Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nisha Devi Saini | -- | 1942 | 2022-11-11 10:29:07 | | | |
| 2 | Rita Xu | Meta information modification | 1942 | 2022-11-15 10:47:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Devi, N.; Singh, S.; Manickam, S.; Cruz-Martins, N.; Kumar, V.; Verma, R.; Kumar, D. Aspergillus Strain for the Production of Itaconic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/34696 (accessed on 08 February 2026).
Devi N, Singh S, Manickam S, Cruz-Martins N, Kumar V, Verma R, et al. Aspergillus Strain for the Production of Itaconic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/34696. Accessed February 08, 2026.
Devi, Nisha, Shubhangi Singh, Shivakumar Manickam, Natália Cruz-Martins, Vinod Kumar, Rachna Verma, Dinesh Kumar. "Aspergillus Strain for the Production of Itaconic Acid" Encyclopedia, https://encyclopedia.pub/entry/34696 (accessed February 08, 2026).
Devi, N., Singh, S., Manickam, S., Cruz-Martins, N., Kumar, V., Verma, R., & Kumar, D. (2022, November 15). Aspergillus Strain for the Production of Itaconic Acid. In Encyclopedia. https://encyclopedia.pub/entry/34696
Devi, Nisha, et al. "Aspergillus Strain for the Production of Itaconic Acid." Encyclopedia. Web. 15 November, 2022.
Copy Citation
Itaconic acid (IA) is a well-known bio-based monounsaturated organic acid (C5H6O4), with a white color and crystalline structure. It is widely used in the agro-based, plastics, textile, paint and pharmaceutical sectors, owing to its flexible structure, due to the presence of functional groups with covalent double bonds. IA is an alternative to the petrochemicals acrylic and methacrylic acids.
itaconic acid
metabolic engineering
Aspergillus terreus
1. Introduction
With a renewed interest in sustainable development, the chemical industry is making many efforts to replace petrochemical-based monomers with natural ones. Itaconic acid (2-methylidenebutanedioic acid) (IA) is one of the more valuable organic acids and is an important platform of chemical compounds. IA is a crystalline, white color, unsaturated dicarboxylic acid with a methylene group connected to one carboxyl group generated by microorganisms and has industrial importance as a precursor of polymers and chemicals [1]. Citraconic and mesaconic acids are isomeric with IA and can be substituted with acrylic or methacrylic acid. At moderate temperatures, IA can sustain acidic, neutral and slightly basic conditions [2][3]. The US Department of Energy in 2004 considered IA an added-value product from biomass, utilized as a precursor of polymers and chemical intermediates. These include synthetic fibers, resins, paints, acrylic plastics, acrylate latexes, super-absorbent materials, anti-scaling agents, styrene, 2-methyl-1,4-butanediol and 3-methyltetrahydrofuran [4].
IA has a very large market potential [5]. Currently, the industries use pure glucose or sucrose to produce IA at a very high cost; hence, it cannot compete and is considered for potential use in food applications [6]. Nonetheless, it is necessary to use low-cost agricultural waste as a raw material to regulate and reduce the substrate cost for its use for food purposes [7]. The cost of primary raw materials, competing among the food and biorefinery industries, environmental security, and the production of upgraded products from biowaste benefit from converting these biowastes into IA [8]. Starchy biomass from sweet potato, cassava, sago, corn and sorghum has been reported to generate IA by fermentation [9][10]. IA is a microbially produced organic acid that may be used as a substitute for many petroleum-based compounds, including acrylic and methacrylic acids, and, thus, can help to promote environmental sustainability. IA is renewed rapidly, unlike petroleum, which can lessen the reliance on petroleum and its negative environmental consequences [11][12]. The US-DOE 2004 produced a list of the “top 12” building organic chemicals to promote the bio-based economy, and IA is one of them [13]. Because IA has a vinyl group, it may be used to build poly-(IA), a polymer of IA, which has a wide range of industrial applications [14].
2. Chemical Route for IA Production
As described in Figure 1, IA was discovered in 1837 by Baup as “Citric acid” (Pfizer & Co.) [15][16]. Crassus (1840) named the same substance that results from the heat breakdown of citric acid as “IA” [16]. IA as an ethyl ester that was polymerized for the first time in 1873 by Swart [15]. Kinoshita [17] was the first to report on the microbial synthesis of IA. Under surface culture conditions, a thread-like colony of fungus was isolated from the salted plum juice, comprising concentrated sugar solutions and chlorides in high concentrations producing an amount of IA up to 0.24 g IA/g substrate, using Aspergillus itaconicus for its manufacture [17][18].
