
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Broggi | + 4519 word(s) | 4519 | 2020-12-10 10:31:14 | | | |
| 2 | Nicole Yin | -1524 word(s) | 2995 | 2020-12-17 04:16:33 | | |
Video Upload Options
Choroidal melanoma (CM), despite its rarity, is the most frequent intraocular malignancy. Over time, several histological variants of CM have been distinguished, including spindle A and B cell, fascicular, epithelioid and necrotic type. However, they have been progressively abandoned as having no prognostic value and currently, the American Joint Committee of Cancer (AJCC) classification identifies three CM cell types: spindle, epithelioid and mixed cell type. Other rare histological variants of CM include: (i) diffuse melanoma; (ii) clear cell; and (iii) balloon cell melanoma. Immunohistochemically, CMs are stained with Human Melanoma Black 45 (HMB45) antigen, S-100 protein, Melan-A (also known as melanoma antigen recognized by T cells 1/MART-1), melanocyte inducing transcription factor (MITF), tyrosinase, vimentin, and Sex determining region Y-Box 10 (SOX10). Several genetic and histopathological prognostic factors of CM have been reported in the literature, including epithelioid cell type, TNM staging, extraocular extension, monosomy 3 and 6p gain and loss of BAP-1 gene. The aim of this review was to summarize the histopathological, immunohistochemical and genetic features of CM, establishing “the state of the art” and providing colleagues with practical tools to promptly deal with patients affected by this rare malignant neoplasm.
1. Introduction
Despite being generally considered a rare neoplasm, uveal melanoma (UM) represents the most common primary ocular malignant lesion in adults[1]. Regarding its anatomical location, UM more frequently affects the choroid, then the ciliary bodies and finally the iris[1][2][3]. Iris melanomas often have a better outcome than those affecting choroid or ciliary body, probably because iris melanomas are often visible from the outside of the eye and therefore early diagnosed. Since the choroid is the most common site of onset, herein we will use the term “choroidal melanoma” (CM) to indicate the entire spectrum of melanomas arising from the uveal tract. The incidence of CM is 200 times higher in Caucasians and Fitzpatrick phototypes I-II, than in the black population[1][2][3]. The following risk factors[1][2][3]have traditionally been associated with a higher occurrence of CM: (i) presence of choroidal nevus, (ii) cutaneous dysplastic nevus syndrome, (iii) oculo-dermal melanocytosis (Ota nevus), and (iv) type 1 neurofibromatosis (NF-1). Although CM often remains clinically non-symptomatic for years, sudden retinal detachment frequently represents the presentation form of the tumor[1][2][3]. CM is characterized by poor prognosis with distant metastases (liver is the most affected site) occurring in about 50% of patients within 10–15 years from diagnosis[1][2][3].
2. Eye Anatomy
The human eye is composed of two connected portions: the anterior segment and the posterior segment. The former is composed of the cornea, iris and lens; the latter consists of the vitreous, retina, choroid, ciliary bodies and sclera. Normal ocular histology identifies three concentric layers:
- (i).
-
the outer layer (sclera and cornea): the sclera is a fibrous connective tissue mainly composed of non-parallel-oriented, dense type 1 collagen fibers; the cornea consists of type I collagen fibers oriented in parallel and lined by a non-keratinized, stratified squamous epithelium.
- (ii).
-
the uveal tract (choroid, iris and ciliary bodies): the choroid is made up of a dense network of blood vessels, embedded in loose connective tissue. The iris is composed of connective fibrovascular tissue, into which the sphincter and dilator pupillae muscles are inserted, and lined by a pigmented epithelium. Ciliary bodies separate the vitreous from the remaining posterior segment structures and are composed of the ciliary muscle that regulates the accommodation of the lens, and the ciliary epithelium, that produces aqueous humor.
- (iii).
-
the inner layer (lens, vitreous and retina): the retina constitutes the intraocular nervous tissue, consisting of multiple layers (retinal pigment epithelium, outer limiting membrane, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, nerve fiber layer and internal limiting membrane).
Anatomically, the ophthalmic artery (a branch of the internal carotid) represents the main ocular source of arterial blood. Multiple arterial branches arise from the ophthalmic artery to supply different ocular portions: dorsal nasal (the nasolacrimal sac and the dorsum of the nose), central retinal artery (optic nerve and inner layers of the retina), muscular artery (extraocular muscles, ciliary plexus and conjunctiva), ciliary arteries (sclera, choroid and anterior segment of the eye), lacrimal artery (lacrimal gland and eyelids) and supraorbital artery (in its intraocular tract, superior rectus and levator palpebrae superioris muscles).
