Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Oksana Vasylivna Lobachevska | -- | 2231 | 2022-11-14 13:10:55 | | | |
| 2 | Rita Xu | Meta information modification | 2231 | 2022-11-15 02:49:07 | | | | |
| 3 | Rita Xu | Meta information modification | 2231 | 2022-11-15 02:49:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lobachevska, O.V.; Kyyak, N.Y.; Kordyum, E.L.; Khorkavtsiv, Y.D.; Kern, V.D. Gravi-Sensitivity of Bryophytes Ontogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/34485 (accessed on 07 February 2026).
Lobachevska OV, Kyyak NY, Kordyum EL, Khorkavtsiv YD, Kern VD. Gravi-Sensitivity of Bryophytes Ontogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/34485. Accessed February 07, 2026.
Lobachevska, Oksana V., Natalia Y. Kyyak, Elizabeth L. Kordyum, Yaroslava D. Khorkavtsiv, Volker D. Kern. "Gravi-Sensitivity of Bryophytes Ontogenesis" Encyclopedia, https://encyclopedia.pub/entry/34485 (accessed February 07, 2026).
Lobachevska, O.V., Kyyak, N.Y., Kordyum, E.L., Khorkavtsiv, Y.D., & Kern, V.D. (2022, November 14). Gravi-Sensitivity of Bryophytes Ontogenesis. In Encyclopedia. https://encyclopedia.pub/entry/34485
Lobachevska, Oksana V., et al. "Gravi-Sensitivity of Bryophytes Ontogenesis." Encyclopedia. Web. 14 November, 2022.
Copy Citation
Gravi-morphoses affect the variability of plants and are the morphogenetic adaptation to different environmental conditions. Gravity-dependent phenotypic plasticity of gametophytes as well as gravi-sensitivity of moss protonemata in microgravity and simulated microgravity conditions are discussed. Attention is focused on the influence of gravity on bryophyte ontogenesis, including the gravitropic reactivity of moss protonemata, gravi-sensitivity at the stage of leafy shoot development and sporogonium formation, gravity-influenced morphogenesis of apical cell budding, and gravity-dependent spiral growth patterns.
microgravity
clinorotation
gravi-sensitivity
gravi-morphoses
mosses
protonemata
ontogenesis
1. Introduction
Gravity is a constant environmental factor that influences the evolutionary development of life on Earth. A fundamental uniqueness of plants is the high plasticity of their growth as a reaction to external influences. Tropisms are growth-mediated plant movements that allow plants to respond to changes in their environment. Gravitropism is the response to sensing the constant 1 g stimulus; gravity sensing involves the sedimentation of dense amyloplasts within specialized gravity-sensing cells, e.g., root and shoot statocytes. Gravitropism has been observed and analyzed in a variety of plant organs, including roots, hypocotyls, and inflorescence stems. Single, initially apolar cells, such as the Fucus zygote, spores, pollen grains, protonema of mosses and Chara algae serve as models to investigate the underlying mechanisms of gravitropism [1][2][3]. The perception of the gravity signal plays a key role in plant development: from the growth orientation of seedlings to the development of adult plants [4][5][6]. Positive gravitropic growth of the main root as well as the gravitropic reaction of lateral roots determine the plant’s overall structural architecture [7][8][9]. In the evolutionary chain, mosses belong to the oldest/earliest group of terrestrial plants. The unique ability of moss protonemata, in particular their apical cell, to both perceive and respond to the gravi-stimulus makes mosses as a model system for this type of research [10][11][12][13].
The spore, the reproductive unit that ultimately develops into an adult plant, is usually one-celled. When spores of mosses germinate in the light, they initially form a sporeling (chloronema and rhizoid), followed by juvenile filamentous structures referred to as protonemata and, subsequently, the adult gametophore forms. The chloronema, the primary photosynthetic stage of the protonema, is characterized by perpendicular cross walls, short cells, numerous chloroplasts, colorless cell walls, and irregular branching. Dendroids consist of tree-like branched chloronemata. Caulonemata, the secondary stage of protonema development, give rise to buds and upright gametophores. Caulonemata are characterized by longer cells with slanting cross walls and usually brownish cell walls. In mosses, rhizoids, the anchoring and absorbing structures, are multicellular featuring oblique end walls. The moss sporangium (the seta and capsule), which produces spores, is the main body of the sporophyte.
The haploid gametophyte, a relatively simple juvenile stage of the moss, is a convenient subject for studying morphogenesis [3]. In daylight conditions, filamentous, multi-cellular moss protonemata express plagiotropic growth; in darkness, they orient negatively gravitropic. Figure 1 illustrates a detailed description of the moss life cycle.