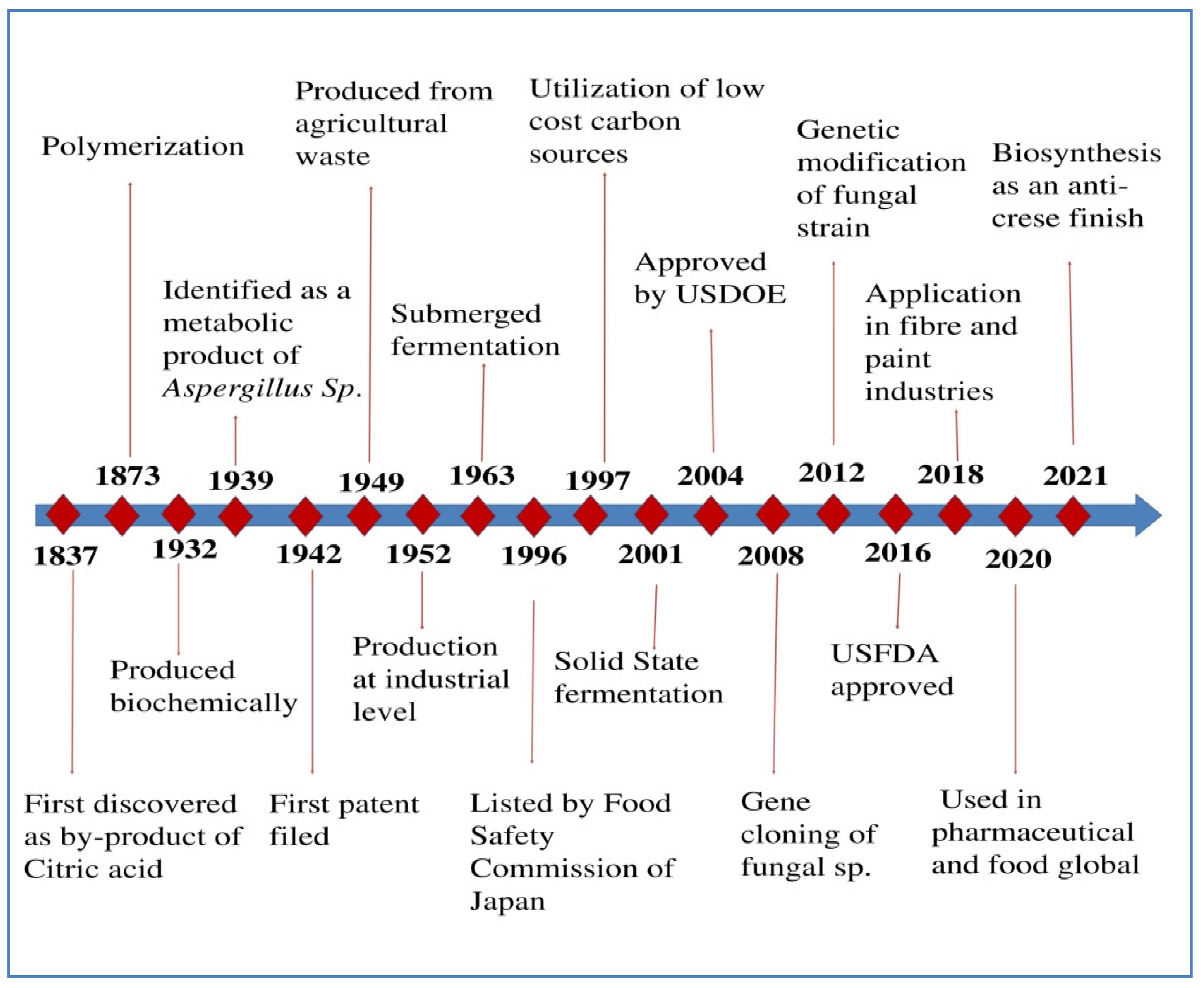
Thermal degradation of citric acid and hydrolysis of the anhydrides were the earliest methods used for IA production [19]. Another method used was the decarboxylation of aconitic acid [20][21], and the resultant product “IA” was named as an anagram of the source. The carbonization of citric acid and successive treatment of its anhydride with water are the most common methods of chemical synthesis [22] or using water, propargyl chloride, nickel carbonyl and carbon monoxide, according to the Montecatini (Italy) technique [23]. Tate et al. [3] reported that no other chemical methods could compete with fungal cultivation; hence, they are not used commercially. No more than 0.09 g/g of citric acid has been recorded as an IA yield [24], considered very low in industrial production. The primary disadvantage of chemical production of IA is that it depends upon high-cost raw materials for manufacture and on high temperatures during the reaction, making the process more costly [12].
