
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergey Shityakov | -- | 3257 | 2022-11-16 10:06:04 | | | |
| 2 | Catherine Yang | Meta information modification | 3257 | 2022-11-16 10:09:22 | | |
Video Upload Options
Cyclodextrins (CDs) are cyclic oligosaccharide structures that could be used for theranostic applications in personalized medicine. These compounds have been widely utilized not only for enhancing drug solubility, stability, and bioavailability but also for controlled and targeted delivery of small molecules. These compounds can be complexed with various biomolecules, such as peptides or proteins, via host-guest interactions. CDs are amphiphilic compounds with water-hating holes and water-absorbing surfaces. Architectures of CDs allow the drawing and preparation of CD-based polymers (CDbPs) with optimal pharmacokinetic and pharmacodynamic properties. These polymers can be cloaked with protein corona consisting of adsorbed plasma or extracellular proteins to improve nanoparticle biodistribution and half-life. Besides, CDs have become famous in applications ranging from biomedicine to environmental sciences.
1. Structural Features of Cyclodextrin-Based Polymers
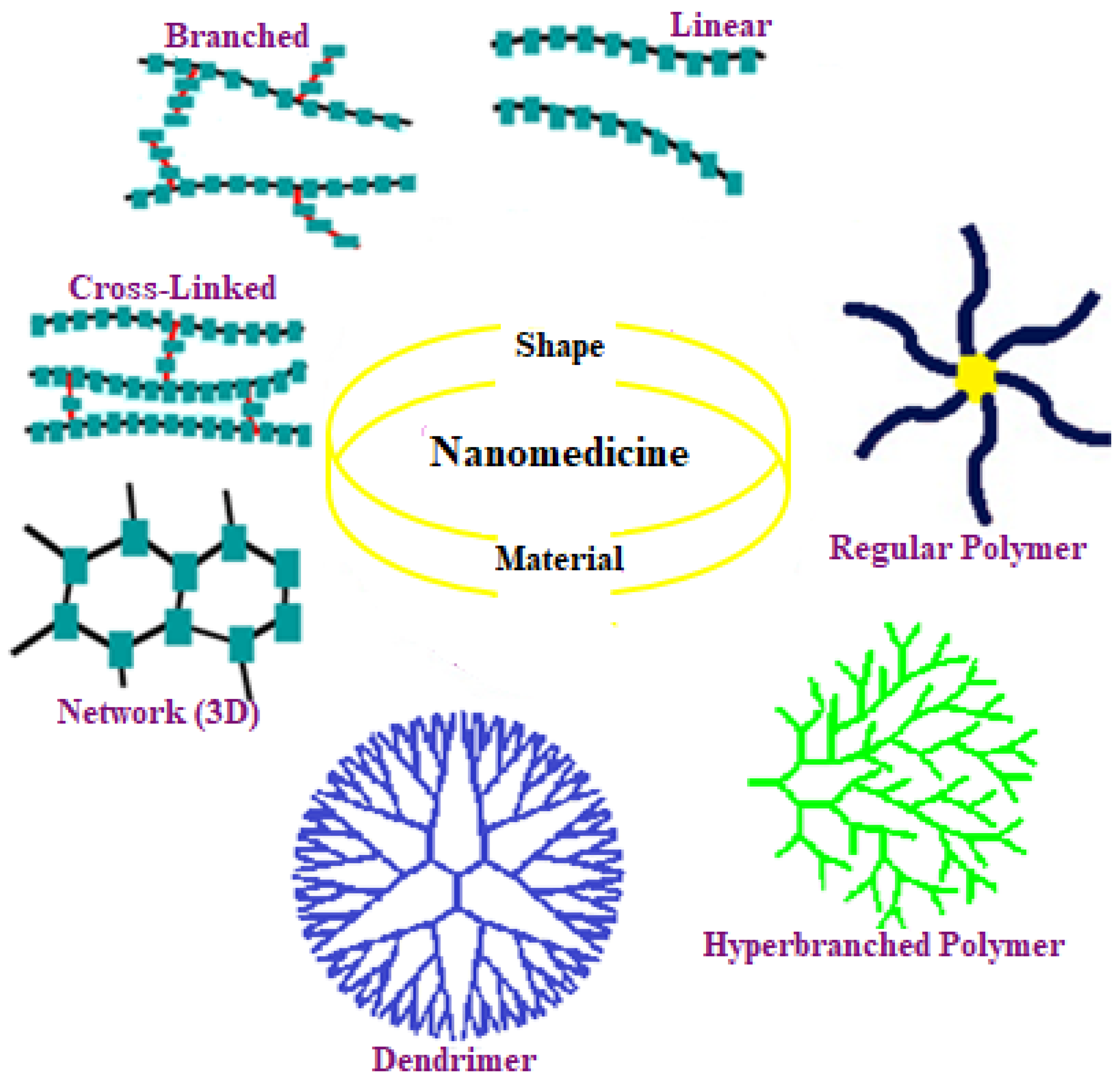
