Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kutub Uddin | -- | 3463 | 2022-11-14 11:02:56 | | | |
| 2 | Camila Xu | Meta information modification | 3463 | 2022-11-15 02:35:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Uddin, K.; Saha, B.B. Low-GWP Refrigerants (Pure and Blended). Encyclopedia. Available online: https://encyclopedia.pub/entry/34475 (accessed on 08 February 2026).
Uddin K, Saha BB. Low-GWP Refrigerants (Pure and Blended). Encyclopedia. Available at: https://encyclopedia.pub/entry/34475. Accessed February 08, 2026.
Uddin, Kutub, Bidyut Baran Saha. "Low-GWP Refrigerants (Pure and Blended)" Encyclopedia, https://encyclopedia.pub/entry/34475 (accessed February 08, 2026).
Uddin, K., & Saha, B.B. (2022, November 14). Low-GWP Refrigerants (Pure and Blended). In Encyclopedia. https://encyclopedia.pub/entry/34475
Uddin, Kutub and Bidyut Baran Saha. "Low-GWP Refrigerants (Pure and Blended)." Encyclopedia. Web. 14 November, 2022.
Copy Citation
Low global warming potential (GWP) refrigerants for the next-generation air conditioning systems have been investigated with target domestic applications. High-GWP refrigerants are mostly used in climate control applications such as heating, ventilation and air conditioning (HVAC) and refrigeration systems.
air conditioning
COP
global warming
refrigerant blend
1. Introduction
Refrigerants as working substances considerably influence the performance of air conditioning and refrigeration systems [1]. The thermal and physical properties of such refrigerants starting from the PVT relationships are crucial in designing efficient yet cost-effective heat pump systems. Apart from the excellent performance requirement, other characteristics such as flammability, toxicity, and, most importantly, environmental friendliness must be accounted for in ranking refrigerants. Most of the early-generation refrigerants, such as CFCs and HCFCs, offer favorable flammability and toxicity but unfavorable ozone depletion potential (ODP) and GWP characteristics. Despite excellent technological advancement in pushing the COP of the mechanical vapor compression cycle closer to the Carnot limit, the adverse impacts of refrigerants on the environment, especially the GWP, remain a huge concern. Following the Montreal Protocol (signed in 1987), a successive phase-out of refrigerants that are responsible for depleting the ozone layer has been carried out: they have been replaced with a new type of ozone-friendly refrigerants [2][3]. During this time, air conditioning and refrigeration industries have undergone and overcome many complications in developing appropriate refrigerants to replace CFC and HCFC refrigerants [4][5][6][7][8][9]. Notably, the developed countries achieved the CFCs phase-out target in 1996, though the deadline for the developing countries was extended to 2010. On the other hand, the HCFCs phase-out will be accomplished by 2030 [10]. During the 28th meeting of the Montreal Protocol, known as the Kigali Amendment (MOP28-2016), the Parties agreed to work on phasing out HFCs based on the compromise consensus of MOP28 targeting to avoid 0.5 °C global warming by the end of the century.
R134a, R1234yf, R410A, R407C, and other refrigerants are currently the best alternatives to ozone-depleting refrigerants (CFCs and HCFCs), especially for household and car heat pump applications. But the GWP of R134a, R410A and R407C is considerably high. There are some low-GWP (pure and blended) refrigerants now studying in different laboratories which can be used in domestic and car air conditioning applications.
2. Low-GWP Refrigerants (Pure and Blended)
2.1. Pure Refrigerants
2.1.1. R1234yf
The hydrofluoroolefin 2,3,3,3-tetrafluoro-1-propene (R1234yf; CF3CF=CH2) is an ultra-low-GWP refrigerant. It is mildly flammable (A2L) and low-toxic [11][12][13]. Compared to R134a and R744, it has zero ODP and excellent life cycle climate performance (LCCP) [14]. The refrigerant’s critical temperature and pressure are 94.7 °C and 3.38 MPa, respectively [15][16][17][18]. To address the safety and performance of R1234yf considering its application in mobile air conditioning, SAE International started the CRP 1234 program in 2007. It has received attention from the research community as a potential replacement candidate for R134a, the most commonly used refrigerant in vehicles [19][20][21][22][23]. The performance of R11234yf is found to be slightly lower than that of R134a [24][25][26][27]. General Motors introduced R1234yf for vehicles in 2012, and presently such vehicles have numbered more than 100,000 worldwide [28]. However, when researchers analyzed the performance of R1234yf to replace R410A, a widely used refrigerant in residential applications, they found the COP was lower and required larger unit bodies as compared to R410A [29][30]. These differences are mainly due to its smaller volumetric capacity because a greater volumetric flow is required to maintain a similar cooling/heating load, resulting in a higher irreversible loss. Barve [30] concluded that refrigerant R1234yf is unsuitable for direct drop-in replacement of R410A without modifications of commercial heat pump systems or charge management in a high-temperature test. However, regarding power consumption by the compressor, R1234yf shows a better performance due to low refrigerant flow rates and lower pressure ratios. Insufficient production capacity and cost are the main limitations to using this refrigerant on a larger scale.
2.1.2. R1234ze(E)
Because of its ultra-low GWP (<1), the hydrofluoroolefin 1,3,3,3-tetrafluoro-1-propene (R1234ze(E); CF3CH=CHF) has gained interest as a prospective refrigerant for industrial centrifugal chillers [31][32]. The critical pressure and temperature of the refrigerant are 3.63 MPa and 109.3 °C, respectively [33][34]. It is a promising refrigerant for high-temperature heat pumps that act as hot dryers and steam generators for industrial applications, including beverage concentration, sterilization of foods, drying lumber, solvent recovery, and distillation of petrochemical products. Fukuda et al. [35] experimentally studied the feasibility of R1234ze(E) and also R1234ze(Z) in a high-temperature heat pump system. In the same laboratory, Koyama et al. [36] investigated the compatibility of R1234ze(Z) with plastic, elastomers, metal, and its thermal stability. The researchers found that the flammability of R1234ze(Z) is appropriate for its commercial use. Brown et al. and Li et al. [37][38] recommended R1234ze(Z) for high-temperature heat pump applications. The researchers recommended an additional study to use it as an alternative to R114. To replace the widely used R410A with R1234ze(E), Koyama et al. noticed pure R1234ze(E) is not appropriate because of its limited volumetric capacity [39], but it is recommended for turbo refrigeration systems. In that case, the impeller size of the compressor is recommended to be enlarged as compared to that for R134a [40][41]. When compared to R134a, the refrigerant has a 9–15% energy-saving potential [41][42]. R1234ze(E) is also studied as a potential replacement of R134a [43][44]. Figure 1 shows the schematic layout of a water-cooled experimental setup used at the Koyama laboratory (Kyushu University, Japan) to investigate the performance of pure and blended refrigerants [45][46].