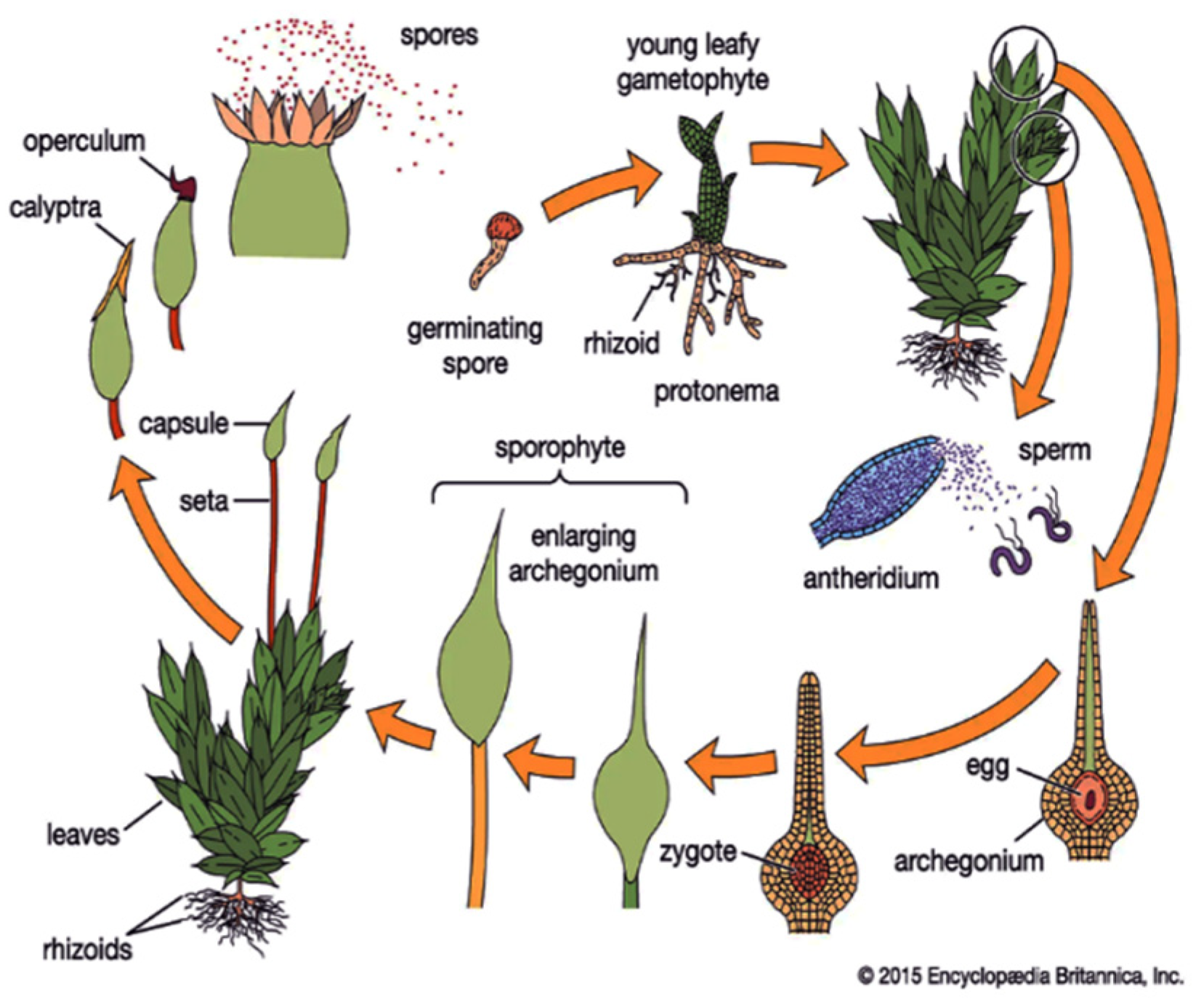
Figure 1. Bryophyte life cycle. Data from Encyclopedia Britannica, Inc., Chicago, IL, USA, [14].
Studies of gravitropism and gravity-dependent moss morphogenetic processes indicate the important role of gravity in plant ontogenesis. Moss spore germination, the differentiation of rhizoid and chloronemal stolons, the formation of vegetative reproductive organs, the initiation of gametophore bud development on apical protonema cells, and the formation of the sporogon are gravi-morphoses influenced by the polarizing effect of gravity [10][15][16][17][18][19][20]. Gravi-morphoses are species-specific and evolve depending on the stage of moss development and environmental factors; they represent a widespread adaptive form of growth in the life strategy of bryophytes [8][21][22]. In other systems, the dorsoventral shape of tree shoot development, gravi-dependent wood formation, the location of lateral buds in orchids and fungal fruiting bodies are well-known examples of adaptations in response to gravity [23][24][25]. Gravi-sensitive chloronemata of the species Ceratodon purpureus (Hedw.) Brid, Physcomitrella patens (Hedw.) Bruch & Schinp, (Physcomitrium patens (Hedw.) Mitt. and Funaria hygrometrica Hedw. are often used as objects in researching plant gravitropism [11][26]. In several species, moss caulonemata appear more sensitive to gravity while the gravi-response of rhizoid filaments is described as weak [15][27].
