
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Steven Johnson | + 1808 word(s) | 1808 | 2020-11-19 03:42:36 | | | |
| 2 | Vicky Zhou | -75 word(s) | 1733 | 2020-12-09 03:12:25 | | | | |
| 3 | Vicky Zhou | Meta information modification | 1733 | 2020-12-09 04:32:19 | | |
Video Upload Options
The “exposome” is the cumulative exposures (diet, exercise, environmental exposure, vaccination, genetics, etc.) an individual has experienced and provides a mechanism for the establishment of immune training or immunotolerance. It is becoming increasingly clear that trained immunity constitutes a delicate balance between the dose, duration, and order of exposures. Upon innate stimuli, trained immunity or tolerance is shaped by epigenetic and metabolic changes that alter hematopoietic stem cell lineage commitment and responses to infection. Due to the immunomodulatory role of the exposome, understanding innate immune training is critical for understanding why some individuals exhibit protective phenotypes while closely related individuals may experience immunotolerant effects (e.g., the order of exposure can result in completely divergent immune responses). Research on the exposome and trained immunity may be leveraged to identify key factors for improving vaccination development, altering inflammatory disease development, and introducing potential new prophylactic treatments, especially for diseases such as COVID-19, which is currently a major health issue for the world. Furthermore, continued exposome research may prevent many deleterious effects caused by immunotolerance that frequently result in host morbidity or mortality.
1. Introduction
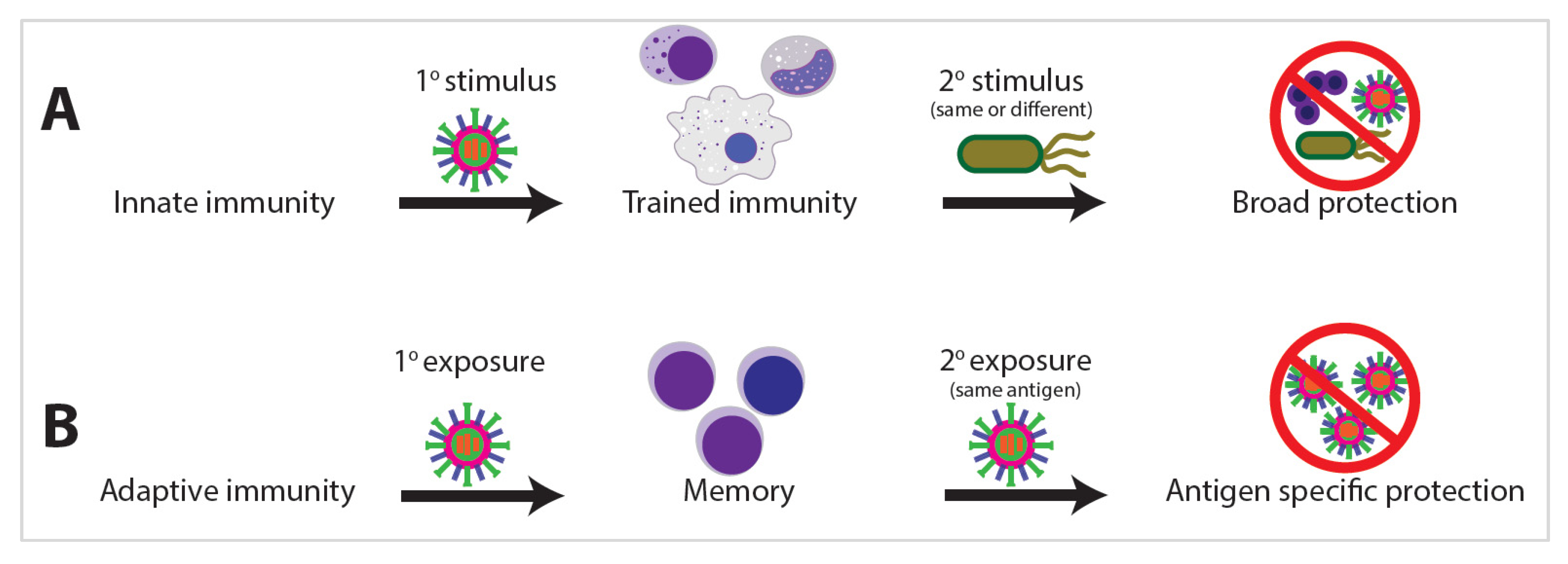
Innate immunity is the first line of host defense against external exposures. While traditionally viewed as primitive and nonspecific, a growing body of clinical and experimental evidence argues the innate immune system develops memory as a result of previous exposures, allowing the innate system to respond with enhanced and broad immunological protection upon exposure to a secondary stimulus [1][2]. This biological process of enhanced innate immunity response on secondary pathogen exposure has been termed “trained immunity” [2][3]. Trained immunity shares many phenotypic and epigenetic characteristics with adaptive immune memory; however, one of the starkest distinctions is the propensity for trained immunity to develop against heterologous stimuli (Figure 1). Innate memory is not antigen specific and is often protective against unrelated organisms, such as when vaccination for tuberculosis with Bacillus Calmette–Guerin (BCG) also affords protection against fungal or even viral infections such as SARS-CoV-2 [4][5][6].