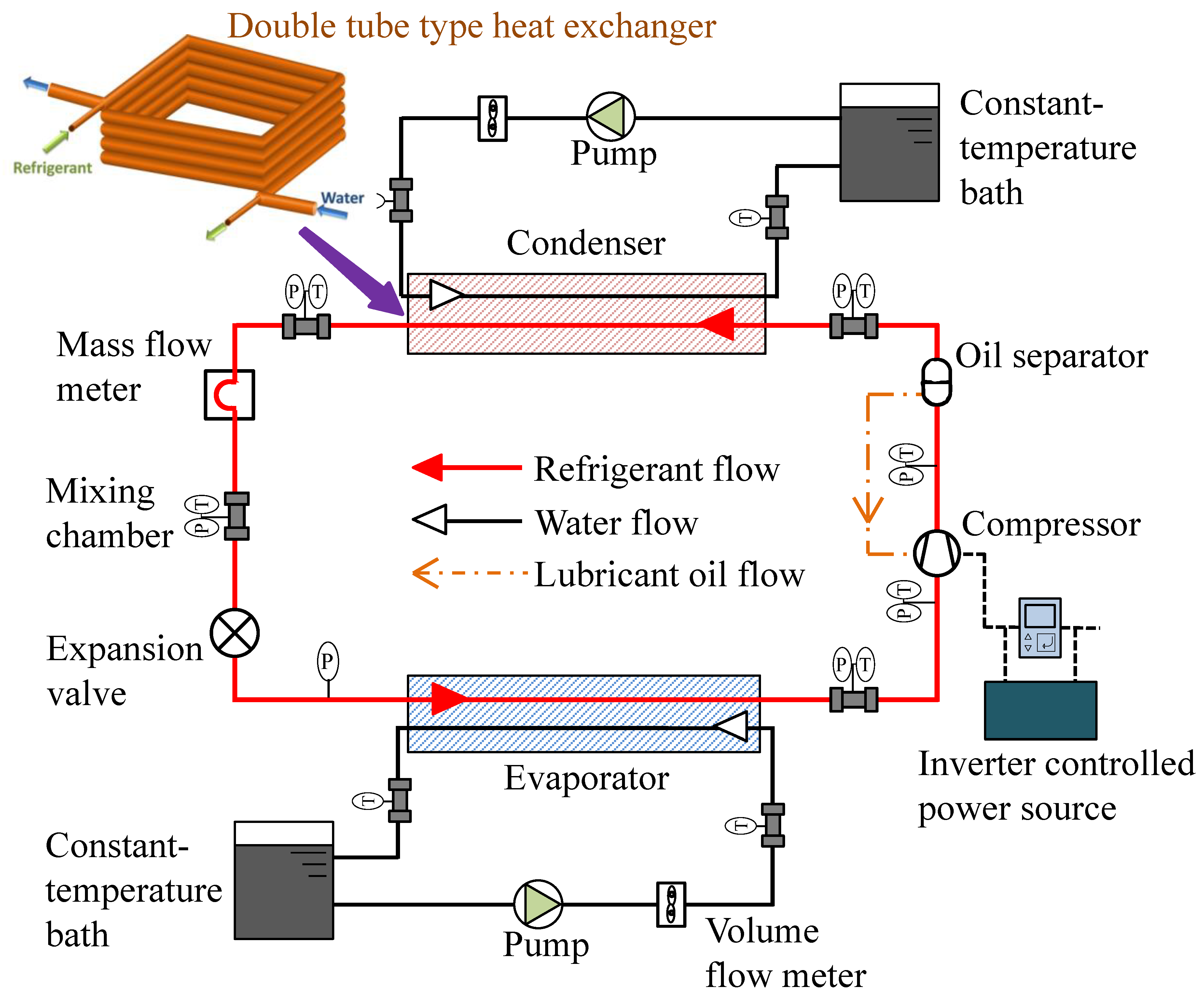
Figure 1. Prototype of a next-generation refrigerant performance study [46].
Figure 2 shows the experimental COP of R1234ze(E) as compared to the widely used R410A and promising R32, where it is found that the COP of R1234ze(E) is lower in both the heating and cooling modes, notably in higher heating loads.
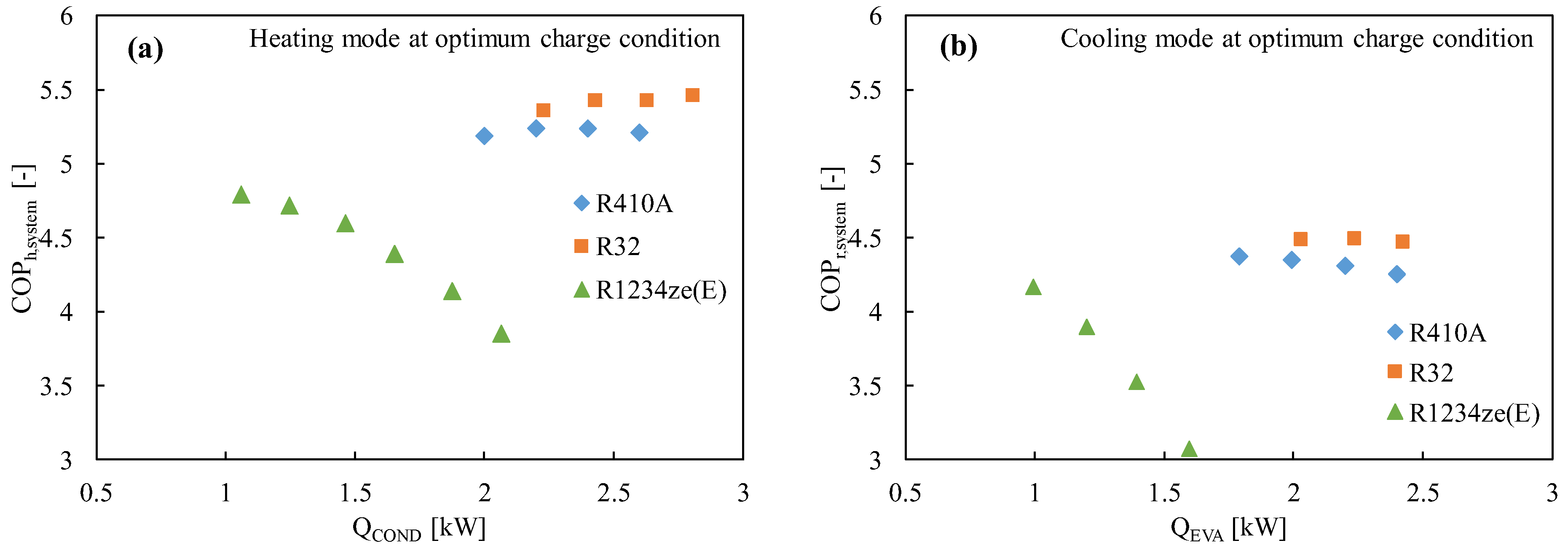
Figure 2. The coefficient of performance of the R1234ze(E), R410A, and R32 refrigerants in a drop-in experiment in (a) the heating mode and (b) the cooling mode [46].
A double tube-type counter flow heat exchanger was used in the experiment. In the heating mode, the heat sink water temperatures at the inlet and the outlet of the condenser were set at 20 °C and 45 °C, respectively, and the corresponding inlet and outlet water temperatures in the evaporator were kept constant at 15 °C and 9 °C, respectively. In the cooling mode, the heat sink water temperature at the inlet and the outlet of the condenser was maintained at 30 °C and 45 °C, respectively, and the inlet and outlet water temperatures in the evaporator were maintained at 20 °C and 10 °C, respectively.
The heat transfer rate varied from 1.4 kW to 2.6 kW by adjusting the refrigerant flow, compressor rotational speed, and water flow. The degree of superheat was kept constant at 3–4 °C over the entire range of test conditions.