In vascular plants, within gravity-sensing cells, starch-filled amyloplasts (heavy starch particles or plastids) act as statoliths and sediment with respect to the gravity vector [28][29]. Amyloplasts are understood to be a trigger of the gravi-sensory system in the apical protonema cell. Typically, apical tip-growing cells that are gravitropic can both sense the g-vector and reorient their growth accordingly. Gravity vector-dependent sedimentation of amyloplasts precedes gravitropic bending of apical cells which, for example in Ceratodon purpureus (Hedw.) Brid., occurs 20–30 min after initiation of gravistimulation. In microgravity during spaceflight and clinorotation, amyloplasts appear evenly distributed along the entire cell and sedimentation patterns are not apparent [1][30]. Moss gravi-sensitivity manifests at the stage of gametophyte and sporophyte development as a morphogenetic adaptation to environmental influences. Many bryophytes express unique characteristics such as high tolerance to various stresses. In fact, bryophytes grow surprisingly well in diverse habitats ranging from deserts to wetlands and from tropical to polar regions. This is exemplified by the fact that the most found terrestrial plants in Antarctica are bryophytes [31][32]. DNA methylation is seen as one of the mechanisms controlling gravi-dependent protonema morphogenesis, evidenced by the increased formation of buds after gravistimulation. In the natural environment, gravi-morphoses and DNA methylation increase phenotypic plasticity of the gametophyte, an important feature for ephemeral species with a short life cycle [26][33][34].
2. Gravi-Sensitivity of Bryophytes Ontogenesis
2.1. Gravi-Sensitivity of Sporelings
In moss, gravity serves as a trigger for the polarization of apolar spores and the subsequent development of morphologically distinct chloronema and rhizoid sporelings [35]. Species-dependent adjustment to distinct habitats and individual life strategy is influenced by their sporeling polarization and is highly variable [36]. Protonemata are the most gravi-sensitive structures in mosses.
The development of sporelings in F. hygrometrica and C. purpureus in the dark demonstrates the strong gravitropic reaction and associated polar symmetry of growth. Chloronemata express negative gravitropism; rhizoids express positive gravitropism at a perfect 180° orientation [15][27][35]. The location of sporeling initiation occurs depending on the gravity vector. If the position of a spore culture is changed by 360° relative to the horizontal surface, a second chloronema sporeling emerges near the first rhizoidal one (Figure 2a,b). Growth reorientation to a negatively gravitropic orientation indicates the competence of cells to respond to changes relative to the gravitational vector.
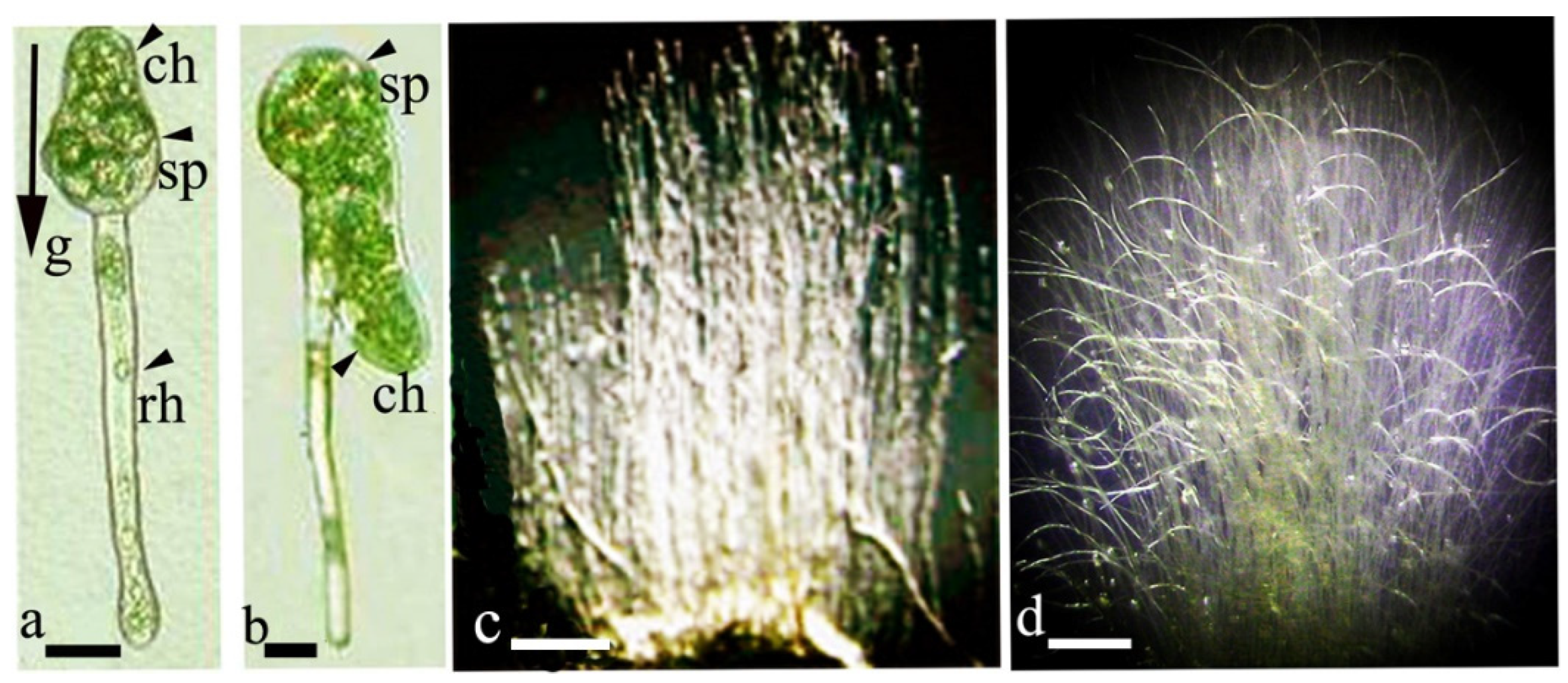
Figure 2. Dark-grown sporelings of Ceratodon purpureus and protonemata mats; (a)—three-day-old rhizoidal sporeling directed down (positively gravitropic) and chloronema growing upward (negatively gravitropic); (b)—after reorientation of the Petri dish by 360°, a chloronemal sporeling was formed and grew down parallel to the rhizoid; (c)—negative gravitropism of eight-day-old protonemata; (d)—spiral bend pattern of filaments in culture rotated for 5 days on a clinostat. Legend: sp—spore; ch—chloronema; rh—rhizoid. Scale bar: (a,b) = 30 μm; (c,d) = 7.5 mm. Data from Lobachevska et al., 2014 [15].