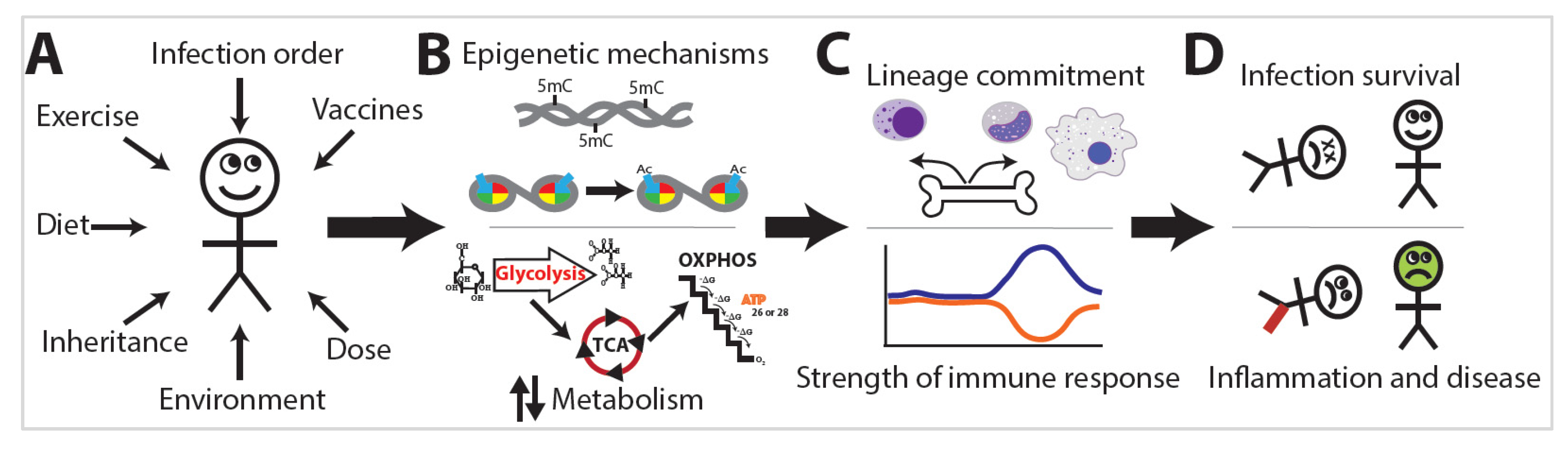
The exposome is the compilation of everything an individual has encountered throughout their life [7]. Research into the exposome examines the combination of all exposures an individual has encountered during a set period of their life and the subsequent effects on that individual (Figure 2A) [7]. The exposome varies spatiotemporally, is highly dynamic and diverse, and provides a mechanism by which trained immunity or immunotolerance is established [8]. This occurs via distinct changes in leukocyte epigenetics and metabolism (Figure 2B) that initiate changes in leukocyte lineage commitment and the strength of the immune response (Figure 2C), eventually resulting in divergent levels of infection survival, inflammation, and disease (Figure 2D). Studies of the exposome are relatively sparse, yet the effects on human health are dramatic. Two major projects aimed at unraveling the exposome are The Human Early Life Exposome (HELIX) project and the EXPOsOMICS project. The HELIX project is a study aimed at measuring and correlating the effects of early-life exposures on human health [9]. The EXPOsOMICS project utilizes high-throughput sequencing and “omics” experiments to build models of lifetime exposures and their effects on human health [10][11].