3. Histopathological Features
Grossly, an enucleated eye shows the presence of a frequently pigmented, dome- or mushroom-shaped, mass protruding into the posterior segment (Figure 1). Hemorrhages, signs of extrascleral invasion and secondary retinal detachment may be identified in some cases. In 1931 Callender[4] proposed the first classification of CM identifying six types of melanoma based on their cellular composition and included: (1) spindle A, (2) spindle B, (3) fascicular, (4) mixed, (5) epithelioid, and (6) necrotic. In this classification, (1) spindle A cells are fusiform, cohesive and have a slender nucleus with fine nuclear chromatin, a small or indistinct basophilic nucleolus and have a longitudinal fold in the nucleus related to invagination of the nuclear membrane; (2) spindle B cells are fusiform cohesive and have plumper spindle or cigar-like nuclei, coarser chromatin, and a more prominent basophilic or eosinophilic nucleolus; (3) fascicular choroidal melanomas have a histopathological pattern reminiscent of schwannoma and therefore characterized by nuclei arranged in columns perpendicular to a central vessel or spindle cells arranged in bundles with palisading of nuclei; (4) the mixed type is composed of spindle cells and epithelioid cells that are non-cohesive, larger, polygonal cells with an abundant, glassy cytoplasm, distinct cell border and large, round nucleus with marginated coarse chromatin; (5) melanomas composed exclusively of the previously described epithelioid cells and (6) the last group is composed of tumors too necrotic to be classified. In this first classification, patients with mixed, epithelioid and necrotic choroidal melanomas have a much worse prognosis than those with tumors of the spindle A, spindle B or fascicular types[5]. However, this first classification was difficult to interpret and apply by pathologists that requested a more reproducible classification with objective criteria. Considering this, it was decided to eliminate the division of spindle cell tumors into type A and type B because tumors are usually composed of a mixture of these two cell types and, more important, no differences in prognosis were observed. CMs originally reported as fascicular were reclassified as spindle-cell type or mixed-cell type[6]. It was also decided to include a smaller type of epithelioid cells with less cytoplasm and a small nucleus than the classical epithelioid cell described by Callender[7]. These small epithelioid cells have large eosinophilic nucleoli and lack cohesiveness as classical epithelioid cells and have a worse prognosis. Currently, the American Joint Committee of Cancer (AJCC) classification[8] recognizes three cell types: (i) spindle cell (usually composed of an admixture of spindle A and spindle B cells) (Figure 2); (ii) epithelioid cell (Figure 3); and (iii) mixed cell type (Figure 4). According to this classification, spindle cell melanoma is composed of spindle cells in ≥90% of the tumor, and epithelioid cell melanoma is composed of epithelioid cells in ≥90% of the tumor; all other tumors are mixed cell melanomas. Other rare variants of choroidal melanomas include: (1) diffuse melanoma characterized by the involvement of at least one-quarter of the uveal tract[9]; (2) clear cell melanoma composed of tumor cells with oval, centrally located nuclei and clear cell cytoplasm due to the dissolution of glycogen granules during fixation[10]; (3) balloon cell melanoma characterized of cells with abundant cytoplasm containing multiple vacuoles that stain for lipids[11]. The pigmentation of CMs usually varies from heavily pigmented to amelanotic cases. Lymphocytic infiltration in CMs is not as frequent as in cutaneous melanoma and two types of infiltration, patchy or diffuse, are reported[12] (Figure 5). As reported in the literature[13], lymphocytic infiltration is usually associated with a better prognosis in many cancer types, such as cutaneous melanoma, nonsmall cell lung cancer, and breast cancer. However, in CMs the inflammation is associated with a worse prognosis and is frequently related to loss of chromosome 3[13]. Folberg[14] reported a prognostic vascular classification according to histological features. At Periodic Acid Schiff (PAS) stained sections nine vascular patterns were observed: (1) normal choroidal vessels, (2) no tumor vessels, (3) straight vessels, (4) parallel vessels, (5) parallel vessel with cross-linking, 6) arcs or incomplete loops, (7) arcs with branching, (8) complete loops, and (9) networks of three or more back-to-back closed loops. According to this study, the presence of at least one closed vascular loop in a CM in association with epithelioid cells and mitotic figures is the most significant vascular pattern associated with death from metastatic melanoma after enucleation.
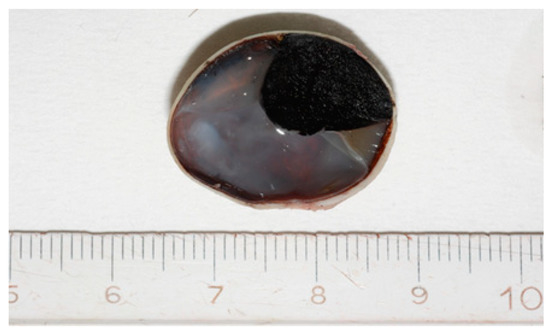
Figure 1. Grossly, Choroidal melanoma (CM) appears on the cut section as a dome-shaped pigmented mass protruding into the posterior segment of the eye.
