47][48][49][50], forces exerted on epithelial tissues [51][52][53][54], and fluid flow [37][55]. Those global cues somehow control the localization of core PCP proteins at cell boundaries. For example, in Xenopus skin, core PCP proteins are stabilized at cell boundaries that lie perpendicular to the direction of gastrulation. When mechanical strain was artificially applied to Xenopus skin, localization of core PCP proteins was stabilized at cell boundaries, suggesting that the mechanical strain generated by gastrulation acts as a global cue [54]. However, mechanisms by which global cues control the localization of core PCP proteins, and what acts as a global cue in each organ, are still largely unknown.
2.2. How Do Core PCP Proteins Orient Cilia?
Various forms of evidence suggest that the distribution of core PCP proteins at the cell boundary provides a directional cue for orienting cilia. If so, how do core PCP proteins control cilia that emerge from distant locations? Recent studies suggest that core PCP proteins orient cilia via microtubules [38][56][57][58]. In ependymal cells of the ventricle, a molecular motor dynein is localized at the cell cortex where the FZD-complex is enriched. Dishevelled-associating protein DAPLE is required for the localization of dynein at the cell cortex, and the loss of DAPLE and the inhibition of the activity of dynein both abrogate the orientation of cilia [56][59]. It has been proposed that dynein at the cell cortex pulls microtubules that connect the cell cortex and basal bodies, and this pulling force might orient cilia to FZD-enriched cell boundaries (Figure 2C) [56]. DAPLE is also required for the establishment of rotational polarity in the trachea, although it is unclear whether the role of DAPLE is the same as in ependymal cells. In the trachea, DAPLE binds to FZD6, and bundles and stabilizes nearby microtubules, and this concentrates microtubules around the FZD6-enriched cell cortex [58]. A theoretical analysis incorporating a hydrodynamic interaction between cilia and microtubules suggests that such an asymmetric concentration of microtubules is sufficient to orient cilia [57]. A similar asymmetric concentration of microtubules was reported in the mouse oviduct [39]. In a CELSR1 mutant oviduct, microtubules were still concentrated in more than 75% of multi-ciliated cells, while the orientation of the concentration was not aligned along the tissue axis. Importantly, the orientation of the concentration was consistent with that of cilia in each CELSR1 mutant cell [39]. Therefore, CELSR1 control the orientation of the concentration of microtubules, thus aligning cilia along the body axis. These results suggest that core PCP proteins provide directional information to cilia via microtubules that is sensed by basal bodies.
Interestingly, in the Xenopus skin, an effective stroke pointed to the direction of VANGL-complex-enriched cell boundaries [60]. This relationship is reversed in the mouse oviduct, trachea, and ventricle, where the direction of recovery stroke and that of VANGL complex-enriched cell boundaries are consistent [37][38][42]. Mechanisms of how core PCP proteins control cilia orientation might be different among tissues and animals.
2.3. Variable Roles of Members of Core PCP Proteins in Multi-Ciliated Cells
A simplified view of how core PCP proteins orient cilia is as follows: (1) global cues orient the cell boundary-localization of core PCP proteins along the tissue axis and (2) core PCP proteins orient cilia toward core PCP proteins-enriched cell boundaries via microtubules. However, complexities reside in this core PCP proteins-dependent mechanism. Genetic analyses suggest that each member of core PCP proteins plays different roles in multi-ciliated cells. In the brain ventricle, intercellular coordination of cilia orientation require CELSR1, while rotational polarity depends on CELSR3 and VANGL2 [41].