2.1.3. R152a
R152a (1,1 difluoroethane, C2H4F2) has been considered as a refrigerant blend for a long time, but not as a pure refrigerant. The refrigerant’s critical pressure and temperature are 4.52 MPa and 115 °C, respectively [47][48][49]. It does not have any ODP. Compared to the HFC and HFO refrigerants, R152a has a low global warming potential (138 [50]) and a lower price. The volumetric capacity and pressure of R152a are quite close to those of R134a. On the other hand, the energy efficiency, mass flow, and vapor density are much better than those of R134a [51]. The system performance in a vapor compression system was found to be higher than that of R134a [52][53]. Test results demonstrated a 4.7–13% improvement in the COP, with a decrease in the cooling capacity of about 0–10%. Figure 3 shows the comparison of the (a) COP and (b) energy consumption between R134a, R152a, and R32 in 120 L refrigerators for different charge amounts. It was found the COP increases with the increase in refrigerant amount, but the minimum power consumption reached 100 g. R152a is commonly used as an aerosol spray propellant and foam-blowing agent. It is also used as a component in a variety of refrigerant blends (R401A, R415A, R430A, R500, etc.) [54]. However, its flammability is rated as A2 by the ASHRAE [55], and hence flammable dangers may have been the primary factor preventing its use as a pure refrigerant up until now [53]. The minimum ignition energy for this refrigerant is 0.38 MJ, which is slightly higher than that for flammable hydrocarbons. Cabello et al. [56] also studied cascade refrigeration plants using R152a/R744 and R134a/R744 pairs for industrial and commercial applications. Though the comparative energy results did not show a great difference, its low GWP can be a great advantage from the environmental point of view.
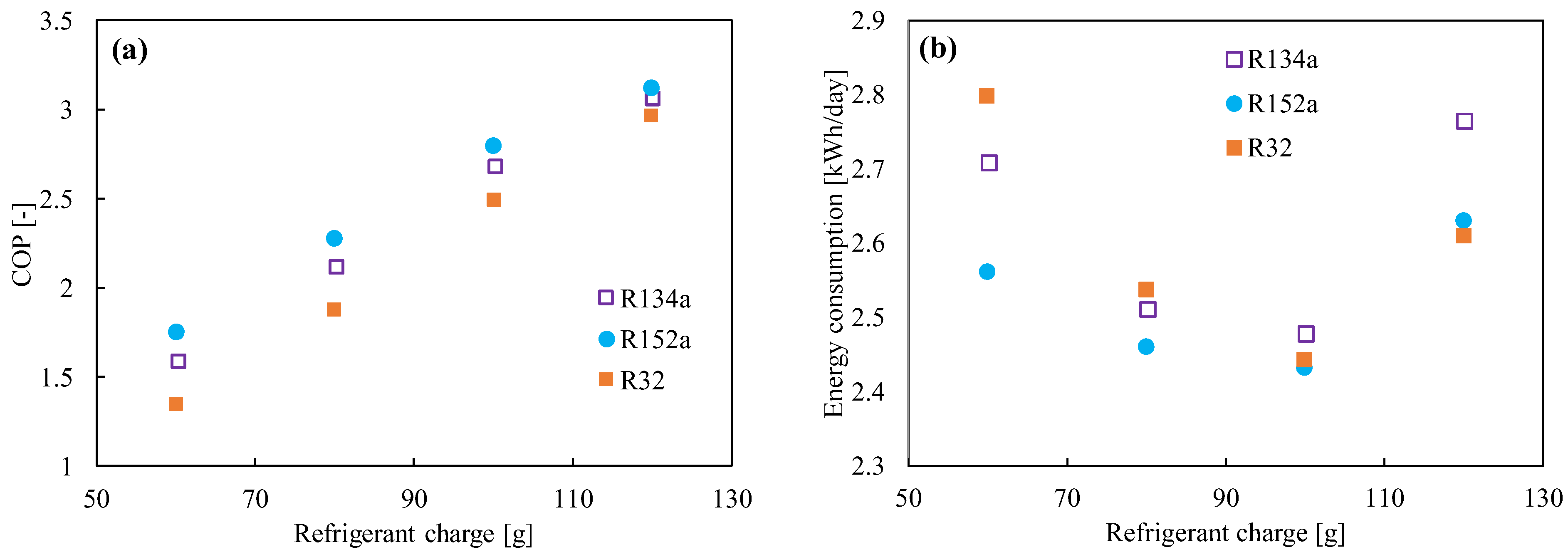
Figure 3. (a) Coefficient of performance (COP) and (b) energy consumption as a function of the refrigerant’s charged amount [52].
2.1.4. R744
R744 (carbon dioxide, CO2) has been utilized in various types of vapor compressor systems for over 130 years [57][58][59][60]. It is a low-cost and natural refrigerant. However, it works at an exceptionally high-pressure level and operates in a transcritical cycle. The decline of using R744 started in the mid-1950s after the invention of high-performance CFC refrigerants. When the adverse effect of synthetic refrigerants was found in the late 1980s, there was a renewed interest in R744 as it is not toxic and flammable [58][61]. In the early 1990s, Gustav Lorentzen revived R744 as a refrigerant [62][63]. To assess the COP of R744 and R12, Lorentzen and Pettersen [64] designed a laboratory prototype considering its application in vehicle air conditioning systems. The researchers speculated that the increased energy density might have a high cost but provide practical benefits due to their smaller size and weight. Koyama et al. [65] and Xue et al. [66] constructed a steady-state model where they assessed the heating and cooling performance of R744. Many researchers have studied theoretically and empirically the performance of R744. The performance has also been compared with other refrigerants as a feasible alternative to synthetic fluids [67][68][69][70][71][72]. Hwang et al. [73] tested the performance of several two-stage compressor R744 cycles and observed an 18–35% improvement in the COP over the basic cycles. Girotto et al. [69] calculated the COP of all-CO2 systems for a supermarket in northern Italy, estimated the annual energy consumption and the cost of CO2 installation. Even though the installation cost was 20% greater, the researchers discovered a way to raise the efficiency of the CO2 system to match that of the R404A system. According to Maina and Huan [61] and Nekså [74], this refrigerant has been used for different purposes, including hot water generation, commercial refrigeration, heat pump dryers, etc.
2.1.5. R1336mzz(Z)
New refrigerant R1338mzz(Z) has been found very promising for high-temperature heat pump cycles. It is non-flammable, and the GWP of this refrigerant is only 2 [75][76][77]. The boiling and critical temperatures of R1336mzz-Z are 33.5 and 171.3 °C, respectively. Therefore, it can be potentially considered as an alternative to refrigerants HFC-245fa and HCFC-123. The pool boiling performance of R1336mzz(Z) on a flattened horizontal Turbo-ESP surface has been studied for air conditioning and industrial heat recovery applications [77]. The potential application of these refrigerants has also been studied for transcritical Rankine power cycles [78].