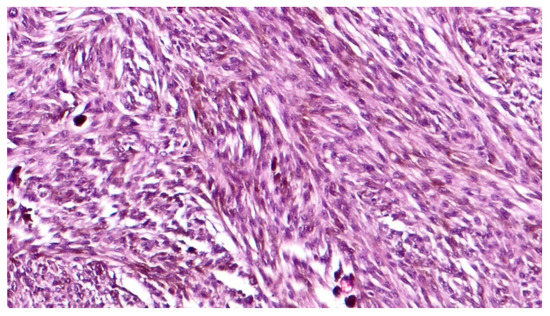
Figure 2. High magnification showing spindle cell type, pigmented, choroidal melanoma, composed of a mixture of spindle A and B cells (hematoxylin and eosin; original magnification 200×).
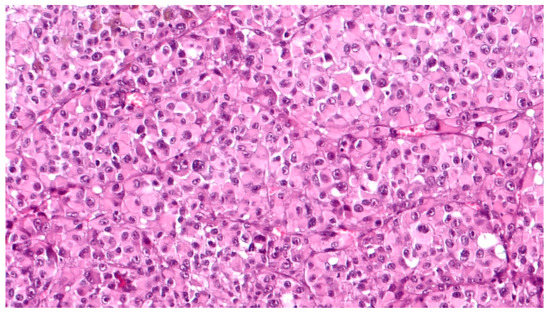
Figure 3. High magnification showing epithelioid cell type, poorly pigmented, choroidal melanoma, composed of polygonal cells with abundant, eosinophilic cytoplasm, distinct cell borders and large nuclei with conspicuous nucleoli (hematoxylin and eosin; original magnification 200×).
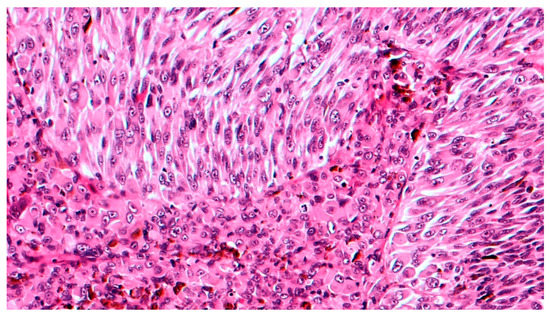
Figure 4. High magnification showing mixed cell type choroidal melanoma, composed of both epithelioid and spindle cells (hematoxylin and eosin; original magnification 200×).
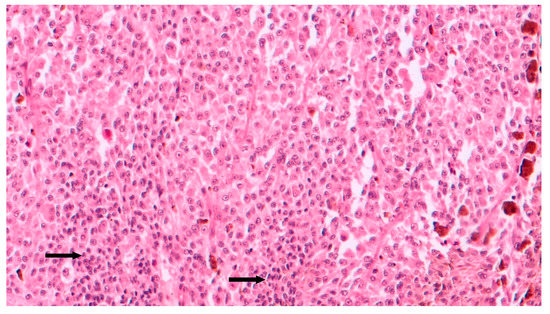
Figure 5. Intermediate magnification showing epithelioid cell type choroidal melanoma with sparse chronic lymphocytic infiltrates (arrows) intermingled to neoplastic cells (hematoxylin and eosin; original magnification 100×).
4. Immunohistochemical Features
CMs are positive for Human Melanoma Black 45 (HMB45) antigen, S-100 protein, Melan-A (also known as melanoma antigen recognized by T cells 1/MART-1), melanocyte inducing transcription factor (MITF), tyrosinase, vimentin, and Sex determining Region Y-Box 10 (SOX10)[15][16]. Differently to the strong labeling of cutaneous melanoma, S100 is frequently weak and variable in CM. HMB45 antigen, tyrosinase, Melan-A, SOX10 and MITF are better immunomarkers for CM considering the strong positivity in all histologic types[17]. Particularly, SOX10 is a nuclear transcription factor with crucial functions in the specification of the neural crest and maintenance of Schwann cells and melanocytes[18][19]. It is usually immunoexpressed at the nuclear level in melanocytes and breast myoepithelial cells and is currently used in the histopathologic diagnosis of many neoplasms, being diffusely positive in breast basal-like (triple-negative) carcinomas, melanomas and soft tissue tumors of neural crest origin, including malignant peripheral nerve sheath tumor[18][19]. SOX10 represents the most specific melanoma cell marker, being also expressed in those histotypes characterized by loss of immunohistochemical expression of other melanoma cell markers, such as cutaneous desmoplastic melanoma[18][19].