1.1. Cyclodextrin-Centred Core Polymers
1.2. Cyclodextrin-(Pendant and Terminated) Polymers
2. Application of Cyclodextrin-Based Polymers in Theranostic Nanomedicine
3. Application of Cyclodextrin-Protein Corona in Biomedical Approaches
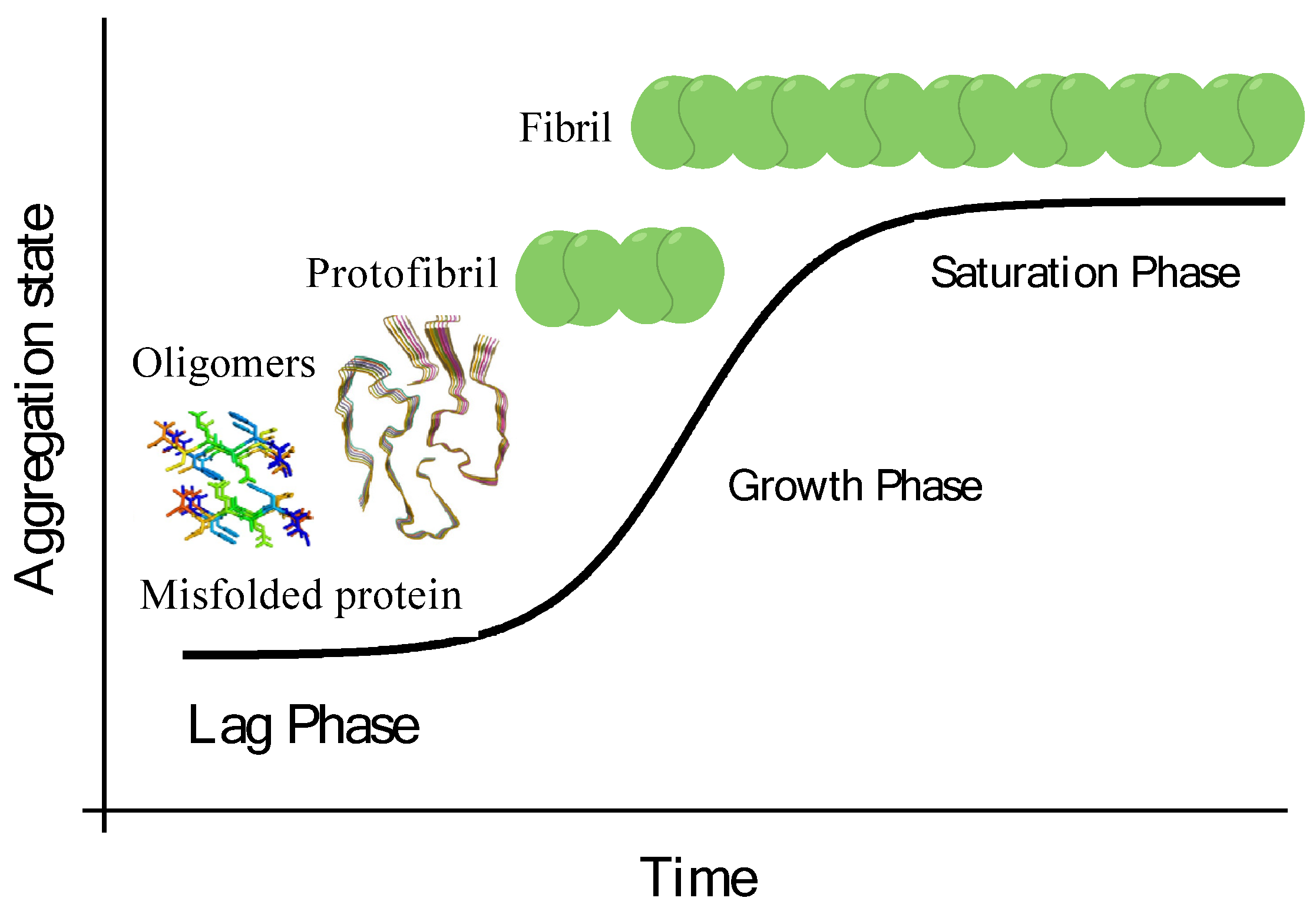
4. Mechanism of Protein Fibrillation
References
- Takata, T.; Aoki, D. Topology-transformable polymers: Linear-branched polymer structural transformation via the mechanical linking of polymer chains. Polym. J. 2018, 50, 127–147.
- Cheng, J.; Khin, K.T.; Jensen, G.S.; Liu, A.; Davis, M.E. Synthesis of Linear, β-Cyclodextrin-Based Polymers and Their Camptothecin Conjugates. J. Bioconjug. Chem. 2003, 14, 1007–1017.
- Flory, P.J. Network topology and the theory of rubber elasticity. Br. Polym. J. 1985, 17, 96–102.
- Duplantier, B. Statistical mechanics of polymer networks of any topology. J. Stat. Phys. 1989, 54, 581–680.
- Tezuka, Y.; Oike, H. Topological polymer chemistry: Systematic classification of nonlinear polymer topologies. J. Am. Chem. Soc. 2001, 123, 11570–11576.
- Gref, R.; Amiel, C.; Molinard, K.; Daoud-Mahammed, S.; Sébille, B.; Gillet, B.; Beloeil, J.-C.; Ringard, C.; Rosilio, V.; Poupaert, J.; et al. New self-assembled nanogels based on host–guest interactions: Characterization and drug loading. J. Control. Release 2006, 111, 316–324.