Before an individual is born, they show evidence of epigenetic reprogramming because they are exposed to many stimuli encountered by their mother while also inheriting maternal epigenetic marks. Following their birth, individuals encounter environmental pollutants, pathogens, and allergens unique to them [12][13]. Even fluctuations in diet can cause long-term variations in health outcomes, variations that even exist between family members in the same household [13][14]. Furthermore, cohabiting individuals maintain uniquely identifiable external microbial clouds and internal microbiomes [12][15][16]. The combination of lifetime exposures establishes serious consequences in the development of inflammatory diseases [17][18].
Exposure to many airborne antigens can induce inflammatory airway diseases such as asthma. Individuals who walk along high-traffic streets experience airway acidification and immune cell infiltration, similar to that induced by intense exercise [19][20]. Unexpectedly, other airborne antigens such as dog-associated house dust can provide protection of the airways. While some of this protection is attributable to changes in the gut microbiome, the mechanism by which one person develops allergies to dog-associated dust while their sibling is protected remains unclear [21]. Because the training stimulus may be potentiated or abrogated by a secondary stimulus, it is reasonable to conclude that the order of exposures may actually be more important than the combination of exposures. This has been demonstrated in childhood vaccination as well as in animal models, many of which demonstrate significantly altered inflammatory profiles based solely upon the order of exposures received [22].
Monocytes/macrophages, dendritic cells (DCs), and natural killer (NK) cells are innate immune cells that all exhibit the ability to recollect a previous foreign encounter and subsequently mount an altered immunological memory response [23]. Exposure to high levels of bacterial lipopolysaccharide (LPS) and other toll-like receptor (TLR) agonists can induce a tolerogenic or “paralyzed” immune response, whereas BCG and many other pathogens induce a proinflammatory milieu of gene expression. This enhanced immune response is predicated upon extensive metabolic shifts in energy utilization and epigenetic regulation at the level of histone modification and differential DNA methylation. The metabolic and epigenetic effects of immune training can improve immune function, but they are also often maladaptive and result in arthritis, atherosclerosis, or allergies [1].
The metabolic and epigenetic changes observed are the result of the specific “training” the innate cells received upon the primary exposure. This, in conjunction with the various secondary stimuli encountered, results in the production of distinct cytokine profiles that are dependent on the order of pathogen exposure. Many studies have been conducted to understand the effects of environmental exposures on human health and immune function, emphasizing the total combination of exposures. However, they do not normally take exposure order into consideration, yet it is becoming clear that trained immunity is significantly influenced by exposure order.
2. Exposome and Immunity Training
Trained immunity is a well-established phenomenon found in organisms ranging from plants to humans. Much of the research performed has focused solely on either the primary or the secondary stimulus, illustrating the extent to which the combination of exposures determines epigenetic reprogramming; however, in many cases, the order of exposures is likely more significant than the combination of stimuli can adequately explain. The most dramatic example is when primary LPS and secondary LPS + IFN-γ are inverted (Figure 3A). A similar effect is observed when primary LPS and secondary β-glucan are likewise inverted (Figure 3B). These examples demonstrate a proclivity for the secondary exposure to induce a stronger influence than the primary exposure.