2.1.6. HC (Propane R290, N-Butane R600, Isobutane R600a)
In general, the natural refrigerant known as HC (hydrocarbon) has great efficiency, good miscibility with mineral oils, a lower compressor discharge pressure, and strong heat transfer properties. However, because of its significant flammability, this refrigerant cannot be used on a larger scale. Because many other nations have outlawed using flammable gases in the public, HCs are only used as refrigerants in Europe following some regulations. The theoretical and practical performance of R290 in contrast to R22 was examined by Lampugnani and Zgliczynski [79]. According to the experimental findings, R290 is a superb candidate to replace R22 from the thermodynamic standpoint. Granryd [80] discussed the advantages and disadvantages of employing HC as a refrigerant for refrigeration and heat pump applications. Numerous HCs have been discovered to exhibit advantageous properties as refrigerants from the thermodynamic and heat transport perspective. In their investigation, Halimic et al. [81] came to a conclusion that, after addressing technical operation and safety concerns, R290 is a desirable replacement for R12 in small residential refrigerators. Bjerre and Larsen [82] assessed the potential usage of R600 for residential applications and discovered that it performed 10% better than R134a. Palm and Somchai and Chimres [83][84] found the excellent performance of hydrocarbon compared with R134a, R22, and ammonia. According to the researchers, the safety risk may be decreased by constructing systems hermetically with the least amount of connections and refrigerant charge or by using an indirect system. The accepted practice of employing HC in a vapor compression system was examined by Corberán et al. [85]. According to the IEC355.2.20 standard, up to 150 g of HC can be sealed in a regular refrigerator, and tiny freezers can be placed anywhere, regardless of the size of the room, with a few additional safety precautions. Some European refrigerator producers now have access to flammable HCs to make residential refrigerators. However, the failure rate of the compressor is increased with the vast commercialization of such refrigerants.
2.1.7. Other Natural Refrigerants
In home and automotive heat pump applications, water, ethanol, ammonia, and methanol, known as natural refrigerants, are receiving attention [59][86][87][88][89]. The thermodynamic properties of ammonia (R717) are excellent, and it is energy-efficient when applied in large industrial systems and commercial buildings, but its toxic behavior limits its usage as a refrigerant in domestic and automobile applications [90][91]. Due to their low volumetric efficiency, water (R718) and ethanol are not common refrigerants in conventional vapor compression systems, but they can be regarded as common refrigerants in sorption-based systems [92][93][94][95][96][97].
2.2. Refrigerant Mixtures (Blended)
Due to their azeotropic character and generally well-developed thermophysical properties, pure refrigerants are a good choice for vapor compression systems. HFOs are reasonable alternatives regarding their toxicity, flammability, and ultra-low GWP. According to Bella and Kaemmer [98], pure HFOs are inefficient substitutes for R410A from the perspective of system performance. For drop-in replacement, rebuilding the system with a larger compressor, pipe, and heat exchangers is necessary. It has also been found that the volumetric capacity of the refrigerant is low, which leads to larger mass flow rates, larger pressure drops in the heat exchangers and connection pipes, and, ultimately, lower coefficients of performance (COP) [99][100]. The volumetric capacity as well as the COP can be improved by adding some other refrigerants with HFOs. In China, R32 has been investigated due to its lower cost as compared to R410A, while blends of R32 with HFOs have been studied in Japan and the USA [101]. A blend is often defined as a combination of two or more single-component refrigerants. Blends can be either azeotropic or zeotropic. Azeotropic blends act like pure refrigerants in such a way that they boil and condense at consistent temperatures at any given pressure. As opposed to azeotropic blends, zeotropic blends boil and condense at a variety of temperatures at a given pressure (see Figure 4a). This temperature range is known as temperature glide, which is essentially the difference between the refrigerant compound’s bubble point and dew point. Figure 4b shows the formation of temperate glides of the R32/R1234ze(E) and R32/R1234yf blends at a constant pressure. The maximum temperature glides (△T) of the R32/R1234yf and R32/R1234ze(E) mixtures at 0.5 MPa are 7.6 and 12.2 °C, respectively (source of thermophysical properties—REFPROP V9.1) [102]. Despite the vast possibilities of the mixing strategy, a few mixtures, such as binary and ternary mixtures, remain popular due to the possible complexity involved in the thermophysical properties of the mixtures.
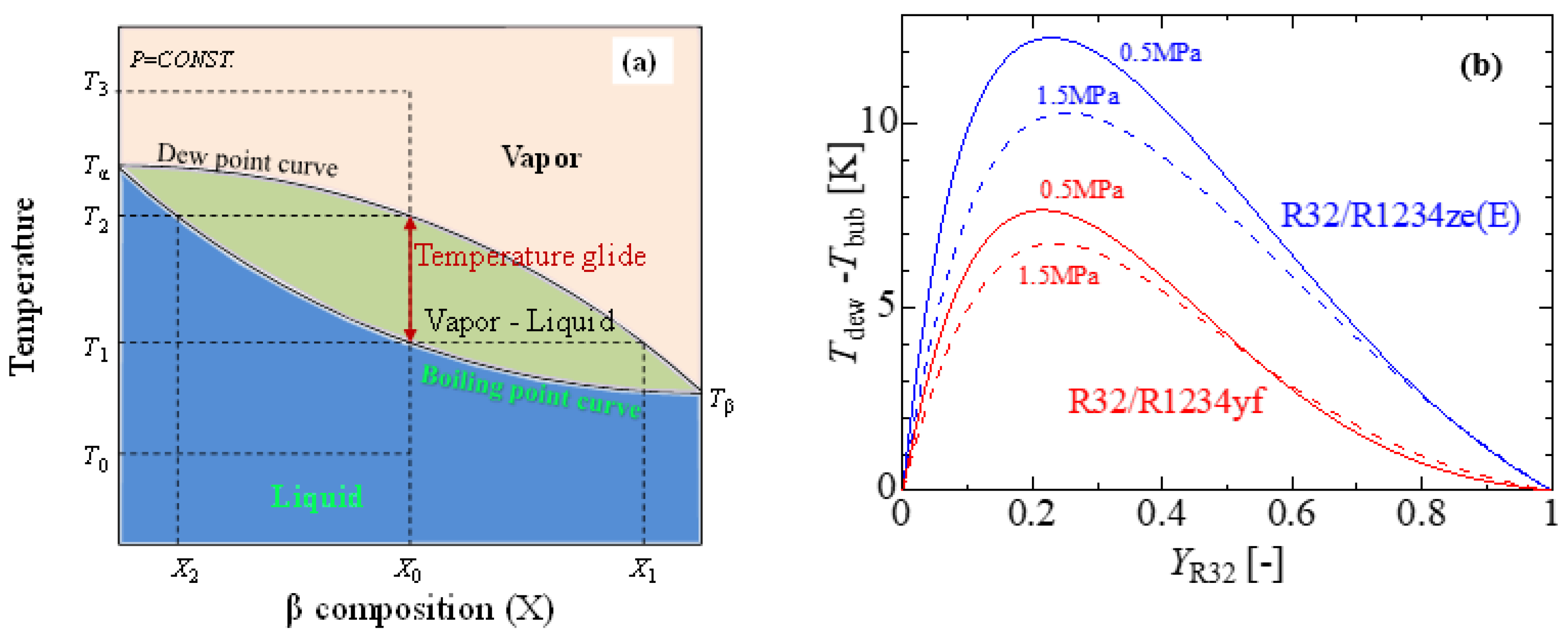
Figure 4. Temperature glides of a binary mixture. (a) Temperature vs. mass fraction diagram of a zeotropic mixture, (b) temperate glides of the R32/R1234ze(E) and R32/R1234yf binary blends.
2.2.1. Binary Mixtures
R32/R1234ze
Numerous studies have demonstrated that R1234ze(E) has a poorer coefficient of performance (COP) than other widely used refrigerants like R410A or R134a, mostly because of its limited volumetric capacity and greater pressure drop [103]. R32 is typically blended in a variety of ratios to improve the performance of R1234ze(E) and the vapor density [40][104]. The cycle performance analysis showed that the inclusion of R32 (50% mass) with R1234ze(E) boosted the volumetric capacity while maintaining the COP greater than that of R410A, suggesting this mixture is a strong contender to replace R410A [105]. The thermodynamic properties of the mixture developed by Akasaka [106] were used in the study.