3. Biological Route of IA Synthesis
The biotechnological production of IA using A. itaconicus was first described by Kinoshita [17], despite that when using Aspergillus terreus a higher final IA content is achieved when compared to other fungal species [25]. In 1945, Charles Pfizer filed the first patent for IA manufacturing on an industrialized level, and, after 10 years, the first production plan was started in the USA. The production of IA using A. terreus has been repeatedly improved to be a highly stable practice in which the chemical process could not compete with the biological process for a long time [26].
Nonetheless, the research for the synthesis of IA through enzyme participation is currently receiving greater attention. In the microbial biosynthesis pathway, several sugars, such as xylose, arabinose and glucose have been used as a source of carbon substrates. As revealed in the pattern of A. terreus, these sugars are transformed into pyruvate through hexose mono-phosphate shunt and glycolysis [27]. The pyruvate is transferred to the mitochondria from the cytosol and converted into cis-aconitate and citrate, as intermediate compounds of the Krebs’s cycle. Then, with the assistance of cadA, the cis-aconitate is converted into itaconate when the mitochondrial tricarboxylate transporter (Mtt) transports cis-aconitate back to the cytosol [28] (Figure 2). To date, IA is commonly produced at an industrial level using the filamentous fungus, A. terreus, as it generates the highest output as well as increases the final amount of IA under optimum growth conditions in the fermentation broth compared to other fungal strains [29]. It is noteworthy that, despite A. terreus being the main microbial species used for IA production, according to the requirements of A. terreus for optimum growth, it is not the most effective. Because a continuous supply of oxygen is necessary, this results in high NADH levels. A high level of NADH inhibits the major enzymes that play a significant role in IA production. The vigorous stirring during fermentation can readily damage the mycelia grown on A. terreus [28]. Moreover, due to the limited activity of natural cis-aconitate decarboxylase, CadA from A. terreus is over-expressed in these modified strains, but the final yields still remain less [30].