5. Genetic Profile
Mutation analysis allowed the identification of two mutually exclusive driver mutations in Guanine nucleotide-binding protein G, q polypeptide (GNAQ) (~55%) or Guanine nucleotide-binding protein G, subunit alpha-11 (GNA11) (~40%) in CMs. Q209 and R183 mutations of these heterotrimeric G protein alpha subunits of the Gq class can lead to the activation of multiple downstream pathways involved in proliferation and cell growth[20][21]. Unfortunately, these mutations cannot discriminate nevi from melanoma considering that they are also present in choroidal nevi[21] and neither have they been shown to be of significant prognostic value. Worse prognosis and high risk of metastasis are instead associated with the loss of chromosome 3 (monosomy of chromosome 3), in particular, when associated with polysomy 8q[22]. The gain of chromosome 6p (6p gain) is usually associated with a better prognosis, while the loss of chromosome 6q is usually found in tumors that metastasize[23]. V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF) mutations, usually observed in cutaneous melanomas, are very rare in CMs[24]. Kit mutations are also rare[25]. BRCA1 associated protein-1 (BAP1) is a gene located on chromosome 3 that encodes for a ubiquitin carobxy-terminal hydrolase involved in chromatin remodeling and cell cycle control[26]. Inactivating mutations of this gene, affecting the catalytic domain of the enzyme or frameshift/non-sense mutations resulting in a non-functional protein, have been found in about 80% of metastasizing choroidal melanomas[27]. According to the literature[28], the loss of BAP1 promotes metastasis of CM because it is also involved in transendothelial migration of neoplastic cells. Koopmans et al.[29] found that BAP1 mutation status was strongly associated with BAP1 protein immunohistochemical expression; accordingly, the absence of nuclear immunohistochemical expression of BAP1 represents an easily identifiable surrogate for the presence of BAP1 mutations. Mutations of codon 625 of Splicing Factor 3b Subunit 1 (SF3B1) on chromosome 2 are reported in some CMs. The encoded protein is a splice factor and is also associated with chromatin remodeling[30]. This mutation usually gives an intermediate risk of developing metastasis[31]. A translation initiation factor encoded by Eukaryotic Translation Initiation Factor 1A X-Linked (EIF1AX), located at Xp22.1, is also frequently mutated in rarely metastasizing choroidal melanomas[32]. These secondary driver mutations that occur in BAP1, SF3B1, or EIF1AX are generally mutually exclusive.
The risk of CM metastasis could be predicted by gene expression profiling (GEP) of 15 genes[33] (Table 1). GEP differentiates class 1 and class 2 tumors, the first class was likely to undergo metastasis, whereas the second class has a greater rate of metastasis and disease-related mortality. GEP class 1 tumors mainly contain tumors with disomy 3, the gain of chromosome 6p, and EIF1AX or SF3B1 mutations, where monosomy 3 tumors, deletions of chromosome 8p with BAP1 mutations are mainly classified as GEP class 2 tumors. Considering cell differentiation, class 1 tumors are well-differentiated with low Ki-67 antibody positivity, class 2 tumors are composed of ectodermal stem-cell-like cells with high Ki-67 antibody positivity[34]. The Cancer Genome Atlas (TCGA) recently performed a study on 80 CMs and identified four subsets: two associated with poor-prognosis monosomy 3 and two with better prognosis disomy 3[35].
Table 1. Genes included in the 15-gene expression profile.
| Gene Symbol | Gene Name |
|---|---|
| UpRegulated in Class 2 Uveal Melanomas | |
| CDH1 | E-cadherin |
| ECM1 | Extracellular matrix protein 1 |
| HTR2B | 5-Hydroxytryptamine (serotonin) receptor 2B |
| RAB31 RAB31 | member of the RAS oncogene family |
| DownRegulated in Class 2 Uveal Melanomas | |
| EIF1B | Eukaryotic translation initiation factor 1B |
| FXR1 | Fragile X mental retardation, autosomal homolog 1 |
| ID2 | Inhibitor of DNA binding 2 |
| LMCD1 | LIM and cysteine-rich domains 1 |
| LTA4H | Leukotriene A4 hydrolase |
| MTUS1 | Microtubule-associated tumor suppressor 1 |
| ROBO1 | Roundabout, axon guidance receptor, 1 |
| SATB1 | SATB homeobox 1 |
| Control Genes | |
| MRPS21 | Mitochondrial ribosomal protein S21 |
| RBM23 | RNA-binding motif protein 23 |
| SAP130 | Sin3A-associated protein, 130 kDa |
Although advantages have been made in the knowledge of the molecular status of CM, no strongly validated therapeutical options have been introduced and the therapeutic “landscape” of metastatic CM, in particular, still remains largely unknown. The relatively recent introduction of immune checkpoint inhibitors (ICIs) has represented a turning point in the treatment of metastatic cutaneous melanoma. However, metastatic CM is extremely resistant to ICIs[36]. The mechanisms by which CM cells develop resistance to ICIs are still largely unknown. It is believed that one of these potential mechanisms would be the induction of an immune-suppressive CM microenvironment through the upregulation of immunosuppressive molecules and downregulation of activated T cells. Terai et al.[37] demonstrated that one of the mechanisms leading to poor T cell immune reaction in CM hepatic metastases is tryptophan 2,3-dioxygenase (TDO)-mediated tryptophan (TRP) catabolism, since the liver is rich in TDO. TRP is essential for T cell and NK cell functions and it has been shown that its catabolites act as suppressor molecules for T cell and NK cell proliferation[38]. Terai et al.[37] found a high expression of TDO protein in both CM liver metastasis tissue and metastatic CM cell lines; interestingly, TDO was diffusely expressed by both normal hepatocytes and neoplastic cells, suggesting a strong role for TDO in the development of ICI resistance.