The polarizing effect of gravity o” the’growth direction of sporelings is reduced in later stages of development [36]. Variability of chloronema angles in C. purpureus was higher (40–55°) than in F. hygrometrica (18–19°), potentially indicating higher gravi-sensitivity of F. hygrometrica sporelings. Gravity-dependent orientation of sporelings is a distinguishing feature of the moss life strategy and its morphology; in F. hygrometrica, sporelings may allow for faster formation of growth mats and better adaptation to changing environmental conditions.
Mosses show different growth adaptations depending on their individual life strategy (timing of the life events for best environmental conditions) [37][38]. F. hygrometrica has a so-called fugitive life strategy, generally surviving only for a short time (1–2 years). Funaria has a characteristically high gravi-sensitivity at the chloronema stage; in contrast, the colonist C. purpureus thrives where the habitat start is unpredictable, and the cultures usually last for many years. In Ceratodon, caulonemata are most sensitive to gravity. C. purpureus is a typical colonist species forming dense mats and demonstrating rapid expansion on dry, often sandy or stony substrates. In contrast, F. hygrometrica has a short life cycle, forms loose mats, and is adapted to wet soil conditions. In order to adapt to the local microenvironment and a short growth season, F. hygrometrica must establish fast and firmly anchor itself in the soil via its rhizoids. Coupled with expedited polar growth of its chloronemal sporelings, the formation of loose, but stronger mats is promoted. These morphological species-dependent differences that in part are dependent on gravity have evolved allowing for customized life strategies of these species.
2.2. Gravi-Sensitivity of Protonemata—The Juvenile Stage of Moss Development
The level of gravitropic sensitivity in moss protonemata appears to be species-specific and differs from species to species. For example, in F. hygrometrica and C. purpureus, sporelings and chloronemata are highly sensitive with respect to gravity [35], while gravi-sensitivity appears to be less dominant in Dicranella heteromalla (Hedw.) Schimp. and Dicranella varia (Hedw.) Schimp. In Pohlia nutans (Hedw.) Lindb., D. heteromalla and Barbula unguiculata Hedw., chloronema or caulonema cells only appear to respond to gravity at later stages of protonemata development [15]. In Tortula modica Zander (Tortula caucasica Broth.) and B. caespiticium, regenerative secondary protonemata and gametophores are gravi-sensitive; in W. tortilis, caulonemata are highly gravi-sensitive. Filamentous protonemata display a negatively gravitropic growth pattern in the dark (Figure 2c), while they grow plagiotropically under illumination and show spiral bend pattern filaments in culture rotated on a clinostate (Figure 2d). The unique ability of the protonema apical cell to perceive and respond to gravity made mosses a model system for the research of gravitropism in plants. Initial studies focused on Ceratodon purpureus [11][39], F. hygrometrica, T. modica, P. patens, and Pohlia nutans (Hedw.) Lidb. [30]. The apical protonema cell and spores of most species contain amyloplasts that sediment in response to the gravi-stimulation angle and function as statoliths [10][35][39][40]. Species-dependent differences and morphological adaptations of amyloplasts have been described [35].