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547.
- Ma, Y.; Mou, Q.; Wang, D.; Zhu, X.; Yan, D. Dendritic polymers for Theranostics. Theranostics 2016, 6, 930–947.
- Wang, J.; Zhang, J.; Yu, S.; Wu, W.; Jiang, X. Synthesis and Self-Assembly of a Nanoscaled Multiarm Polymer Terminated by β-Cyclodextrin. ACS Macro Lett. 2012, 2, 82–85.
- Deng, J.; Liu, X.; Zhang, S.; Cheng, C.; Nie, C.; Zhao, C. Versatile and rapid postfunctionalization from cyclodextrin modified host polymeric membrane substrate. Langmuir 2015, 31, 9665–9674.
- Kawano, S.; Lie, J.; Ohgi, R.; Shizuma, M.; Muraoka, M. Modulating Polymeric Amphiphiles Using Thermo- and pH-Responsive Copolymers with Cyclodextrin Pendant Groups through Molecular Recognition of the Lipophilic Dye. Macromolecules 2021, 54, 5229–5240.
- Liao, R.; Liu, Y.; Lv, P.; Wu, D.; Xu, M.; Zheng, X. Cyclodextrin pendant polymer as an efficient drug carrier for scutellarin. Drug Deliv. 2020, 27, 1741–1749.
- Jia, Y.-G.; Zhu, X.X. Self-healing supramolecular hydrogel made of polymers bearng cholic acid and β-cyclodextrin pendants. Chem. Mater. 2015, 27, 387–393.