6. Prognostic Factors
The pathology report must classically contain some useful information to guide the oncologist on the patient’s outcome. The exact location of the tumor must be indicated considering that melanomas confined to the choroid tend to have a more favorable prognosis than melanomas extending into the ciliary body[39]. The possible evidence of extraocular extension with particular attention to infiltration of the sclera along emissary vessels and nerves. In order to identify this kind of infiltration serial sections may be required. It is also advisable to measure the diameter of the extraocular extension with an ocular micrometer because the microscopic extraocular extension has no negative prognostic impact[40]. Further classical morphologic features with an impact on patient outcomes, such as cell type, specific growth pattern (diffuse CMs tend to be associated with a less favorable prognosis[41]), greatest thickness and largest basal diameter of the tumor (on which the 8th edition of Tumor, node, metastasis-TNM-staging depends[42]) and proliferative activity, both as a mitotic index (number of mitoses/mm2) and Ki67 proliferation rate[43], must be reported by the pathologist. In recent years the evidence of inflammatory anti-tumor response has become increasingly important, hence pathology reports should also contain the potential presence of prominent tumor-infiltrating lymphocytes (>100 lymphocytes per 20 (40×) high power fields) and tumor-associated macrophages, that seem to be associated with an increased likelihood of metastasis[13] [13]. The pathologic finding of closed loops of PAS-positive material encircling packets of tumor cells or at least three back-to-back loops has a strong independent association with tumor-related mortality. These loops stain for laminin and fibronectin and have been shown to conduct plasma cells and red blood cells and are formed by highly invasive tumor cells through a process known as vasculogenic mimicry[14]. In addition to the abovementioned prognostic factors, our group recently reported some new immunohistochemical markers with prognostic value in predicting the risk of distant metastases[44][45][46][47][48][49]. CM pathologic prognostic factors are summarized in Table 2.
Table 2. Main histologic findings with prognostic value in choroidal melanoma.
| Pathologic Prognostic Factors of Choroidal Melanoma |
|---|
| Cell type (epithelioid, spindle, mixed cell type) |
| Tumor location |
| TNM staging (T-stage based on largest tumor basal diameter and thickness) |
| Extraocular invasion |
| Mitotic index |
| Ki67 proliferation rate |
| Intratumoral inflammatory infiltrates (tumor-infiltrating lymphocytes and tumor-associated macrophages) |
| PAS-positive peritumoral loops |
7. Conclusions
The genetic landscape of CM is increasingly being studied and numerous points have been clarified for a better understanding of the main molecular traits of this rare neoplasm. The results obtained made it possible only to prognostically sub-stratify CM patients, distinguishing patients with more favorable prognosis, characterized by longer overall survival and slower metastatic progression, and patients with more biologically aggressive CM forms; however, they did not identify validated target genes and mutations for a potential therapeutic response; in this regard, it must be emphasized that CM prognosis remains poor, as the therapeutic options available to these patients are still few. Finally, the most relevant efforts of the scientific community are being focused on the identification of novel molecular targets, potentially useful as a predictor of therapeutic response in CM.
References
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer. Treat. Rev. 2012, 38, 549–553.
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24.
- Mahendraraj, K.; Lau, C.S.; Lee, I.; Chamberlain, R.S. Trends in incidence, survival, and management of uveal melanoma: A population-based study of 7516 patients from the surveillance, epidemiology, and end results database (1973–2012). Clin. Ophthalmol. 2016, 10, 2113–2119.
- Callender, G.R.; Malignant melanotic tumors of the eye: A study of histologic types in 111 cases. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1931, 131, 131–142.
- Eleanor V. Paul; B Leslie Parnell; May Fraker; PROGNOSIS OF MALIGNANT MELANOMAS OF THE CHOROID AND CILIARY BODY. International Ophthalmology Clinics 1962, 2, 387-402, 10.1097/00004397-196206000-00007.