2.3. Gravity-Dependent Development of Bud Formation
The initiation and development of gametophore buds on apical cells of gravitropic protonemata was first described for T. modica gravitropic protonemata cultivated in nature, and later during spaceflight conditions and under clinorotation [12][36][41]. When dark-grown gravitropic protonemata was oriented vertically, phototropism was completely repressed. When reorienting these cultures horizontally under white light illumination, buds form on the apical cells (Figure 3a). Single apical cell buds developed in microgravity during spaceflight (STS–87); under clinorotation, buds initiated along the caulonema stolons (Figure 3b) are not restricted to the apical cells. When DNA methylation is inhibited by application of 5-Azacytidine (Figure 3c,d), in P. patens, increased bud formation is observed along most cells of the gravitropic stolon.

Figure 3. Gravitropic protonema stolons of Tortula modica (a,b) and Physcomitrella patens (c,d): (a)—bud development on the apical cell; (b)—buds form along the entire stolon under clinorotation; (c)—gametophores initiated from buds on the apical part of the stolon when illuminated; (d)—enhanced bud formation along the entire stolon after treatment by 25 µM 5-Azacytidine. Scale bar: 100 µm. Data from Lobachevska et al., 2014 [15].
The formation of buds that subsequently develop into leafy shoots is a key stage in moss ontogenesis. Bud formation in apical cells of T. modica was described as gravity-dependent photomorphogenesis [41]. Gravity accelerates photomorphogenesis beyond the caulonema stage, obligatory for bud formation. Treatment of protonemata with cytokinin induces the formation of gametophores on caulonema stolons [18][42]. Gravity is thought to intensify the acropetal transport of cytokinin and influence bud initiation in the apical part of the stolon [16][43]. This mechanism accelerates the dominant gametophyte stage of moss ontogenesis allowing for stronger mat development, and thereby better adaptation to the environment. Clinorotation is known to randomize the effect of gravity on the cytokinins gradient; thus, gametophore buds initiate along the stolon.
2.4. Gravi-Sensitivity of Mosses at the Stage of Leafy Shoots and Sporogonia Formation
Typically, moss species that do not express a clear gravitropic reaction at the early chloronema stage, develop strong gravitropism in later development stages when they form gametophores and regenerative caulonemata. Leafy shoots (gametophores) of acrocarpous bryophytes are oriented negatively gravitropically. Low gravi-sensitivity of regenerative protonemata developed from shoots, compared with those developed from spores, was observed in Bryum argenteum Hedw. and Dicranella varia. However, in B. caespiticium, T. truncata. and T. modica, in contrast, regenerative protonemata from shoots and gametophores expressed strong gravi-sensitivity.
Usually, a moss sporophyte grows negatively gravitropically at the initial stage of development and during capsule and sporogenous tissue formation; in some species, subsequently, the capsule reorients to become positively gravitropic. It was demonstrated that sporogonia form as bipolar structures with apical and basal growth centers, the direction of which changes relative to the gravity vector resulting in an inclined orientation of the capsules (Figure 4a,b). As a result of the positive gravitropic growth of the seta, the initially orthotropic orientation of the capsule inverts (at an angle of 185°) and becomes asymmetric-dorsoventral [17]. Clinorotation at the stage of the capsule formation leads to undifferentiated sporogenous tissue and/or morphological changes, e.g., spherical, often curved capsules are observed in Bryum argenteum. Almost vertically oriented capsules without observable bending of the seta were formed in Pohlia nutans.