R32/R1234yf
Due to its extremely low GWP and performance that is equivalent to that of R134a, refrigerant R1234yf can be regarded as a promising next-generation refrigerant. Prior to now, R1234yf has had a few drawbacks, most notably a volumetric capacity that was much smaller than that of R134a. However, pure R32 exhibits a very high temperature at the compressor output and a significantly greater GWP, making it a poor candidate for home air conditioning. In order to address their respective weaknesses, these two refrigerants are combined with different ratios. . The experimental result show the performance of the R32/R1234yf binary mixture of 42/58 (by mass) is higher than R410A.
R32/R1123
AGC (Asahi Glass Co., Ltd., Japan) developed a novel refrigerant for air conditioning systems in 2014 that uses hydrofluoroolefin R1123 as its primary component and can cut GWP even further. Trifluoroethylene, or R1123, has a performance that is comparable to that of common refrigerants and a very low GWP (0.3) [107]. However, the R1123 refrigerant has disproportionate properties at a higher temperature. To make the system safe, the blend of R1123 and R32 was proposed to replace R410A. Higashi and Akasaka [108] measured the thermodynamic properties of the 60/40 and 40/60 R32/R1123 mixtures (by mass). When R410A is replaced in residential and commercial air conditioning systems, the new refrigerant blend, which is azeotropic, can provide good performance, according to AGC [109]. The performance of this mixture is being tested at many laboratories in Japan; however, cycle performance results are not available in the open literature.
HC Mixtures
HC blends are ecofriendly refrigerants that may be employed in already-built systems. Due to their high flammability (A3), HC blends are recommended over halogenated refrigerants for usage in small systems with low charge amounts. Many studies investigated the R290/R600a combination as a replacement for R12 and discovered that it has a greater COP and refrigeration capacity than R12. R290/R600a mixtures have been investigated to replace R134a; it was found the mixtures are an appropriate alternative to R134a in terms of power consumption, charge amount, and cooling rate [84][110][111][112][113][114][115]. When the temperature glide matches the fluid temperature change, the 75/25 mixture (by mass) of R744/R290 increases the COP 12.8% more than R744 [116][117]. The performance of R32/R290 mixtures (68/32) as a drop-in replacement for R410A was found to be 6–7% lower than that of R410A, but the charge amount is reduced by 30–35%.
2.2.2. Ternary Mixtures
R744/R32/R1234ze(E)
For the case of binary blends of R32/R1234yf and R32/R1234ze(E), more than 50% R32 always shows a better performance than R410A. To maintain a GWP less than 300, the mass fraction of R32 must be below 50%, resulting in a lower COP, which is a consequence of limited volumetric capacity. When R744 added in the binary mixture, the performance of R744/R32/R1234ze(E) mixtures having GWP 300, found the COP is comparable to that of R410A in both heating and cooling modes.
R744/R32/R1234yf
Two ternary blends were formed by adding R744 into binary blend R32/R1234yf to enhance the volumetric capacity of the binary blend. The coefficient of performance of the ternary blends was experimentally studied, and their performance was compared with R410A [45]. For the blends, the researchers considered the GWP value of 200 and 300. Temperature glide was a concern during the condensation and evaporation procedures since these combinations are zeotropic. The inclusion of R744 lowers the GWP but increases the temperature glide. The irreversible loss is minimized by matching the gliding temperature with the temperature changes in the heat sink and the heat source.
Other Ternary Mixtures
Ternary blend R744/R32/R134a (7/31/62) was studied theoretically and experimentally and compared with R32 [118]. This blend was found as a promising alternative to R22 as a drop-in replacement. It shows a 10% better COP. The blend is recommended for the use of low-temperature heat pumps because the condensing pressure is too high. The main problem with this blend is its GWP value (~1100). The performance of R410A was compared with R744/R32/propane (10/80/10, GWP ~540) [119], and the heating capacity of the blend was found to be higher than that of R410A. Two low-GWP refrigerant mixtures R32/R152a/R1234ze(E) (12/5/83) and R134a/R152a/R1234yf (7/11/82) have also been chosen as a promising alternative for next-generation heat pump systems [104].
References
- Calm, J.M.; Didion, D.A. Trade-offs in refrigerant selections: Past, present, and future. Int. J. Refrig. 1998, 21, 308–321.
- UNEP. Report of the Technology and Economic Assessment Panel. Montreal Protocol On Substances that Deplete the Ozone Layer; UNEP: Nairobi, Kenya, 2016.
- Benhadid-Dib, S.; Benzaoui, A. Refrigerants and their environmental impact Substitution of hydro chlorofluorocarbon HCFC and HFC hydro fluorocarbon. Search for an adequate refrigerant. Energy Procedia 2012, 18, 1611–1623.
- Hewitt, N.J.; McMullan, J.T. The replacement of CFCS in refrigeration equipment by environmentally benign alternatives. Appl. Therm. Eng. 1997, 17, 955–972.
- Billiard, F.; Lucas, L. Fluorocarbons and global warming. Rev Générale Therm. 1998, 37, 417–423.
- McMullan, J.T. Refrigeration and the environment—Issues and strategies for the future. Int. J. Refrig. 2002, 25, 89–99.
- Kim, K.H.; Shon, Z.H.; Nguyen, H.T.; Jeon, E.C. A review of major chlorofluorocarbons and their halocarbon alternatives in the air. Atmos. Environ. 2011, 45, 1369–1382.
- Calm, J.M. Refrigerant Transitions … Again. In Proceedings of the ASHRAE-NIST Refrigrants Conference, Gaithersburg, MD, USA, 29–30 October 2012; pp. 1–16.
- Aprea, C.; Greco, A.; Maiorino, A. An experimental evaluation of the greenhouse effect in the substitution of R134a with CO2. Energy 2012, 45, 753–761.
- Powell, R.L. CFC phase-out: Have we met the challenge? J. Fluor. Chem. 2002, 114, 237–250.
- Minor, B.H.; Herrmann, D.; Gravell, R. Flammability characteristics of HFO-1234yf. Process. Saf. Prog. 2010, 29, 150–154.
- Honeywell SolsticeTM yf Refrigerants. Honeywell Technical Bulletin. 2012. Available online: https://www.honeywell-refrigerants.com/europe/wp-content/uploads/2013/03/honeywell-solstice-yf-technical-bulletin.pdf (accessed on 12 December 2021).
- Feng, B.; Yang, Z.; Zhai, R. Experimental study on the influence of the flame retardants on the flammability of R1234yf. Energy 2018, 143, 212–218.
- Minor, B.; Spatz, M. HFO-1234yf Low GWP Refrigerant Update. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 14–17 July 2008; pp. 1–8.
- Tanaka, K.; Higashi, Y. Thermodynamic properties of HFO-1234yf (2,3,3,3-tetrafluoropropene). Int. J. Refrig. 2010, 33, 474–479.
- Akasaka, R.; Tanaka, K.; Higashi, Y. Thermodynamic property modeling for 2,3,3,3-tetrafluoropropene (HFO-1234yf). Int. J. Refrig. 2010, 33, 52–60.
- Lai, N.A.; Vrabec, J.; Raabe, G.; Fischer, J.; Wendland, M. Description of HFO-1234yf with BACKONE equation of state. Fluid Phase Equilib. 2011, 305, 204–211.
- Richter, M.; McLinden, M.O.; Lemmon, E.W. Thermodynamic properties of 2,3,3,3-tetrafluoroprop-1-ene (R1234yf): Vapor pressure and p- rho- T Measurements and an Equation of State. J. Chem. Eng. Data 2011, 56, 3254–3264.