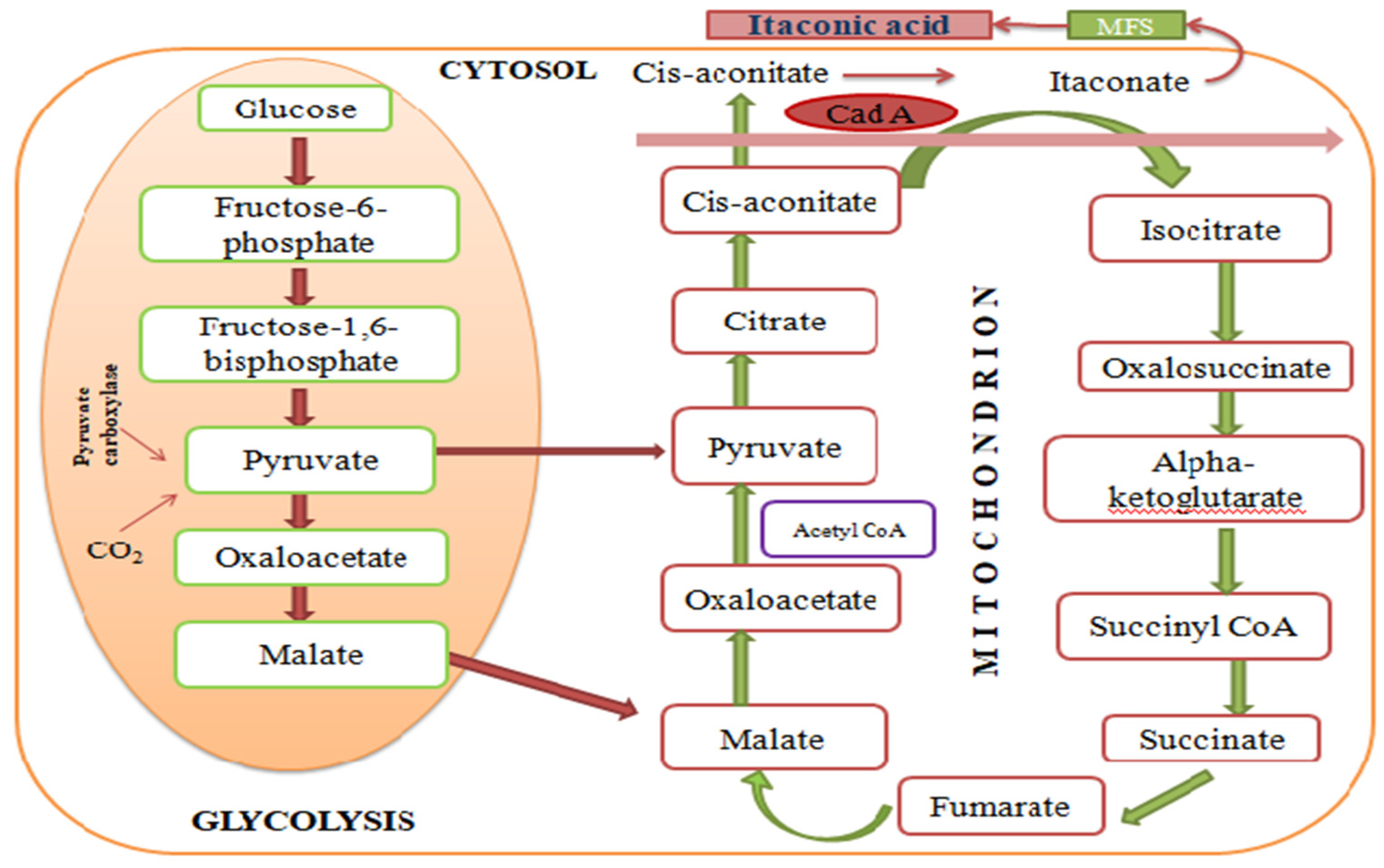
Figure 2. Biological route for IA synthesis.
4. Genetic Modifications of Aspergillus Strain for the Production of IA
4.1. Metabolic Engineering
In this biosynthesis pathway, cis-aconitic acid decarboxylation into IA is the unique and critical process. Kanamasa et al. [31] observed the characterization of the cadA gene in this biosynthesis; genetic modification of A. terreus into another microbe became feasible. Researchers tried to produce IA with A. niger, which was selected owing to its great ability to synthesize citric acid (CA) in large amounts of about 200 g/L, and the route for the synthesis of CA and IA are intertwined [32]. However, due to the lack of CadA, the synthesis of IA from A. niger does not occur spontaneously; as a result, CadA has been taken from A. terreus and transported into A. niger. IA yield from the modified A. niger strain was initially low [27]. For A. niger, codon optimized the cadA gene, and the Mtt and the major facilitator (Mfsa) genes were added, resulting in a 20-fold increase in the final yield of IA. Figure 3 shows that Mtta and Mfsa are important components in synthesizing IA [28]. In A. niger strain AB 1.13, the cadA gene from A. terreus is expressed [33]. The A. niger gpdA promoter has been used to regulate the cadA gene. For this reason, it allows a constitutive expression. A strain of A. niger that expresses the single cadA gene may generate around 0.7 g/L of IA, a yield that is not comparable to existing A. terreus production strains, but it is a promising starting point for further modification steps. Furthermore, researchers have attempted to enhance the number of expressed genes (Table 1), such as those already identified in the cadA gene and mitochondrial carrier protein (MCP) [25][33].
Figure 3. Genetic modifications for IA production using A. niger.
Table 1. Role of genetically modified non-Aspergillus genera in the production of IA.