In addition to cell boundaries, core PCP proteins are also detected at cilia. DVLs are localized at the base of cilia in Xenopus skin, mouse trachea and ventricle [37][38][61], and VANGL2 is localized along the cilia in mouse ventricle [37]. DVL1, DVL2, and DVL3 seem to show different subcellar localization in a tissue-dependent manner. Whereas DVL1 and DVL3 are localized at cell boundaries, DVL2 is localized only at the base of cilia in mouse trachea [38]. In the mouse ventricle, DVL1 and DVL2 are detected at the patch of basal bodies [37][62][63]. In Xenopus skin, knockdown of DVLs disrupted the apical migration of basal bodies, and impacted ciliogenesis. Furthermore, when a deletion form of DVL, Xdd1, was expressed in multi-ciliated cells, ciliogenesis was weakly affected, but the orientation of cilia was no longer aligned in each cell. In addition to Xdd1, misexpression of a dominant negative form of RhoA severely misoriented cilia. Since rGBD, which binds to active RhoA, was concentrated in foci at the apical surface of multi-ciliated cells and those foci were lost when DVLs became depleted, DVLs might control cilia orientation via the activation of RhoA at basal bodies (Figure 2C) [61]. Furthermore, functions of DVL in cilia might be regulated post-transcriptionally. PTEN dephosphorylates serine 143 of DVL2, and the loss of PTEN affects ciliogenesis and/or the polarity of cilia in Xenopus skin, and mouse trachea and ventricle [64]. In addition to DVLs, CELSRs also regulate ciliogenesis. In ventricles lacking both CELSR2 and CELSR3, basal bodies do not migrate to the apical surface of cells during the differentiation of multi-ciliated cells [36]. These findings indicate that core PCP proteins function at two different locations, at cilia and at cell boundaries.
References
- Brooks, E.R.; Wallingford, J.B. Multiciliated Cells. Curr. Biol. 2014, 24, R973–R982.
- Boutin, C.; Kodjabachian, L. Biology of Multiciliated Cells. Curr. Opin. Genet. Dev. 2019, 56, 1–7.
- Reiter, J.F.; Leroux, M.R. Genes and Molecular Pathways Underpinning Ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547.
- Meunier, A.; Azimzadeh, J. Multiciliated Cells in Animals. Cold Spring Harb. Perspect. Biol. 2016, 8, a028233.
- Spassky, N.; Meunier, A. The Development and Functions of Multiciliated Epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436.
- Li, S.; Winuthayanon, W. Oviduct: Roles in Fertilization and Early Embryo Development. J. Endocrinol. 2017, 232, R1–R26.
- Shi, D.; Komatsu, K.; Uemura, T.; Fujimori, T. Analysis of Ciliary Beat Frequency and Ovum Transport Ability in the Mouse Oviduct. Genes Cells 2011, 16, 282–290.
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia Dysfunction in Lung Disease. Annu. Rev. Physiol. 2015, 77, 379–406.
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241.
- Ji, W.; Tang, Z.; Chen, Y.; Wang, C.; Tan, C.; Liao, J.; Tong, L.; Xiao, G. Ependymal Cilia: Physiology and Role in Hydrocephalus. Front. Mol. Neurosci. 2022, 15, 1–12.
- Satir, P.; Christensen, S.T. Structure and Function of Mammalian Cilia. Histochem. Cell Biol. 2008, 129, 687–693.
- Reiter, J.F.; Blacque, O.E.; Leroux, M.R. The Base of the Cilium: Roles for Transition Fibres and the Transition Zone in Ciliary Formation, Maintenance and Compartmentalization. EMBO Rep. 2012, 13, 608–618.
- Clare, D.K.; Magescas, J.; Piolot, T.; Dumoux, M.; Vesque, C.; Pichard, E.; Dang, T.; Duvauchelle, B.; Poirier, F.; Delacour, D. Basal Foot MTOC Organizes Pillar MTs Required for Coordination of Beating Cilia. Nat. Commun. 2014, 5, 4888.
- Garcia, G.; Reiter, J.F. A Primer on the Mouse Basal Body. Cilia 2016, 5, 1–9.
- Klotz, C.; Bordes, N.; Laine, M.C.; Sandoz, D.; Bornens, M. A Protein of 175,000 Daltons Associated with Striated Rootlets in Ciliated Epithelia, as Revealed by a Monoclonal Antibody. Cell Motil. Cytoskelet. 1986, 6, 56–67.
- Yang, J.; Liu, X.; Yue, G.; Adamian, M.; Bulgakov, O.; Li, T. Rootletin, a Novel Coiled-Coil Protein, Is a Structural Component of the Ciliary Rootlet. J. Cell Biol. 2002, 159, 431–440.