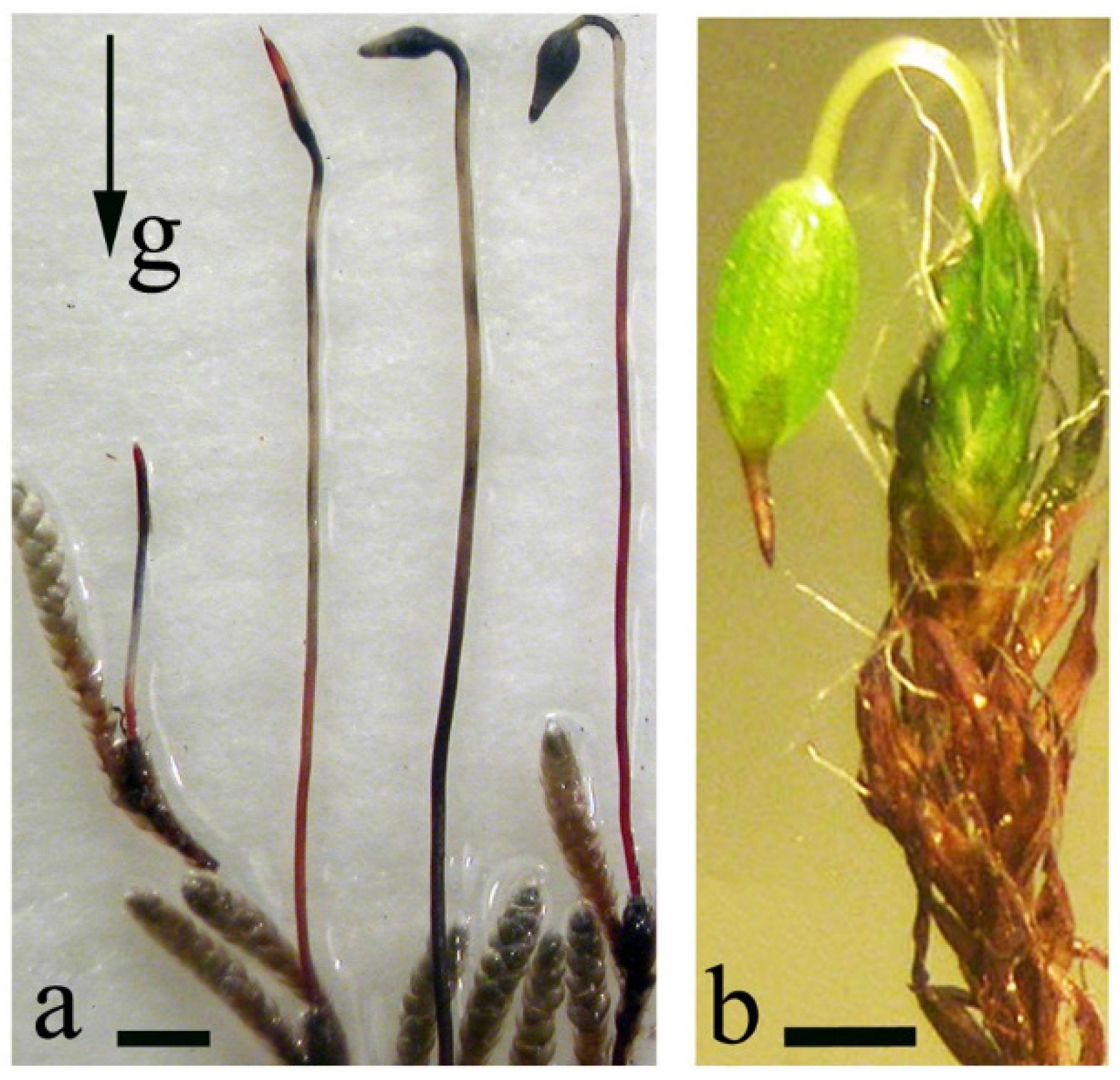
Figure 4. Sporogonia of Bryum argenteum (a) and Grimmia anomala (b). (a)—the gradual gravitropic bending of the sporogonia, (b)—a sporogonium with a reoriented capsule. Scale bar = 200 µm. Data from Lobachevska et al., 2014 [15].
Gravi-dependent growth changes of the sporophyte are manifested in the reorientation of the seta and, in some cases, the shape of the capsule. Shape and spatial orientation of the capsule serves as a taxonomic feature. A positively-gravitropic orientation of the capsule ensures spore dispersion in close vicinity to the parental culture [18]. Gravi-dependent growth responses of the sporophyte during its development are seen as crucial for moss reproduction and enrichment of species diversity [17][37][38][39].
References
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Kardash, A.R.; Chaban, C.I. Vzaymodeistvye sveta y hravytatsyy v rostovukh dvyzhenyiakh protonemu mkhov (The interaction of light and gravity in growth movements of protonemata mosses). Fyzyologia Rastenyi 1997, 44, 205–211.
- Chebli, Y.; Geitmann, A. Gravity research on plants: Use of single-cell experimental models. Front. Plant Sci. 2011, 2, 56.
- Braun, M.; Bohmer, M.; Häder, D.-P.; Hemmersbach, R.; Palme, K. Gravitational Biology 1. Gravity sensing and graviorientation in microorganism and plants. In Springer Brief in Space Life Sciences; Ryuters, G., Braun, M., Eds.; Springer: Cham, Switzerland; German Aerospace Centre: Bonn, Germany, 2018.
- Barlow, P.W. Gravity perception in plants: A multiplicity. Pant Cell Environ. 1995, 18, 951–962.