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, E.; Crini, G. Water-insoluble β-cyclodextrin-epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processess using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23.
- Ji, Y.; Shan, S.; He, M.; Chu, C.-C. Inclusion complex from cyclodextrin-grafted hyaluronic acid and pseudo protein as biodegradable nano-delivery vehicle for gambogic acid. Acta Biomater. 2017, 62, 234–245.
- Uekama, K.; Hirayama, F.; Arima, H. Recent Aspects of Cyclodextrin-Based Drug Delivery System. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 3–8.
- Song, X.; Wen, Y.; Zhu, J.-L.; Zhao, F.; Zhang, Z.-X.; Li, J. Thermoresponsive Delivery of Paclitaxel by β-CD-Based Poly(N-isopropylacrylamide) Star Polymer via Inclusion Complexation. Biomacromolecules 2016, 17, 3957–3963.
- Sivakumar, P.M.; Peimanfard, S.; Zarrabi, A.; Khosravi, A.; Islami, M. Cyclodextrin-Based Nanosystems as Drug Carriers for Cancer Therapy. Anti-Cancer Agents Med. Chem. 2020, 20, 1327–1339.
- Ohno, K.; Wong, B.; Haddleton, D.M. Synthesis of well-defined cyclodextrin-core star polymers. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2206–2214.
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-based Polymer Materials: From Controlled Synthesis to Applications. J. Prog. Polym. Sci. 2019, 93, 1–35.
- Schaefgen, J.R.; Flory, P.J. Synthesis of Multichain polymers and Investigation of their Viscosities. J. Am. Chem. Soc. 1948, 70, 2709–2718.
- Ito, D.; Kimura, Y.; Takenaka, M.; Ouchi, M.; Terashima, T. Single-chain crosslinked polymers via the transesterification of folded polymers: From efficient synthesis to crystallinity control. J. Polym. Chem. 2020, 11, 5181–5190.
- Morton, M.; Helminiak, T.E.; Gadkary, S.D.; Bueche, F. Preparation and properties of monodisperse branched polystyrene. J. Polym. Sci. 1962, 57, 471–482.
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Driva, P.; Sakellariou, G.; Chatzichristidi, M. Polymers with Star-Related Structures: Synthesis, Properties, and Applications. Polym. Sci. Compr. Ref. 2012, 6, 29–111.
- Szillat, F.; Schmidt, B.V.K.J.; Hubert, A.; Barner-Kowollik, C.; Ritter, H. Redox-Switchable Supramolecular Graft Polymer Formation via Ferrocene-Cyclodextrin Assembly. J. Macromol. Rapid Commun. 2014, 35, 1293–1300.
- Daoud-Mahammed, S.; Ringard-Lefebvre, C.; Razzouq, N.; Rosilio, V.; Gillet, B.; Couvreur, P.; Amiel, C.; Gref, R. Spontaneous association of hydrophobized dextran and poly-β-cyclodextrin into nanoassemblies: Formation and interaction with a hydrophobic drug. J. Colloid Interface Sci. 2007, 307, 83–93.
- Simões, S.M.N.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289.
- Yuan, Y.; Nie, T.; Fang, Y.; You, X.; Huang, H.; Wu, J. Stimuli-responsive cyclodextrin-based supramolecular assemblies as drug carriers. J. Mater. Chem. B 2022, 10, 2077–2096.
- Siepmann, J.; Faham, A.; Clas, S.-D.; Boyd, B.J.; Jannin, V.; Bernkop-Schnürch, A.; Zhao, H.; Lecommandoux, S.; Evans, J.C.; Allen, C.; et al. Lipids and polymers in pharmaceutical technology: Lifelong companions. Int. J. Pharm. 2019, 558, 128–142.
- Solms, J.; Egli, R.H. Harze mit Einschlusshohlräumen von Cyclodextrin-Struktur. Helv. Chim. Acta 1965, 48, 1225–1228.