- SAE-CRP1234; Industry Evaluation of Low Global Warning Potential Refrigerant HFO-1234yf. SAE International: Warrendale, PA, USA, 2009.
- Lee, Y.; Jung, D. A brief performance comparison of R1234yf and R134a in a bench tester for automobile applications. Appl. Therm. Eng. 2012, 35, 240–242.
- Spatz, M.; Minor, B. HFO-1234yf Low GWP Refrigerant: A Global Sustainable Solution for Mobile Air Conditioning. In Proceedings of the SAE Alternate Refrigerant Systems Symposium, Scottsdale, AZ, USA, 10 June 2008; pp. 1–26.
- Del Col, D.; Torresin, D.; Cavallini, A. Heat transfer and pressure drop during condensation of the low GWP refrigerant R1234yf. Int. J. Refrig. 2010, 33, 1307–1318.
- Koban, M. HFO-1234yf Low GWP Refrigerant—Information for Manufacturing and Service Facilities. 2010.
- Navarro-Esbrí, J.; Mendoza-Miranda, J.M.; Mota-Babiloni, A.; Barragán-Cervera, A.; Belman-Flores, J.M. Experimental analysis of R1234yf as a drop-in replacement for R134a in a vapor compression system. Int. J. Refrig. 2013, 36, 870–880.
- Zilio, C.; Brown, J.S.; Schiochet, G.; Cavallini, A. The refrigerant R1234yf in air conditioning systems. Energy 2011, 36, 6110–6120.
- Jarall, S. Study of refrigeration system with HFO-1234yf as a working fluid. Int. J. Refrig. 2012, 35, 1668–1677.
- Qi, Z. Performance improvement potentials of R1234yf mobile air conditioning system. Int. J. Refrig. 2015, 58, 35–40.
- Sciance, F. The Transition from HFC-134a to a Low-GWP Refrigerant in Mobile Air Conditioners HFO-1234yf; General Motors Public Policy Center: Detroit, MI, USA, 2013; pp. 1–15.
- Kojima, H.; Fukuda, S.; Kondou, C.; Takata, N.; Koyama, S. Comparative Assessment of Heat Pump Cycle Operated with R32/R1234ze(E) and R32/R1234yf mixtures. In Proceedings of the International Refrigeration and Air Conditioning Conference, Yokohama, Japan, 16–22 August 2015; pp. 1–8.
- Barve, A.; Cremaschi, L. Drop-in Performance of Low GWP Refrigerants in a Heat Pump System for Residential Applications. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 16–19 July 2012; pp. 1–9.
- Kenji, U.; Kazuki, W.; Yokoyama, A.; Akimasa, S.A.; Ueda, K.; Wajima, K.; Yokoyama, A.; As, A. Low GWP refrigerant application for the centrifugal chiller—Study for application of HFO-1234ze(E). Trans Jpn. Soc Refrig. Air Cond. Eng. 2011, 28, 503–508.
- Yang, Z.; Wu, X.; Tian, T. Flammability of Trans-1, 3, 3, 3-tetrafluoroprop-1-ene and its binary blends. Energy 2015, 91, 386–392.
- Higashi, Y.; Tanaka, K.; Ichikawa, T. Critical Parameters and Saturated Densities in the Critical Region for trans-1,3,3,3-Tetrafluoropropene (HFO-1234ze(E)). J. Chem. Eng. Data 2010, 55, 1594–1597.
- Brown, J.S.; Zilio, C.; Cavallini, A. Thermodynamic properties of eight fluorinated olefins. Int. J. Refrig. 2010, 33, 235–241.
- Fukuda, S.; Kondou, C.; Takata, N.; Koyama, S. Low GWP refrigerants R1234ze(E) and R1234ze(Z) for high temperature heat pumps. Int. J. Refrig. 2014, 40, 161–173.
- Koyama, S.; Fukuda, S.; Osafune, K.; Akasaka, R. Development of low GWP refrigerants suitable for heat pump systems. In Proceedings of the JRAIA International Symposium, Kobe, Japan, 3–4 April 2012.
- Brown, J.S.; Zilio, C.; Cavallini, A. The fluorinated olefin R-1234ze(Z) as a high-temperature heat pumping refrigerant. Int. J. Refrig. 2009, 32, 1412–1422.
- Li, J.; Liu, Q.; Ge, Z.; Duan, Y.; Yang, Z. Thermodynamic performance analyses and optimization of subcritical and transcritical organic Rankine cycles using R1234ze(E) for 100â(E)“200°C heat sources. Energy Convers. Manag. 2017, 149, 140–154.
- Koyama, S.; Takata, N.; Fukuda, S. An experimental study on heat pump cycle using zeotropic binary refrigerant of HFO-1234ze(E) and HFC-32. In Proceedings of the 10th IEA Heat Pump Conference, Tokyo, Japan, 14–16 September 2011; pp. 1–10.
- Koyama, S.; Takata, N.; Fukuda, S. Drop-in Experiments on Heat Pump Cycle Using HFO-1234ze(E) and Its Mixtures with HFC-32. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 9–12 July 2010; pp. 1–7.
- Kabeel, A.E.; Khalil, A.; Bassuoni, M.M.; Raslan, M.S. Comparative experimental study of low GWP alternative for R134a in a walk-in cold room. Int. J. Refrig. 2016, 69, 303–312.
- Lai, N.A. Equations of state for HFO-1234ze(E) and their application in the study on refrigeration cycle. Int. J. Refrig. 2014, 43, 194–202.
- Mota-Babiloni, A.; Navarro-Esbrí, J.; Barragán, Á.; Molés, F.; Peris, B. Drop-in energy performance evaluation of R1234yf and R1234ze(E) in a vapor compression system as R134a replacements. Appl. Therm. Eng. 2014, 71, 259–265.
- An Mota-Babiloni, A.; Navarro-Esbrí, J.; Barrag An-Cervera, A.; Mol, F.; Peris, B. Drop-in analysis of an internal heat exchanger in a vapour compression system using R1234ze(E) and R450A as alternatives for R134a. Energy 2015, 90, 1636–1644.
- Fukuda, S.; Kojima, H.; Kondou, C.; Takata, N.; Koyama, S. Comparative assessment on irreversible losses in heat pumps using R744/R32/R1234yf and R744/R32/R1234ze(E). Sci. Technol. Built Environ. 2016, 22, 1118–1127.
- Yamamoto, S.; Koyama, S. Performance Evaluation of Heat Pump Cycle Using Non-Azeotropic Refrigerant Mixture R32/R1234ze(E); Kyushu University: Fukuoka, Japan, 2014.
- Higashi, Y.; Ashizawa, M.; Kabata, Y.; Majima, T.; Uematsu, M.; Watanabe, K. Measurements of vapor pressure, vapor-liquid coexistence curve and critical parameters of Refrigerant 152a. Trans Jpn. Soc. Mech. Eng. Ser. B 1987, 53, 1379–1385.
- Tamatsu, T.; Sato, H.; Watanabe, K. An equation of state for 1,1-difluoroethane (HFC 152a). Int. J. Refrig. 1993, 16, 347–352.
- Van Poolen, L.J.; Holcomb, C.D.; Niesen, V.G. Critical temperature and density from liquid-vapor coexistence data: Application to refrigerants R32, R124, and R152a. Fluid Phase Equilib. 1997, 129, 105–111.
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013 The Physical Science Basis Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013.