- Hasenstein, K.H. Plant responses to gravity–insights and extrapolations from ground studies. Gravit. Space Res. 2009, 22, 21–33. Available online: https://link.gale.com/apps/doc/A348311756/AONE?u=googlescholar&sid=googleScholar&xid=916e9f0c (accessed on 11 September 2022).
- Pouliquen, O.; Forterre, Y.; Bérut, A.; Chauvet, H.; Bizet, F.; Legué, V.; Moulia, B. A new scenario for gravity detection in plants: The position sensor hypothesis. Phys. Biol. 2017, 14, 3–14.
- Girloy, S. Plant tropism. Curr. Biol. 2008, 18, 275–277.
- Kordyum, E.L. Plant cell gravisensitivity and adaptation to microgravity. Plant Biol. 2014, 16, 79–90.
- Hoson, T. Plant growth and morphogenesis under different gravity conditions: Relevance to plant life in space. Life 2014, 4, 205–216.
- Chaban, C.I.; Kern, V.D.; Ripetskyj, R.T.; Demkiv, O.T.; Sack, F.D. Gravitropism in caulonemata of the moss Pottia intermedia. J. Bryol. 1998, 20, 287–299.
- Demkiv, O.T.; Kordyum, E.L.; Khorkavtsiv, Y.D.; Kardash, O.R.; Chaban, C.I. Gravi-and photostimuli in moss protonema growth movements. Adv. Space Res. 1998, 21, 1191–1195.
- Demkiv, O.T.; Kordyum, E.L.; Khorkavtsiv, Y.D.; Tairbekov, M.H. Umovy hravitatsii—Eksperymentalna baza dlia piznannia zakonomirnostei morfohenezu roslyn v hravitatsiinomu poli (Microgravity is the experimental basis for understanding of the peculiarities of plant morphogenesis in the gravitational field). Space Sci. Technol. 2006, 12, 30–35.
- Lobachevska, O.V.; Kyyak, N.Y.; Kordyum, E.L.; Khorkavtsiv, Y.D. Role of gravimorphoses in moss adaptation to extreme environment. Ukr. Bot. J. 2021, 78, 47–57.
- Schofield, W.B. Bryophyte. Encyclopedia Britannica. 7 September 2022. Available online: https://www.britannica.com/plant/bryophyte (accessed on 20 October 2022).
- Lobachevska, O.V.; Khorkavtsiv, Y.D. Hravichutlyvist v ontohenezi mokhiv (Gravisensitivity in the moss ontogenesis). Space Sci. Technol. 2014, 20, 55–60.
- Sabovljević, M.; Vujičić, M.; Sabovljević, A. Plant growth regulators in bryophytes. Bot. Serbica 2014, 38, 99–107.
- Lobachevska, O.V.; Demkiv, O.T.; Ripetskyj, R.T. Influence of gravity on spatial orientation and morphogenesis of moss sporophytes. Adv. Space Res. 1998, 21, 1141–1144.
- Cove, D.; Bezanilla, M.; Harries, P.; Quatrano, R. Mosses as model systems for the study of metabolism and development. Annu. Rev. Plant Biol. 2006, 57, 497–520.
- Khorkavtsiv, Y.D.; Kordyum, E.L.; Lobachevska, O.V.; Kyyak, N.Y.; Kit, N.A. Haluzhennia protonemy Ceratodon purpureus v umovakh zminenoi syly tiazhinnia. (Branching of Ceratodon purpureus (Brid.) Hedw. protonemata effected under altered gravity conditions). Ukr. Bot. J. 2015, 72, 588–595.
- Lobachevska, O.V.; Kyyak, N.Y.; Khorkavtsiv, Y.D.; Kit, N.A. Hravizalezhna modyfikatsiia reproduktyvnoho rozvytku mokhiv (Gravity-dependent modification of reproductive development of mosses). Ukr. Bot. J. 2017, 74, 488–496.
- Kyyak, N.Y.; Khorkavtsiv, Y.D. Otsinka okysniuvalnoho stresu mokhu Pohlia nutans (Hedw.) Lindb. zalezhno vid vplyvu hravitatsii (Estimation of the oxidative stress in moss Pohlia nutans (Hedw.) Lindb. depending on the influence of gravity). Space Sci. Technol. 2016, 22, 58–66.
- Lobachevska, O.V.; Kyyak, N.Y.; Khorkavtsiv, Y.D. Morphofunktsionalni ocoblyvosti klimyn protonemy Weissia tortulis Spreng. z riznoiu chutlyvistiu do hravitatsii (Morpho-functional peculiarities of the moss Weissia tortulis Spreng. protonemata cells with different gravisensitivity). Space Sci. Technol. 2019, 25, 60–70.
- Wareing, P.F.; Phillips, I.D.J. Growth & Differentiation in Plants; Wareing, P., Ed.; Pergamon: Oxford, UK, 1981; p. 343.