- Hagiwara, H.; Harada, S.; Maeda, S.; Aoki, T.; Ohwada, N.; Takata, K. Ultrastructural and Immunohistochemical Study of the Basal Apparatus of Solitary Cilia in the Human Oviduct Epithelium. J. Anat. 2002, 200, 89–96.
- Butler, M.T.; Wallingford, J.B. Planar Cell Polarity in Development and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 375–388.
- Axelrod, J.D. Planar Cell Polarity Signaling in the Development of Left–Right Asymmetry. Curr. Opin. Cell Biol. 2020, 62, 61–69.
- Shi, D.; Komatsu, K.; Hirao, M.; Toyooka, Y.; Koyama, H.; Tissir, F. Celsr1 Is Required for the Generation of Polarity at Multiple Levels of the Mouse Oviduct. Development 2014, 3, 4558–4568.
- Adler, P.N. The Frizzled/Stan Pathway and Planar Cell Polarity in the Drosophila Wing. Curr. Top. Dev. Biol. 2012, 101, 1–31.
- Humphries, A.C.; Mlodzik, M. From Instruction to Output: Wnt/PCP Signaling in Development and Cancer. Curr. Opin. Cell Biol. 2018, 51, 110–116.
- Strutt, H.; Strutt, D. How Do the Fat-Dachsous and Core Planar Polarity Pathways Act Together and Independently to Coordinate Polarized Cell Behaviours? Open Biol. 2021, 11, 200356.
- Aw, W.Y.; Devenport, D. Planar Cell Polarity: Global Inputs Establishing Cellular Asymmetry. Curr. Opin. Cell Biol. 2017, 44, 110–116.
- Lawrence, P.A.; Casal, J. Planar Cell Polarity: Two Genetic Systems Use One Mechanism to Read Gradients. Development 2018, 145, dev168229.
- Yang, Y.; Mlodzik, M. Wnt-Frizzled/Planar Cell Polarity Signaling: Cellular Orientation by Facing the Wind (Wnt). Annu. Rev. Cell Dev. Biol. 2015, 31, 623–646.
- Eaton, S.; Jülicher, F. Cell Flow and Tissue Polarity Patterns. Curr. Opin. Genet. Dev. 2011, 21, 747–752.
- Usui, T.; Shima, Y.; Shimada, Y.; Hirano, S.; Burgess, R.W.; Schwarz, T.L.; Takeichi, M.; Uemura, T. Flamingo, a Seven-Pass Transmembrane Cadherin, Regulates Planar Cell Polarity under the Control of Frizzled. Cell 1999, 98, 585–595.
- Wallingford, J.B. Planar Cell Polarity and the Developmental Control of Cell Behavior in Vertebrate Embryos. Annu. Rev. Cell Dev. Biol. 2012, 28, 627–653.
- Hale, R.; Strutt, D. Conservation of Planar Polarity Pathway Function Across the Animal Kingdom. Annu. Rev. Genet. 2015, 49, 529–551.
- Strutt, D.I. Asymmetric Localization of Frizzled and the Establishment of Cell Polarity in the Drosophila Wing. Mol. Cell 2001, 7, 367–375.
- Axelrod, J.D. Unipolar Membrane Association of Dishevelled Mediates Frizzled Planar Cell Polarity Signaling. Genes Dev. 2001, 15, 1182–1187.
- Feiguin, F.; Hannus, M.; Mlodzik, M.; Eaton, S. The Ankyrin Repeat Protein Diego Mediates Frizzled-Dependent Planar Polarization. Dev. Cell 2001, 1, 93–101.
- Tree, D.R.P.; Shulman, J.M.; Rousset, R.; Scott, M.P.; Gubb, D.; Axelrod, J.D. Prickle Mediates Feedback Amplification to Generate Asymmetric Planar Cell Polarity Signaling. Cell 2002, 109, 371–381.