- Lederer, M.; Nguyen, H.K.H. Adsorption chromatography on cellulose XIV. Some results use aqueous solutions of soluble cyclodextrin polymers as eluents. J. Chromatogr. 1996, 723, 405–409.
- Safapour, S.; Mazhar, M.; Nikanfard, M.; Liaghat, F. Recent advancements on the functionalized cyclodextrin-based adsorbents for dye removal from aqueous solutions. Int. J. Environ. Sci. Technol. 2022, 19, 5753–5790.
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. Cyclodextrin-Based Polymers for Food and Pharmaceutical Applications: A Historical Review. Hist. Cyclodext. 2020, 52, 281–304.
- Rahman, M.; Alrobaian, M.; Almalki, W.H.; Mahnashi, M.H.; Alyami, B.A.; Alqarni, A.O.; Alqahtani, Y.S.; Alharbi, K.S.; Alghamdi, S.; Panda, S.K.; et al. Suprabranched polyglycerol nanostructures as drug delivery and theranostics tools for cancer treatment. J. Drug Discov. Today 2021, 26, 1006–1017.
- Khor, S.Y.; Quinn, J.; Whittaker, M.; Truong, N.P.; Davis, T.P. Controlling Nanomaterials Size and Shape for Biomedical Applications via Polymerization-Indced Self-Assembling. J. Macromol. Rapid Commun. 2018, 40, e1800438.
- Tang, H.; Zhang, J.; Tang, J.; Shen, Y.; Guo, W.; Zhou, M.; Wang, R.; Jiang, N.; Gan, Z.; Yu, Q. Tumor-specific and renal excretable star-like polymer-doxorubicin conjugates for safe and efficient anticancer therapy. Biomacromolecules 2018, 19, 2849–2862.
- Xu, Z.; Liu, S.; Liu, H.; Yang, C.; Kang, Y.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. J. Chem. Commun. 2015, 51, 15768–15771.
- Li, Z.; Senthil, K.; Sun, Y.; Wang, Y.; Huo, H.; Hao, Y.; Liu, Y.; Chen, H.; Li, H.; Zhang, Z.; et al. Nearly monodisperse unimolecular micelles via chloro-based atom transfer radical polymerization. Giant 2021, 7, 100062.
- Namgung, R.; Lee, Y.M.; Kim, J.; Jang, Y.; Lee, B.-H.; Kim, I.-S.; Sokkar, P.; Rhee, Y.; Hoffman, A.S.; Kim, W.J. Poly-cyclodextrin and poly-paclitaxel nano-assembly for anticancer therapy. Nat. Commun. 2014, 5, 3702.
- Badwaik, V.D.; Aicart, E.; Mondjinou, Y.A.; Johnson, M.A.; Bowman, V.D.; Thompson, D.H. Structure-property relationship for in vitro siRNA delivery performance of cationic 2-hydroxypropyl-β-cyclodextrin: PEG-PPG-PEG polyrotaxane vectors. Biomaterials 2016, 84, 86–98.
- Gómez-García, M.; Benito, J.M.; Butera, A.P.; Mellet, C.O.; Fernández, J.M.G.; Blanco, J.L.J. Probing Carbohydrate-Lectin Recognition in Heterogeneous Environments with Monodisperse Cyclodextrin-Based Glycoclusters. J. Org. Chem. 2012, 77, 1273–1288.
- Albuzat, T.; Keil, M.; Ellis, J.; Alexander, C.; Wenz, G. Transfection of luciferase DNA into various cells by cationic cyclodextrin polyrotaxanes derived from ionene-11. J. Mater. Chem. 2012, 22, 8558–8565.
- Huang, H.; Xu, D.; Liu, M.; Jiang, R.; Mao, L.; Huang, Q.; Wan, Q.; Wen, Y.; Zhang, X.; Wei, Y. Direct encapsulation of AIE-active dye with β-cyclodextrin terminated polymers: Self-assembly and biological imaging. J. Mater. Sci. Eng. C 2017, 78, 862–867.