| Fungal Strain | Gene Used | Enzymes/Protein | Modification Pathway | References |
|---|---|---|---|---|
| Candida lignohabitans CBS 10342 | cadA | cis-Aconitate decarboxylase | Heterologous expression of cadA under the control of GAP promoter and terminator | [34] |
| Pseudomonas putida | tad1, adi1 | trans-Aconitate decarboxylase, Aconitase-Δ-isomerase | Heterologous expression of tad1 and adi1 from U. maydis; deletion of PHA synthetases phaC1 and phaC2 | [35] |
| A. niger AB 1.13 | mttA, mtt1 | Mitochondrial tricarboxylate transport protein | Over-expression of acl12 (ATP-citrate lyase), citB (Cytosolic citrate synthase), cadA, mttA and mfsA | [36] |
| U. maydis MB215 | cyp3,cypC, ria1 | P450- monooxygenase (Regulatory gene of itaconic acid gene cluster) | Deletion of byproduct genes (cyp3); over-expression of ria1, Over-expression of native/regulator rai1 and mttA under Petef promoter (from A. terreus); deletion of cyp3 and fuz7 | [37][38] |
| Pichia stipitis FPLUC7 | acoA, acnB | Aconitase | Heterologous expression of CAD and over-expression of native truncated ACO (without mitochondrial signal) | [39] |
| Pichia kudriavzevii YB4010 | Icd | Isocitrate dehydrogenase | Heterologous expression of At_cad, over-expression of native Pk_mttA and deletion of icd (isocitrate dehydrogenase) | [40] |
The yield of IA generated by A. terreus is approximately 85 g/L. However, this amount cannot be compared to the synthesis of CA, which can easily attain a concentration of more than 200 g/L in industrial production. Li et al. [33] stated that the IA synthesis is performed in order to produce a theoretical yield of 240 g/L, and, thus, breeding the current strains or focused genetic engineering might help achieve this objective. The activity of 6-phosphofructo-1-kinase is inhibited by adenosine triphosphate (ATP) and citrate, a gene that influences the efficiency of IA synthesis in A. terreus. Capuder et al. [41] investigated the shortened A. niger pfkA gene in order to reduce the citrate inhibition, and a higher CA yield was obtained using ATP. When expressed in A. terreus, the shorter pfkA variant positively affected the quantity of IA [42].
Lin et al. [43] showed that the strains produced a better yield after interruption of aeration. However, it was realized that the genetic make-up of A. terreus is insufficient to support the production of higher amounts of IA. So, a strategy of IA production through genetic engineering into another host organism already familiar enough to support the high amount of IA production was suggested, and A. niger was best suited [28].
4.2. Mutagenesis of Aspergillus Strain
Genetic alteration and mutagenesis have boosted IA production in A. terreus strains. After repeatedly altering the wild-type A. terreus strain IFO6365, Yahiro et al. [44] identified the strain known as A. terreus TN484-M1, which, after 6 days of fermentation, generated up to 82 g/L IA. Using the same strain, Dwiarti et al. [9] investigated the IA production from sago starch and obtained about 48.2 g/L of IA. The enhanced performance was thought to be caused by the mutant strain’s transcription of CAD1 (the gene encoding cis-aconitic acid decarboxylase), which was five times stronger than IFO6365 [31].
Reddy and Singh [45] developed two mutants of A. terreus SKR10 by applying different chemicals or UV mutagens alone or in combination, which are A. terreus N45 and A. terreus UNCS1, producing 50 and 32 g/L of IA from corn starch and fruit waste extracts, respectively, as compared to the parent strain, which produced 31 and 20 g/L. A modified pfkA gene from A. niger was inserted into the genome of a wild-type strain of A. terreus [42]. This modified pfkA gene was encoded for a shorter and more active 6-phospho-fructo-1-kinase enzyme fragment, and the obtained transformants accumulated higher amounts of IA than the native strain, albeit after a longer lag phase. As a result, the IA yield obtained from the best transformant A729 was 45.5 g/L, which was more than twice that achieved by the parent strain (21.35 g/L) [42].
References
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295.
- Tate, B.E. Itaconic acid, itaconic esters, and related compounds. High Polym. 1970, 24, 205–261.
- Tate, B.E. Itaconic acid and derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology; Grayson, M., Eckroth, E., Eds.; Wiley & Sons: Hoboken, NJ, USA, 1981; Volume 3, pp. 865–873.
- Qi, P.; Chen, H.L.; Nguyen, H.T.H.; Lin, C.C.; Miller, S.A. Synthesis of biorenewable and water-degradable polylactam esters from itaconic acid. Green Chem. 2016, 18, 4170–4175.
- Bafana, R.; Pandey, R.A. New approaches for itaconic acid production: Bottlenecks and possible remedies. Crit. Rev. Biotechnol. 2018, 38, 68–82.
- Huang, X.; Lu, X.; Li, Y.; Li, X.; Li, J.J. Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain. Microb. Cell Factories 2014, 13, 1–9.
- Noh, M.H.; Lim, H.G.; Woo, S.H.; Song, J.; Jung, G.Y. Production of itaconic acid from acetate by engineering acid-tolerant Escherichia coli W. Biotechnol. Bioeng. 2018, 115, 729–738.