- Bitzer Group of Companies. Refrigerant Report, 15th ed.; Bitzer Group of Companies: Sindelfingen, Germany, 2014.
- Bolaji, B.O. Experimental study of R152a and R32 to replace R134a in a domestic refrigerator. Energy 2010, 35, 3793–3798.
- Cabello, R.; Sánchez, D.; Llopis, R.; Arauzo, I.; Torrella, E. Experimental comparison between R152a and R134a working in a refrigeration facility equipped with a hermetic compressor. Int. J. Refrig. 2015, 60, 92–105.
- Park, K.-J.; Jung, D. Performance of alternative refrigerant R430A on domestic water purifiers. Energy Convers. Manag. 2009, 50, 3045–3050.
- ANSI/ASHRAE-34-2007; Designation and Safety Classification of Refrigerants. ASHRAE: Atlanta, GA, USA, 2008; Volume 4723.
- Cabello, R.; Sánchez, D.; Llopis, R.; Catalán, J.; Nebot-Andrés, L.; Torrella, E. Energy evaluation of R152a as drop in replacement for R134a in cascade refrigeration plants. Appl. Therm. Eng. 2017, 110, 972–984.
- Pearson, A. Carbon dioxide—New uses for an old refrigerant. Int. J. Refrig. 2005, 28, 1140–1148.
- Austin, B.T.; Sumathy, K. Transcritical carbon dioxide heat pump systems: A review. Renew. Sustain. Energy Rev. 2011, 15, 4013–4029.
- Bolaji, B.O.; Huan, Z. Ozone depletion and global warming: Case for the use of natural refrigerant—A review. Renew. Sustain. Energy Rev. 2013, 18, 49–54.
- Weber, C.; Favrat, D. Conventional and advanced CO2 based district energy systems. Energy 2010, 35, 5070–5081.
- Maina, P.; Huan, Z. A review of carbon dioxide as a refrigerant in refrigeration technology. S. Afr. J. Sci. 2015, 111, 1–10.
- Lorentzen, G. Revival of carbon dioxide as a refrigerant. Int. J. Refrig. 1994, 17, 292–301.
- Ma, Y.; Liu, Z.; Tian, H. A review of transcritical carbon dioxide heat pump and refrigeration cycles. Energy 2013, 55, 156–172.
- Lorentzen, G.; Pettersen, J. A new, efficient and environmentally benign system for car air-conditioning. Int. J. Refrig. 1993, 16, 4–12.
- Koyama, K.; Xue, J.; Kuwahara, K. Performance Prediction Method of CO2 Cycle for Air Cooling. Trans. Jpn. Soc. Refrig. Air Cond. Eng. 2009, 26, 325–333.
- Xue, J.; Koyama, S.; Kuwahara, K. Performance Prediction of a R744 Transcritical Cycle for Air Conditioning. In Proceedings of the International Symposium Next-generation Air Conditioning Refrigeration Technology, Tokyo, Japan, 17–19 February 2010; pp. 17–19.
- Brown, J.S.; Yana-Motta, S.F.; Domanski, P.A. Comparitive analysis of an automotive air conditioning systems operating with CO2 and R134a. Int. J. Refrig. 2002, 25, 19–32.
- Jing-Yang, M.; Jiang-Ping, C.; Zhi-Jiu, C. System design and analysis of trans-critical carbon-dioxide automotive air-conditioning system. J. Zhejiang Univ. Sci. 2003, 4, 305–308.
- Girotto, S.; Minetto, S.; Neksa, P. Commercial refrigeration system using CO2 as the refrigerant. Int. J. Refrig. 2004, 27, 717–723.
- He, Y.; Deng, J.; Zheng, L.; Zhang, Z. Performance optimization of a transcritical CO2 refrigeration system using a controlled ejector. Int. J. Refrig. 2016, 75, 250–261.
- Pitarch, M.; Navarro-Peris, E.; Gonzalvez, J.; Corberan, J.M. Analysis and optimisation of different two-stage transcritical carbon dioxide cycles for heating applications. Int. J. Refrig. 2016, 70, 235–2342.
- Chen, G.; Volovyk, O.; Zhu, D.; Ierin, V.; Shestopalov, K. Theoretical analysis and optimization of a hybrid CO2 transcritical mechanical compression—Ejector cooling cycle. Int. J. Refrig. 2017, 74, 86–94.
- Hwang, Y.H.; Celik, A.; Radermacher, R. Performance of CO2 cycles with a two-stage compressor. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 12–14 July 2004; pp. 1–8.
- Nekså, P. CO2 heat pump systems. Int. J. Refrig. 2002, 25, 421–427.
- Dawo, F.; Fleischmann, J.; Kaufmann, F.; Schifflechner, C.; Eyerer, S.; Wieland, C.; Spliethoff, H. R1224yd(Z), R1233zd(E) and R1336mzz(Z) as replacements for R245fa: Experimental performance, interaction with lubricants and environmental impact. Appl. Energy 2021, 288, 116661.
- Sakoda, N.; Higashi, Y.; Akasaka, R. Measurements of PvT Properties, Vapor Pressures, Saturated Densities, and Critical Parameters for trans-1,1,1,4,4,4-Hexafluoro-2-butene (R1336mzz(E)). J. Chem. Eng. Data 2021, 66, 734–739.
- Kedzierski, M.A.; Lin, L. Pool boiling of HFO-1336mzz(Z) on a reentrant cavity surface. Int. J. Refrig. 2019, 104, 476–483.
- Kontomaris, K. Zero-ODP, low-GWP, non-flammable working fluids for high temperature heat pumps. In Proceedings of the ASHRAE 2014 Annual Conference, Seattle, WA, USA, 23 July 2014.
- Lampugnani, G.; Zgliczynski, M. R290 as a Substitute of R502 and R22 in Commercial Refrigeration and Air Conditioning. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 23 July 1996; pp. 83–88.
- Granryd, E. Hydrocarbons as refrigerants—An overview. Int. J. Refrig. 2001, 24, 15–24.
- Halimic, E.; Ross, D.; Agnew, B.; Anderson, A.; Potts, I. A comparison of the operating performance of alternative refrigerants. Appl. Therm. Eng. 2003, 23, 1441–1451.
- Bjerre, P.; Larsen, P. Evaluation of N-butane as a potential refrigerant for household compressors. In Proceedings of the International Compressor Engineering Conference, Purdue, IN, USA, 17–20 July 2006; pp. 1–6.
- Palm, B. Hydrocarbons as refrigerants in small heat pump and refrigeration systems—A review. Int. J. Refrig. 2008, 31, 552–563.
- Wongwises, S.; Chimres, N. Experimental study of hydrocarbon mixtures to replace HFC-134a in a domestic refrigerator. Energy Convers. Manag. 2005, 46, 85–100.
- Corberán, J.M.; Segurado, J.; Colbourne, D.; Gonzálvez, J. Review of standards for the use of hydrocarbon refrigerants in A/C, heat pump and refrigeration equipment. Int. J. Refrig. 2008, 31, 748–756.
- Suzuki, M. Application of adsorption cooling systems to automobiles. Heat Recover. Syst. ClIP 1993, 13, 335–340.
- Zhang, L. Design and testing of an automobile waste heat adsorption cooling system. Appl. Therm. Eng. 2000, 20, 103–114.
- Wang, R.; Oliveira, R. Adsorption refrigeration—An efficient way to make good use of waste heat and solar energy. Prog. Energy. Combust. Sci. 2006, 32, 424–458.