- Ian W. McLean; Walter D. Foster; Lorenz E. Zimmerman; John W. Gamel; Modifications of Callender's Classification of Uveal Melanoma at the Armed Forces Institute of Pathology. American Journal of Ophthalmology 2018, 195, lvi–lx, 10.1016/j.ajo.2018.08.025.
- I W McLean; W D Foster; L E Zimmerman; J W Gamel; Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology.. American Journal of Ophthalmology 1983, 96, 502–509.
- Kivela, T.; Simpson, E.R.; Grossniklaus, H.E.; Jager, M.J.; Sough, A.D.; Caminal, J.M. Uveal melanoma. In AJCC Cancer Staging Manual, 8th ed.; Amin, E.B., Edge, S., Greene, F., Byrd, D.R., Brookland, K.R., Washington, M.K., Eds.; Springer: New York, NY, USA; pp. 805–817
- J Biswas; R Raghavendra; V Ratra; S Krishnakumar; L Gopal; M P Shanmugam; Diffuse malignant melanoma of the choroid simulating metastatic tumour in the choroid.. Indian Journal of Ophthalmology 2000, 48, 137–140.
- Hans E. Grossniklaus; Daniel M. Albert; W. Richard Green; Brian P. Conway; Kenneth R. Hovland; Clear Cell Differentiation in Choroidal Melanoma. Archives of Ophthalmology 1997, 115, 894-898, 10.1001/archopht.1997.01100160064010.
- M K Khalil; Balloon cell malignant melanoma of the choroid: ultrastructural studies.. British Journal of Ophthalmology 1983, 67, 579-584, 10.1136/bjo.67.9.579.
- Inge H G Bronkhorst; M J Jager; Inflammation in uveal melanoma. Eye 2012, 27, 217-223, 10.1038/eye.2012.253.
- Bernhard Mlecnik; Gabriela Bindea; Franck Pagès; Jerome Galon; Tumor immunosurveillance in human cancers. Cancer and Metastasis Reviews 2011, 30, 5-12, 10.1007/s10555-011-9270-7.
- Robert Folberg; Jacob Pe'er; Lynn M. Gruman; Robert F. Woolson; Gary Jeng; Paul R. Montague; Thomas O. Moninger; Hong Yi; Kenneth C. Moore; The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: A matched case-control study. Human Pathology 1992, 23, 1298-1305, 10.1016/0046-8177(92)90299-i.
- Fernandes, B.F.; Odashiro, A.N.; Saraiva, V.S.; Logan, P.; Antecka, E.; Burnier, M.N., Jr. Immunohistochemical expression of melan-A and tyrosinase in uveal melanoma. J. Carcinog. 2007, 6, 6.
- Mouriaux, F.; Vincent, S.; Kherrouche, Z.; Maurage, C.A.; Planque, N.; Monté, D.; Labalette, P.; Saule, S. Microphthalmia transcription factor analysis in posterior uveal melanomas. Exp. Eye Res. 2003, 76, 653–661.
- Satori Iwamoto; Robert C. Burrows; Robert E. Kalina; David George; Michael Boehm; Mark Bothwell; Rodney Schmidt; Immunophenotypic differences between uveal and cutaneous melanomas.. Archives of Ophthalmology 2002, 120, 466-470, 10.1001/archopht.120.4.466.
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Biernat, W.; Czapiewski, P.; Kopczynski, J.; Thompson, L.D.; Lasota, J.; Wang, Z.; Fetsch, J.F. Sox10--a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: A systematic analysis of 5134 tumors. Am. J. Surg. Pathol. 2015, 39, 826–835.
- Harbhajanka, A.; Chahar, S.; Miskimen, K.; Silverman, P.; Harris, L.; Williams, N.; Varadan, V.; Gilmore, H. Clinicopathological, immunohistochemical and molecular correlation of neural crest transcription factor SOX10 expression in triple-negative breast carcinoma. Hum. Pathol. 2018, 80, 163–169
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [
- Decatur, C.L.; Ong, E.; Garg, N.; Anbunathan, H.; Bowcock, A.M.; Field, M.G.; Harbour, J.W. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA Ophthalmol. 2016, 134, 728–733.
- Bertil Damato; Catherine Duke; Sarah E. Coupland; Paul Hiscott; Peter A. Smith; Ian Campbell; Angela Douglas; Peter Howard; Cytogenetics of Uveal Melanoma. Ophthalmology 2007, 114, 1925-1931.e1, 10.1016/j.ophtha.2007.06.012.
- G Prescher; N Bornfeld; B Horsthemke; R Becher; Chromosomal aberrations defining uveal melanoma of poor prognosis. The Lancet 1992, 339, 691-692, 10.1016/0140-6736(92)90861-v.