- Yallapu, M.M.; Chauhan, N.; Othman, S.F.; Khalilzad-Sharghi, V.; Ebeling, M.C.; Khan, S.; Jaggi, M.; Chauhan, S.C. Implicatons of protein corona on physico-chemical and biological properties of magnetic nanoparticles. Biomaterials 2015, 46, 1–12.
- Schmitt, S.; Nuhn, L.; Barz, M.; Butt, H.; Koynov, K. Shining Light on polymeric Drug Nanocarriers with fluorescence correlation spectroscopy. Macromol. Rapid Commun. 2022, 43, 2100892.
- Carulla, N.; Caddy, G.L.; Hall, D.; Zurdo, J.; Gairí, M.; Feliz, M.; Giralt, E.; Robinson, C.; Dobson, C.M. Molecular recycling within amyloid fibrils. Nature 2005, 436, 554–558.
- Rajan, R.S.; Illing, M.E.; Bence, N.F.; Kopito, R.R. Specificity in intracellular protein aggregatio and inclusion body formation. Proc. Natl. Acad. Sci. USA 2001, 98, 13060–13065.
- Wang, M.; Kakinen, A.; Pilkington, E.H.; Davis, T.P.; Ke, P.C. Differential effects of silver and iron oxide nanoparticles on IAPP amyloid aggregation. Biomater. Sci. 2017, 5, 485–493.
- Cabaleiro-Lago, C.; Lynch, I.; Dawson, K.A.; Linse, S. Inhibition of IAPP and IAPP(20-29) Fibrilltion by Polymeric Nanoparticles. Langmuir 2010, 26, 3453–3461.
- Iannuzzi, C.; Irace, G.; Sirangelo, I. The Effect of Glycosaminoglycans (GACs) on Amyloid Aggregation and Toxicity. Molecules 2015, 20, 2510–2528.
- Mirsadeghi, S.; Dinarvand, R.; Ghahremani, M.H.; Hormozi-Nezhad, M.R.; Mahmoudi, Z.; Hajipour, M.J.; Atyabi, F.; Ghavami, M.; Mahmoudi, M. Protein corona composition of gold nanoparticles/nanorods affects amyloid beta fibrillation process. Nanoscale 2015, 7, 5004–5013.
- Yan, Y.; Gause, K.T.; Kamphuis, M.M.J.; Ang, C.-S.; O’Brien-Simpson, N.; Lenzo, J.; Reynolds, E.; Nice, E.C.; Caruso, F. Differential Roles of the Protein Corona in the Cellular Uptake of Nanoporous Polymer Particles by Monocyte and Macrophage Cell Lines. ACS Nano 2013, 7, 10960–10970.
- Ghosh, P.; De, P. Modulation of Amyloid Protection Fibrillation by Synthetic Polymers: Recent Advances in the Context of Neurodegenerative Diseases. ACS Appl. Bio Mater. 2020, 3, 6598–6625.
- Christoffersen, H.F.; Andreasen, M.; Zhang, S.; Nielsen, E.H.; Christiansen, G.; Dong, M.; Skrydstrup, T.; Otzen, D.E. Scaffolded multimers of hIAPP20–29 peptide fragments fibrilate fatser and lead to different fibrils compared to the free hIAPP20–29 peptide fragment. Biochim. Biophys. Acta 2015, 1854, 1890–1897.
- Oliveri, V.; Zimbone, S.; Giuffrida, M.L.; Bellia, F.; Tomasello, M.F.; Vecchio, G. Pophyrin Cyclodextrin Conjugates Modulate Amyloid Beta Peptide Aggregation and Cytotoxicity. Chem. Eur. J. 2018, 24, 6349–6353.
- Wang, H.; Xu, X.; Pan, Y.; Yan, Y.; Hu, X.; Chen, R.; Ravoo, B.J.; Guo, D.; Zhang, T. Recognition and Removal of Amyloid-beta by a Heteromultivalent Macrocyclic Coassembly: A Potential Strategy for the Treatment of Alzheimer’s Disease. Adv. Mater. 2021, 33, e2006483.