- El-Imam, A.A.; Du, C. Fermentative itaconic acid production. J. Biodiver. Bioprospec. Develop. 2014, 1, 1–8.
- Dwiarti, L.; Otsuka, M.; Miura, S.; Yaguchi, M.; Okabe, M. Itaconic acid production using sago starch hydrolysate by Aspergillus terreus TN484-M1. Bioresour. Technol. 2007, 98, 3329–3337.
- El-Imam, A.M.A.; Kazeem, M.O.; Odibsi, M.B.; Abidoye, A.O. Production of itaconic acid from Jatropha curcas seed cake by Aspergillus terreus. Not. Sci. Biol. 2013, 5, 57–61.
- El-Imam, A.A.; Chenyu, D. Itaconic acid production from sorghum bran: A biorefining approach. J. Fundam. Renew. Energy Appl. 2015, 5, 42–44.
- Geiser, E.; Przybilla, S.K.; Friedrich, A.; Buckel, W.; Wierckx, N.; Blank, L.M.; Bölker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb. Biotechnol. 2016, 9, 116–126.
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Laboratory: Golden, CO, USA, 2004.
- Fleischhaker, F.; Schade, C.; Müller-Cristadoro, A. Preparation of itaconic Acid Homo- or Copolymers, and Amine- or Amide Containing Alcohols for Metal Surface Treatment. U.S. Patent No. 13/551,127, 11 April 2006.
- Tate, B.E. Polymerisation of itaconic acid and derivatives. In Fortschritte Der Hochpolymeren-Forschung; Springer: Berlin/Heidelberg, Germany, 1967; Volume 5, pp. 214–232.
- Turner, E. Elements of Chemistry: Including the Recent Discoveries and Doctrines of the Science; Thomas, Cowperthwait & Company: Philadelphia, PA, USA, 1840.
- Kinoshita, K. Uber eine neue Aspergillus Art, A. itaconicus. Bot. Mag. Tokyo 1932, 45, 45–50.
- Da Cruz, J.C.; de Castro, A.M.; Sérvulo, E.F.C. World market and biotechnological production of itaconic acid. 3 Biotech. 2018, 8, 1–15.
- Baup, S. About a new pyrogenic citric acid, and about the naming of pyrogenic acids in general. Ann. Derpharmacie 1837, 19, 29–38.
- Pichler, H.; Obenaus, F.; Franz, G. Über die Bildung von Citraconsäurean hydrid bei der katalytischen Oxydation von Methylaromaten und Isopren, Erdöl und Kohle, Erdgas. Petrochemie 1967, 20, 188–192.
- Luskin, L.S. Acidic monomers. Funct. Monomers Prep. Polym. Appl. 1974, 2, 358–528.
- Blatt, A.H.; Henry, G. Organic syntheses: Collective Volume I—Being a Revised Edition of Annual Volumes 10–19; John Wiley: Hoboken, NJ, USA, 1943.
- Paolo, C.G. Process for Preparing Itaconic Acid, and 2, 3-Butadienoic Acid. U.S. Patent No. 3,025,320, 13 March 1962.
- Shriner, R.L.; Ford, S.G.; Roll, L.J. Itaconic anhydride and itaconic acid. Organ. Synth. 2003, 11, 70.
- Calam, C.T.; Oxford, A.E.; Raistrick, H. Studies in the biochemistry of microorganisms: Itaconic acid, a metabolic product of a strain of Aspergillus terreus Thom. Biochem. J. 1940, 33, 1488–1495.
- Kuenz, A.; Krull, S. Biotechnological production of itaconic acid-things you have to know. Appl. Microbiol. Biotechnol. 2018, 102, 3901–3914.
- Saha, B.C. Emerging biotechnologies for production of itaconic acid and its applications as a platform chemical. J. Ind. Microbiol. Biotechnol. 2017, 44, 303–315.
- Steiger, M.G.; Blumhoff, M.L.; Mattanovich, D.; Sauer, M. Biochemistry of microbial itaconic acid production. Front. Microbiol. 2013, 4, 23.
- Regestein, L.; Klement, T.; Grande, P.; Kreyenschulte, D.; Heyman, B.; Maßmann, T.; Eggert, A.; Sengpiel, R.; Wang, Y.; Wierckx, N.; et al. From beech wood to itaconic acid: Case study on biorefinery process integration. Biotechnol. Biofuels 2018, 11, 1.