- Bastock, R.; Strutt, H.; Strutt, D. Strabismus Is Asymmetrically Localised and Binds to Prickle and Dishevelled during Drosophila Planar Polarity Patterning. Development 2003, 130, 3007–3014.
- Tissir, F.; Qu, Y.; Montcouquiol, M.; Zhou, L.; Komatsu, K.; Shi, D.; Fujimori, T.; Labeau, J.; Tyteca, D.; Courtoy, P.; et al. Lack of Cadherins Celsr2 and Celsr3 Impairs Ependymal Ciliogenesis, Leading to Fatal Hydrocephalus. Nat. Neurosci. 2010, 13, 700–707.
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.G.; et al. Coupling between Hydrodynamic Forces and Planar Cell Polarity Orients Mammalian Motile Cilia. Nat. Cell Biol. 2010, 12, 341–350.
- Vladar, E.K.; Bayly, R.D.; Sangoram, A.M.; Scott, M.P.; Axelrod, J.D. Microtubules Enable the Planar Cell Polarity of Airway Cilia. Curr. Biol. 2012, 22, 2203–2212.
- Usami, F.M.; Arata, M.; Shi, D.; Oka, S.; Higuchi, Y.; Tissir, F.; Takeichi, M.; Fujimori, T. Intercellular and Intracellular Cilia Orientation Is Coordinated by CELSR1 and CAMSAP3 in Oviduct Multi-Ciliated Cells. J. Cell Sci. 2021, 134, jcs257006.
- Boutin, C.; Goffinet, A.M.; Tissir, F. Celsr1-3 Cadherins in PCP and Brain Development. Curr. Top. Dev. Biol. 2012, 101, 161–183.
- Boutin, C.; Labedan, P.; Dimidschstein, J.; Richard, F.; Cremer, H.; André, P.; Yang, Y.; Montcouquiol, M.; Goffinet, A.M.; Tissir, F. A Dual Role for Planar Cell Polarity Genes in Ciliated Cells. Proc. Natl. Acad. Sci. USA 2014, 111, E3129–E3138.
- Shi, D.; Usami, F.; Komatsu, K.; Oka, S.; Abe, T.; Uemura, T.; Fujimori, T. Dynamics of Planar Cell Polarity Protein Vangl2 in the Mouse Oviduct Epithelium. Mech. Dev. 2016, 141, 78–89.
- Mitchell, B.; Stubbs, J.L.; Huisman, F.; Taborek, P.; Yu, C.; Kintner, C. The PCP Pathway Instructs the Planar Orientation of Ciliated Cells in the Xenopus Larval Skin. Curr. Biol. 2009, 19, 924–929.
- Chu, C.-W.; Sokol, S.Y. Wnt Proteins Can Direct Planar Cell Polarity in Vertebrate Ectoderm. Elife 2016, 5, 1–13.
- Gao, B.; Song, H.; Bishop, K.; Elliot, G.; Garrett, L.; English, M.A.; Andre, P.; Robinson, J.; Sood, R.; Minami, Y.; et al. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Dev. Cell 2011, 20, 163–176.
- Koca, Y.; Collu, G.M.; Mlodzik, M. Wnt-Frizzled Planar Cell Polarity Signaling in the Regulation of Cell Motility. Curr. Top. Dev. Biol. 2022, 150, 255–297.
- Fulford, A.D.; McNeill, H. Fat/Dachsous Family Cadherins in Cell and Tissue Organisation. Curr. Opin. Cell Biol. 2020, 62, 96–103.
- Ma, D.; Yang, C.; Mcneill, H.; Simon, M.A. Fidelity in Planar Cell Polarity Signalling. Nature 2003, 6, 543–547.
- Ambegaonkar, A.A.; Irvine, K.D. Coordination of Planar Cell Polarity Pathways through Spiny-Legs. eLife 2015, 4, e09946.
- Arata, M.; Sugimura, K.; Uemura, T. Difference in Dachsous Levels between Migrating Cells Coordinates the Direction of Collective Cell Migration. Dev. Cell 2017, 42, 479–497.e10.