- Abdullah, M.O.; Tan, I.A.W.; Lim, L.S. Automobile adsorption air-conditioning system using oil palm biomass-based activated carbon: A review. Renew. Sustain. Energy Rev. 2011, 15, 2061–2072.
- Wang, R.Z.; Li, Y. Perspectives for natural working fluids in China. Int. J. Refrig. 2007, 30, 568–581.
- Critoph, R.E. Performance estimation of convective thermal wave adsorption cycles. Appl. Therm. Eng. 1996, 16, 429–437.
- Balaras, C.A.; Grossman, G.; Henning, H.-M.; Ferreira, C.A.I.; Podesser, E.; Wang, L.; Wiemken, E. Solar air conditioning in Europe—An overview. Renew. Sustain. Energy Rev. 2007, 11, 299–314.
- Sumathy, K.; Huang, Z.C.; Li, Z.F. Solar absorption cooling with low grade heat source—A strategy of development in south China. Sol. Energy 2002, 72, 155–165.
- Wang, R.Z.; Xia, Z.Z.; Wang, L.W.; Lu, Z.S.; Li, S.L.; Li, T.X.; Wu, J.Y.; He, S. Heat transfer design in adsorption refrigeration systems for efficient use of low-grade thermal energy. Energy 2011, 36, 5425–5439.
- Thu, K.; Kim, Y.-D.D.; Myat, A.; Chun, W.G.; Ng, K.C. Entropy generation analysis of an adsorption cooling cycle. Int. J. Heat Mass. Transf. 2013, 60, 143–150.
- Ng, K.C.; Thu, K.; Saha, B.B.; Chakraborty, A. Study on a waste heat-driven adsorption cooling cum desalination cycle. Int. J. Refrig. 2012, 35, 685–693.
- Myat, A.; Thu, K.; Kim, Y.D.; Chakraborty, A.; Chun, W.G.; Ng, K.C. A second law analysis and entropy generation minimization of an absorption chiller. Appl. Therm. Eng. 2011, 31, 2405–2413.
- Bella, B.; Kaemmer, N. An assessment of low GWP refrigerants in different applications. In Proceedings of the 23rd IIR Congress of Refrigeration, Prague, Czech Republic, 26–28 August 2011; p. 8.
- Kamiaka, T.; Dang, C.; Hihara, E. Vapor-liquid equilibrium measurements for binary mixtures of R1234yf with R32, R125, and R134a. Int. J. Refrig. 2013, 36, 965–971.
- Koyama, S.; Takata, N.; Fukuda, S.; Akasaka, R. Measurement of vapor-liquid equilibrium of HFO1234ze(E)/HFC32. In Proceedings of the JSRAE Annual Conference, Tokyo, Japan, 14-16 September 2010.
- Pham, H.; Rajendran, R. R32 And HFOs As Low-GWP Refrigerants For Air Conditioning. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 24–28 May 2012; pp. 1–10.
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Reference Fluid Thermodynamic and Transport Properties (REFPROP), Version 9.1; NIST: Gaithersburg, MD, USA, 2013.
- Mota-Babiloni, A.; Navarro-Esbrí, J.; Molés, F.; Cervera, Á.B.; Peris, B.; Verdú, G. A review of refrigerant R1234ze(E) recent investigations. Appl. Therm. Eng. 2016, 95, 211–222.
- Wang, X.; Amrane, K. AHRI Low Global Warming Potential Alternative Refrigerants Evaluation Program (Low-GWP AREP)—Summary of Phase I Testing Results. In Proceedings of the 15th International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 14–17 July 2014; pp. 1–10.
- Koyama, S.; Takata, N.; Matsuo, Y.; Yoshitake, D.; Fukuda, S. Possibility to introduce HFO-1234ze(E) and its mixture with HFC-32 as low-GWP alternatives for heat pump/refrigeration systems SYSTEMS. In Proceedings of the 2010 International Symposium on Next-generation Air Conditioning and Refrigeration Technology, Tokyo, Japan, 17–19 February 2010; pp. 1–10.
- Akasaka, R. Thermodynamic property models for the difluoromethane (R-32)+trans-1,3,3,3-tetrafluoropropene (R-1234ze(E)) and difluoromethane+2,3,3,3-tetrafluoropropene (R-1234yf) mixtures. Fluid Phase Equilib. 2013, 358, 98–104.
- Tanaka, T.; Hidekazu, O.; Katsuya, U.; Jun, I.; Otsuka, T.; Nogami, T.; Dobashi, R. Development of a new low-GWP refrigerant composed of HFO-1123 (trifluoroethylene). In Proceedings of the AIChE Annual Meeting, Atlanta, GA, USA, 18 November 2014.
- Higashi, Y.; Akasaka, R. Measurements of Thermodynamic Properties for R1123 and R1123 + R32 Mixture. In Proceedings of the International Compressor Engineering Conference, Purdue, IN, USA, 11–14 July 2016; pp. 1–10.
- Fukushima, M.; Hashimoto, M. Next Generation Low-GWP Refrigerants “AMOLEA-TM.”; Reports of the Research Laboratory; Asahi Glass Co., Ltd.: Tokyo, Japan, 2015.
- Mani, K.; Selladurai, V. Experimental analysis of a new refrigerant mixture as drop-in replacement for CFC12 and HFC134a. Int. J. Therm. Sci. 2008, 47, 1490–1495.
- Lee, M.Y.; Lee, D.Y.; Kim, Y. Performance characteristics of a small-capacity directly cooled refrigerator using R290/R600a (55/45). Int. J. Refrig. 2008, 31, 734–741.
- Jung, D.; Kim, C.B.; Song, K.; Park, B. Testing of propane/isobutane mixture in domestic refrigerators. Int. J. Refrig. 2000, 23, 517–527.
- Richardson, R.N.; Butterworth, J.S. The performance of propane/isobutane mixtures in a vapour-compression refrigeration system. Int. J. Refrig. 1995, 18, 58–62.
- Dalkilic, A.S.; Wongwises, S. A performance comparison of vapour-compression refrigeration system using various alternative refrigerants. Int. Commun. Heat Mass. Transf. 2010, 37, 1340–1349.
- Mohanraj, M.; Jayaraj, S.; Muraleedharan, C.; Chandrasekar, P. Experimental investigation of R290/R600a mixture as an alternative to R134a in a domestic refrigerator. Int. J. Therm. Sci. 2009, 48, 103106–103142.
- Kim, J.H.; Cho, J.M.; Kim, M.S. Cooling performance of several CO2/propane mixtures and glide matching with secondary heat transfer fluid. Int. J. Refrig. 2008, 31, 800–806.
- Tian, Q.; Cai, D.; Ren, L.; Tang, W.; Xie, Y.; He, G.; Liu, F. An experimental investigation of refrigerant mixture R32/R290 as drop-in replacement for HFC410A in household air conditioners. Int. J. Refrig. 2015, 57, 216–228.
- Maczek, K.; Muller, J.; Wojtas, K.; Domanski, P.A. Ternary Zeotropic Mixture with CO2 Component for R-22 Heat pump application. In Proceedings of the CLIMA Conference, Brussels, Belgium, 30 August 1997.
- Hakkaki-fard, A.L.I.; Aidoun, Z.; Ouzzane, M. Air-source heat pumps with refrigerant mixtures for cold climates. In Proceedings of the 11th IEA Heat Pump Conference, Montreal, Canada, 12–16 May 2014; pp. 1–11.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.7K
Revisions:
2 times
(View History)
Update Date:
15 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