- Kuenz, A.; Gallenmüller, Y.; Willke, T.; Vorlop, K.D. Microbial production of itaconic acid: Developing a stable platform for high product concentrations. Appl. Microbiol. Biotechnol. 2012, 96, 1209–1216.
- Kanamasa, S.; Dwiarti, L.; Okabe, M.; Park, E.Y. Cloning and functional characterisation of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl. Microbiol. Biotechnol. 2008, 80, 223–229.
- Van der Straat, L.; Vernooij, M.; Lammers, M.; van den Berg, W.; Schonewille, T.; Cordewener, J.; van der Meer, I.; Koops, A.; de Graaff, L.H. Expression of the Aspergillus terreus itaconic acid biosynthesis cluster in Aspergillus niger. Microb. Cell Factories 2014, 13, 1–9.
- Li, A.; van Luijk, N.; ter Beek, M.; Caspers, M.; Punt, P.; van der Werf, M. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet. Biol. 2011, 48, 602–611.
- Bellasio, M.; Mattanovich, D.; Sauer, M.; Marx, H. Organic acids from lignocellulose: Candida lignohabitans as a new microbial cell factory. J. Ind. Microbiol. Biotechnol. 2015, 42, 681–691.
- Elmore, J.R.; Dexter, G.N.; Salvachúa, D.; Martinez-Baird, J.; Hatmaker, E.A.; Huenemann, J.D.; Klingeman, D.M.; Peabody, G.L.; Peterson, D.; Singer, C.; et al. Production of itaconic acid from alkali pretreated lignin by dynamic two stage bioconversion. Nat. Commun. 2021, 12, 1–12.
- Hossain, A.H.; Van Gerven, R.; Overkamp, K.M.; Lübeck, P.S.; Tas¸plnar, H.; Türker, M.; Punt, P.J. Metabolic engineering with ATP-citrate lyase and nitrogen source supplementation improves itaconic acid production in Aspergillus niger. Biotechnol. Biofuels 2019, 12, 1–14.
- Becker, J.; Tehrani, H.H.; Ernst, P.; Blank, L.M.; Wierckx, N. An optimized Ustilago maydis for itaconic acid production at maximal theoretical yield. J. Fungi 2021, 7, 20.
- Geiser, E.; Przybilla, S.K.; Engel, M.; Kleineberg, W.; Büttner, L.; Sarikaya, E.; den Hartog, T.; Klankermayer, J.; Leitner, W.; Bölker, M.; et al. Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab. Eng. 2016, 38, 427–435.
- Qi, H.; Du, Y.; Zhou, X.; Zheng, W.; Zhang, L.; Wen, J.; Liu, L. Engineering a new metabolic pathway for itaconate production in Pichia stipitis from xylose. Biochem. Eng. J. 2017, 126, 101–108.
- Sun, W.; Vila-Santa, A.; Liu, N.; Prozorov, T.; Xie, D.; Faria, N.T.; Ferreira, F.C.; Mira, N.P.; Shao, Z. Metabolic engineering of an acid-tolerant yeast strain Pichia kudriavzevii for itaconic acid production. Metab. Eng. Commun. 2020, 10, e00124.
- Capuder, M.; Šolar, T.; Benčina, M.; Legiša, M. Highly active, citrate inhibition resistant form of Aspergillus niger 6-phosphofructo-1-kinase encoded by a modified pfkA gene. J. Biotechnol. 2009, 144, 51–57.
- Tevž, G.; Benčina, M.; Legiša, M. Enhancing itaconic acid production by Aspergillus terreus. Appl. Microbiol. Biotechnol. 2010, 87, 1657–1664.
- Lin, Y.H.; Li, Y.F.; Huang, M.C.; Tsai, Y.C. Intracellular expression of Vitreoscilla hemoglobin in Aspergillus terreus to alleviate the effect of a short break in aeration during culture. Biotechnol. Lett. 2004, 26, 1067–1072.
- Yahiro, K.; Takahama, T.; Park, Y.S.; Okabe, M. Breeding of Aspergillus terreus mutant TN-484 for itaconic acid production with high yield. J. Ferment. Bioeng. 1995, 79, 506–508.
- Reddy, C.S.K.; Singh, R.P. Enhanced production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour. Technol. 2002, 85, 69–71.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
15 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