- Moore, D.; Gange, A.C.; Gange, E.G.; Boddy, L. Fruit bodies: Their production and development in relation to environment. In Ecology of Saprotrophic Basidiomycetes; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; Volume 28, pp. 79–103.
- Wojtaszek, P. (Ed.) Mechanical Integration of Plant Cells and Plants; Springer: Berlin/Heidelberg, Germany, 2011; p. 345.
- Cove, D.J.; Quatrano, R.S. Agravitropic mutants of the moss Ceratodon purpureus do not complement mutants having a reversed gravitropic response. Plant Cell Environ. 2006, 29, 1379–1387.
- Schwuchow, J.M.; Kern, V.D.; White, N.J.; Sack, F.D. Conservation of the plastid sedimentation zone in all moss genera with known gravitropic protonemata. J. Plant Growth Regul. 2002, 21, 146–155.
- Haberlandt, G. Uber die Perzeption des geotropischen Reizes. Ber. Dtsch. Bot. Ges. 1900, 18, 261–272.
- Nèmec, B. Uber die Art der Wahrnehmung des Schwerkraftreizes bei den Pflanzen. Ber. Dtsch. Bot. Ges. 1900, XVIII, 241–245.
- Kern, V.D.; Smith, J.D.; Schwuchow, J.M.; Sack, F.D. Amyloplasts that sediment in protonemata of the moss Ceratodon purpureus are nonrandomly distributed in microgravity. Plant Physiol. 2001, 125, 2085–2094.
- Bramley-Alves, J.D.; King, D.; Miller, R.E. Dominating the Antarctic environment: Bryophytes in a time of change. Environ. Sci. 2014, 10, 5–17.
- Kume, A.; Kamachi, H.; Onoda, Y.; Hanba, Y.T.; Hiwatashi, Y.; Karahara, I.; Fujita, T. How plants grow under gravity conditions besides 1 g: Perspectives from hypergravity and space experiments that employ bryophytes as a model organism. Plant Mol. Biol. 2021, 107, 279–291.
- Gessel, N.; Lang, D.; Reski, R. Genetics and genomics of Physcomitrella patens. In Plant Cell Biology. The Plant Sciences; Assmann, S., Liu, B., Eds.; Springer: New York, NY, USA, 2017; pp. 1–32.
- Ripetskyj, R.T.; Khorkavtsiv, Y.D. Epihenetychna adaptatsiia mokhiv i fenomen klitynnoi pamiati (Epigenetic adaptation in mosses and the phenomenon of cell memory). Ukr. Bot. J. 2012, 69, 302–314.
- Pundyak, O.I.; Demkiv, O.T.; Khorkavtsiv, Y.D.; Bagrii, B.B. Poliarnist prorostannia spor mokhu Funaria hygrometrica Hedw. (Polarity of spore germination in Funaria hygrometrica Hedw). Space Sci. Technol. 2002, 8, 96–100.
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Pundyak, O.I. Hravitatsiia yak formotvorchyi faktor rozvytku mokhiv (Gravity as a formative factor in the moss development). In Physiology of Plants: Problems and Prospects of Development; Morgyn, V.V., Ed.; Logos: Kyiv, Ukraine, 2009; Volume 2, pp. 403–410.
- During, H.J. Life strategies of Bryophytes: A preliminary review. Lindbergia 1979, 5, 2–18.
- Glime, J.M. Physiological Ecology. In Bryophyte Ecology; Ebook Sponsored by Michigan Technological University and the International Association of Bryologists; Glime, J.M., Ed.; Michigan Technological University: Houghton, MI, USA, 2017; Volume 1, Available online: http://www.bryoecol.mtu.edu/ (accessed on 23 August 2022).
- Sack, F.D. Plant gravity sensing. Int. Rev. Cytol. 1991, 127, 193–252.
- Kordyum, E.L.; Guikema, J.A. An active role of the amyloplasts and nuclei of root statocytes in graviperception. Adv. Space Res. 2001, 27, 951–956.
- Ripetskyj, R.T.; Kit, N.A.; Chaban, C.I. Influence of gravity on the photomorphism of secondary moss protonemata. Adv. Space Res. 1999, 23, 2005–2010.
- Bopp, M. Hormones of the moss protonemata. In Bryophyte Development: Physiology and Biochemistry; Chopra, R.N., Bhatla, S.C., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 55–77.
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Kyyak, N.Y.; Kit, N.A. Vplyv hravitatsii na fotomorfohenez protonemy Pottia intermedia (Turn.) Furnr., Pottiales (Effect of gravity on photomorphogenesis of Pottia intermedia (Turn.) Fürn., Pottiales (Fleisch.) protonemata). Ukr. Bot. J. 2005, 62, 329–336.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
15 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