- Malaponte, G.; Libra, M.; Gangemi, P.; Bevelacqua, V.; Mangano, K.; D’Amico, F.; Mazzarino, M.C.; Stivala, F.; McCubrey, J.A.; Travali, S. Detection of BRAF gene mutation in primary choroidal melanoma tissue. Cancer. Biol. Ther. 2006, 5, 225–227.
- Wallander, M.L.; Layfield, L.J.; Emerson, L.L.; Mamalis, N.; Davis, D.; Tripp, S.R.; Holden, J.A. KIT mutations in ocular melanoma: Frequency and anatomic distribution. Mod. Pathol. 2011, 24, 1031–1035.
- Eletr, Z.M.; Wilkinson, K.D. An emerging model for BAP1’s role in regulating cell cycle progression. Cell Biochem. Biophys. 2011, 60, 3–11.
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413.
- Onken, M.D.; Li, J.; Cooper, J.A. Uveal melanoma cells utilize a novel route for transendothelial migration. PLoS ONE 2014, 9, e115472.
- Anna E Koopmans; Robert M Verdijk; Rutger W W Brouwer; Thierry P P Van Den Bosch; Mike M P Van Den Berg; Jolanda Vaarwater; Christel E M Kockx; Dion Paridaens; Nicole C Naus; Mark Nellist; et al.Wilfred F.J. Van IjckenEmine KiliçAnnelies De Klein Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Modern Pathology 2014, 27, 1321-1330, 10.1038/modpathol.2014.43.
- J. William Harbour; Elisha D. O. Roberson; Hima Anbunathan; Michael D. Onken; Lori A. Worley; Anne M. Bowcock; Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nature Genetics 2013, 45, 133-135, 10.1038/ng.2523.
- Amy C Schefler; Yavuzyigitoglu S; Koopmans Ae; Verdijk Rm; Vaarwater J; Eussen B; Van Bodegom A; Paridaens D; Kilic E; De Klein A; et al.Rotterdam Ocular Melanoma Study Group Faculty Opinions recommendation of Uveal Melanomas with SF3B1 Mutations: A Distinct Subclass Associated with Late-Onset Metastases.. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2018, 123, 1118–1128, 10.3410/f.726180822.793544673.
- Marcel Martin; Lars Maßhöfer; Petra Temming; Sven Rahmann; Claudia Metz; Norbert Bornfeld; Johannes Van De Nes; Ludger Klein-Hitpass; Alan G Hinnebusch; Bernhard Horsthemke; et al.Dietmar R LohmannMichael Zeschnigk Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nature Genetics 2013, 45, 933-936, 10.1038/ng.2674.
- Michael D. Onken; Lori A. Worley; Justis P. Ehlers; J. William Harbour; Gene Expression Profiling in Uveal Melanoma Reveals Two Molecular Classes and Predicts Metastatic Death. Cancer Research 2004, 64, 7205-7209, 10.1158/0008-5472.can-04-1750.
- Harmeet S. Gill; Devron H. Char; Uveal melanoma prognostication: from lesion size and cell type to molecular class. Canadian Journal of Ophthalmology 2012, 47, 246-253, 10.1016/j.jcjo.2012.03.038.
- A. Gordon Robertson; Juliann Shih; Christina Yau; Ewan A. Gibb; Junna Oba; Karen L. Mungall; Julian M. Hess; Vladislav Uzunangelov; Vonn Walter; Ludmila Danilova; et al.Tara M. LichtenbergMelanie KucherlapatiPatrick K. KimesMing TangAlexander PensonOzgun BaburRehan AkbaniChristopher A. BristowKatherine A. HoadleyLisa IypeMatthew T. ChangAndrew D. CherniackChristopher BenzGordon B. MillsRoel G. W. VerhaakKlaus G. GriewankIna FelauJean C. ZenklusenJeffrey E. GershenwaldLynn SchoenfieldAlexander J. LazarMohamed H. Abdel-RahmanSergio Roman-RomanMarc-Henri SternColleen M. CebullaMichelle D. WilliamsMartine J. JagerSarah E. CouplandBita EsmaeliCyriac KandothScott E. WoodmanAdrian AllyJ. Todd AumanMiruna BalasundaramSaianand BaluRameen BeroukhimInanc BirolTom BodenheimerJay BowenReanne BowlbyDenise BrooksRebecca CarlsenLynda ChinJuok ChoEric ChuahSudha ChudamaniCarrie CibulskisKristian CibulskisLeslie CopeTimothy DeFreitasJohn A. DemchokLaurence DesjardinsNoreen DhallaMartin L. FergusonScott FrazerStacey B. GabrielJulie M. Gastier-FosterNils GehlenborgMark GerkenGad GetzElizabeth A. GrimmD. Neil HayesApurva M. HegdeDavid I. HeimanCarmen HelselShital HobensackRobert A. HoltAlan P. HoyleXin HuCarolyn