- Aigouy, B.; Farhadifar, R.; Staple, D.B.; Sagner, A.; Röper, J.C.; Jülicher, F.; Eaton, S. Cell Flow Reorients the Axis of Planar Polarity in the Wing Epithelium of Drosophila. Cell 2010, 142, 773–786.
- Aw, W.Y.; Heck, B.W.; Joyce, B.; Devenport, D. Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr. Biol. 2016, 26, 2090–2100.
- Hirano, S.; Mii, Y.; Charras, G.; Michiue, T. Alignment of the Cell Long Axis by Unidirectional Tension Acts Cooperatively with Wnt Signalling to Establish Planar Cell Polarity. Development 2022, 149, 3–8.
- Chien, Y.H.; Keller, R.; Kintner, C.; Shook, D.R. Mechanical Strain Determines the Axis of Planar Polarity in Ciliated Epithelia. Curr. Biol. 2015, 25, 2774–2784.
- Mitchell, B.; Jacobs, R.; Li, J.; Chien, S.; Kintner, C. A Positive Feedback Mechanism Governs the Polarity and Motion of Motile Cilia. Nature 2007, 447, 97–101.
- Takagishi, M.; Esaki, N.; Takahashi, K.; Takahashi, M. Cytoplasmic Dynein Functions in Planar Polarization of Basal Bodies within Ciliated Cells. iScience 2020, 23, 101213.
- Namba, T.; Ishihara, S. Cytoskeleton Polarity Is Essential in Determining Orientational Order in Basal Bodies of Multi-Ciliated Cells. PLoS Comput. Biol. 2020, 16, 1–18.
- Nakayama, S.; Yano, T.; Namba, T.; Konishi, S.; Takagishi, M.; Herawati, E.; Nishida, T.; Imoto, Y.; Ishihara, S.; Takahashi, M.; et al. Planar Cell Polarity Induces Local Microtubule Bundling for Coordinated Ciliary Beating. J. Cell Biol. 2021, 220, e202010034.
- Takagishi, M.; Sawada, M.; Ohata, S.; Asai, N.; Enomoto, A.; Takahashi, K.; Weng, L.; Ushida, K.; Ara, H.; Matsui, S.; et al. Daple Coordinates Planar Polarized Microtubule Dynamics in Ependymal Cells and Contributes to Hydrocephalus. Cell Rep. 2017, 20, 960–972.
- Butler, M.T.; Wallingford, J.B. Control of Vertebrate Core Planar Cell Polarity Protein Localization and Dynamics by Prickle 2. Development 2015, 142, 3429–3439.
- Park, T.J.; Mitchell, B.J.; Abitua, P.B.; Kintner, C.; Wallingford, J.B. Dishevelled Controls Apical Docking and Planar Polarization of Basal Bodies in Ciliated Epithelial Cells. Nat. Genet. 2008, 40, 871–879.
- Hirota, Y.; Meunier, A.; Huang, S.; Shimozawa, T.; Yamada, O.; Kida, Y.S.; Inoue, M.; Ito, T.; Kato, H.; Sakaguchi, M.; et al. Planar Polarity of Multiciliated Ependymal Cells Involves the Anterior Migration of Basal Bodies Regulated by Non-Muscle Myosin II. Development 2010, 137, 3037–3046.
- Ohata, S.; Nakatani, J.; Herranz-Pérez, V.; Cheng, J.G.; Belinson, H.; Inubushi, T.; Snider, W.D.; García-Verdugo, J.M.; Wynshaw-Boris, A.; Álvarez-Buylla, A. Loss of Dishevelleds Disrupts Planar Polarity in Ependymal Motile Cilia and Results in Hydrocephalus. Neuron 2014, 83, 558–571.
- Shnitsar, I.; Bashkurov, M.; Masson, G.R.; Ogunjimi, A.A.; Mosessian, S.; Cabeza, E.A.; Hirsch, C.L.; Trcka, D.; Gish, G.; Jiao, J.; et al. PTEN Regulates Cilia through Dishevelled. Nat. Commun. 2015, 6, 8388.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
973
Revisions:
2 times
(View History)
Update Date:
21 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