M. HutterStuart R. JefferysCorbin D. JonesSteven J.M. JonesKatayoon KasaianJaegil KimRaju KucherlapatiEric LanderMichael S. LawrenceSemin LeeKristen M. LeraasPei LinJia LiuWenbin LiuLaxmi LollaDanilova LudmilaStern Marc-HenriHarshad S. MahadeshwarOdette MarianiMarco A. MarraMichael MayoSam MeierShaowu MengMatthew MeyersonPiotr A. MieczkowskiRichard A. MooreLisle E. MoseAndrew J. MungallBradley A. MurrayRashi NareshMichael S. NobleAngeliki PantaziMichael ParfenovPeter J. ParkJoel S. ParkerCharles M. PerouTodd PihlRobert PilarskiAlexei ProtopopovAmie RadenbaughKaran RaiNilsa C. RamirezXiaojia RenSheila M. ReynoldsJeffrey RoachJason RoszikSara SadeghiGordon SaksenaXavier SastreDirk SchadendorfJacqueline E. ScheinSteven E. SchumacherJonathan SeidmanSahil SethGeetika SethiMargi ShethYan ShiCarol ShieldsIlya ShmulevichJanae V. SimonsArun D. SinghPayal SipahimalaniTara SkellyHeidi SofiaMatthew G. SolowayXingzhi SongJoshua StuartQiang SunHuandong SunAngela TamDonghui TanJiabin TangRoy TarnuzzerBarry S. TaylorNina ThiessenVesteinn ThorssonKane TseUmadevi VeluvoluDoug VoetYunhu WanZhining WangJohn N. WeinsteinMatthew D. WilkersonLisa WiseTina WongYe WuLiming YangJiashan ZhangHailei ZhangErik Zmuda Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204-220.e15, 10.1016/j.ccell.2017.07.003.
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353.
- Terai, M.; Londin, E.; Rochani, A.; Link, E.; Lam, B.; Kaushal, G.; Bhushan, A.; Orloff, M.; Sato, T. Expression of Tryptophan 2,3-Dioxygenase in Metastatic Uveal Melanoma. Cancers 2020, 12, 405.
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolusic, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502.
- Kivelä, T.; Kujala, E. Prognostication in eye cancer: The latest tumor, node, metastasis classification and beyond. Eye 2013, 27, 243–252.
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology 2013, 120, 2066–2071.
- Shields, C.L.; Kaliki, S.; Furuta, M.; Shields, J.A. Diffuse versus nondiffuse small (≤3 MM thickness) choroidal melanoma: Comparative analysis in 1,751 cases. The 2012 F. Phinizy Calhoun lecture. Retina 2013, 33, 1763–1776.
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) Classification of Malignant Tumours; Wiley-Blackwell: Oxford, UK; Hoboken, NJ, USA, 2017.
- Mooy, C.M.; Luyten, G.P.; de Jong, P.T.; Luider, T.M.; Stijnen, T.; van de Ham, F.; van Vroonhoven, C.C.; Bosman, F.T. Immunohistochemical and prognostic analysis of apoptosis and proliferation in uveal melanoma. Am. J. Pathol. 1995, 147, 1097–1104.
- Salvatorelli, L.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Ragusa, M.; Aldo, C.; Rappazzo, G.; Caltabiano, R.; Salemi, M. Immunoexpression of SPANX-C in metastatic uveal melanoma. Pathol. Res. Pract. 2019, 215, 152431.
- Salvatorelli, L.; Puzzo, L.; Bartoloni, G.; Palmucci, S.; Longo, A.; Russo, A.; Reibaldi, M.; Li Volti, G.; Caltabiano, R. Immunoexpression of macroH2A in uveal melanoma. Appl. Sci. 2019, 9, 3244.
- Broggi, G.; Musumeci, G.; Puzzo, L.; Russo, A.; Reibaldi, M.; Ragusa, M.; Longo, A.; Caltabiano, R. Immunohistochemical expression of ABCB5 as a potential prognostic factor in uveal melanoma. Appl. Sci. 2019, 9, 1316.
- Caltabiano, R.; Puzzo, L.; Barresi, V.; Ieni, A.; Loreto, C.; Musumeci, G.; Castrogiovanni, P.; Ragusa, M.; Foti, P.; Russo, A.; et al. ADAM 10 expression in primary uveal melanoma as prognostic factor for risk of metastasis. Pathol. Res. Pract. 2016, 212, 980–987.
- Caltabiano, R.; Puzzo, L.; Barresi, V.; Cardile, V.; Loreto, C.; Ragusa, M.; Russo, A.; Reibaldi, M.; Longo, A. Expression of Raf Kinase Inhibitor Protein (RKIP) is a predictor of uveal melanoma metastasis. Histol. Histopathol. 2014, 29, 1325–1334.
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front. Oncol. 2020, 10, 562074.