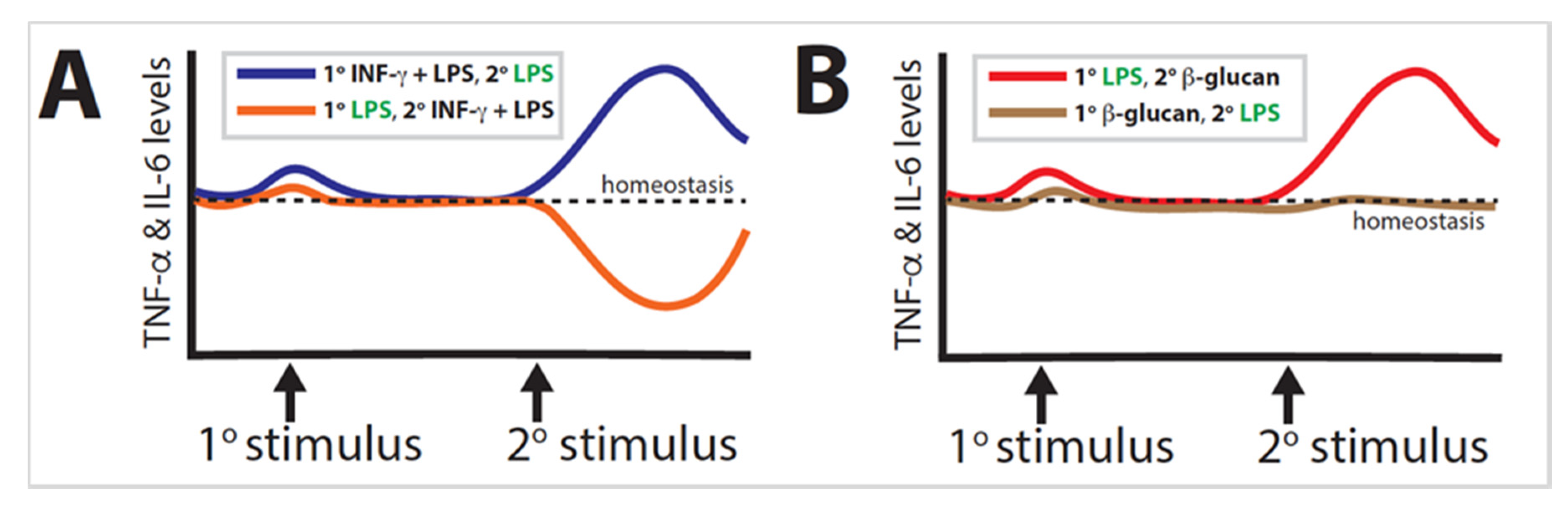
Further research conducted on the exposome should take order into consideration, as an emphasis on solely one stimulus paints an incomplete picture of immune training. This may be particularly substantial in airway diseases, such as asthma or allergies, wherein some individuals develop allergies to pollen while others do not [20]. This may be partially attributable to whether the individual’s immune system was primed prior to exposure to said allergen. Understanding exposure order could significantly benefit atopic individuals.
It is worth reiterating that vaccine order can dramatically affect mortality rates in newborns [22]. Accordingly, vaccination schedules should be reevaluated, as specific vaccines can induce either heterologous immune protection or innate immune tolerance. By reordering vaccine administration, physicians can potentially reduce childhood sepsis and airway infections without necessitating additional vaccine development. Animal studies and, eventually, human trials may be beneficial in reducing childhood mortality, thus reducing global healthcare costs through the non-specific prevention of infections.
Further vaccine development must also take into consideration heterologous innate immune effects. While trained immunity presents a compelling avenue for novel vaccine development, care must be taken to prevent detrimental immune tolerance. Further research should be conducted to better understand exposure order. Inverting BCG vaccination with its various secondary exposures would be an important first step, as it represents an existing vaccine currently in deployment with potential implications for infectious agents such as SARS-CoV-2 [6]. In fact, extensive research is currently underway examining the potential protective effects that previous BCG vaccination and its subsequent innate immune memory may have on preventing COVID-19 or at least in decreasing the severity of the disease [24]. It is hypothesized that since BCG vaccination can induce trained immunity against heterologous infections, including respiratory viral infections, it could offer a measure of protection against SARS-CoV-2, effecting a quicker innate immune response to initial infection, which would decrease the viral load until the adaptive immune response can kick in. Since immune tolerance is a possible outcome of the innate training, controlled clinical studies need to be conducted to make sure that the initial exposure does not inhibit the innate response, increase early viral levels, and then cause an over-stimulation of the adaptive immune response, which is common in serious COVID-19 cases. While innate training represents a powerful option for combating multiple pathogenic threats, these hypotheses need further rigorous clinical study before therapeutic conclusions and recommendations can be made. By altering the administration of primary and secondary exposures, researchers will better explain the extent to which exposure order can reprogram the innate immune system. Ultimately, it is not the individual components that dictate the outcome of the innate immune response, but the combination, order, and potency of the stimuli decide the fate of the system and even the survival of the host organism.
References
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098.
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388.
- Netea, M.G.; Quintin, J.; Van Der Meer, J.W. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361.
- Arts, R.J.; Moorlag, S.J.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5.
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; Van Loenhout, J.; De Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542.
- Mantovani, A.; Netea, M.G. Trained Innate Immunity, Epigenetics, and Covid-19. N. Engl. J. Med. 2020, 383, 1078–1080.
- Wild, C.P. Complementing the genome with an “Exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850.
- Jiang, C.; Wang, X.; Li, X.; Inlora, J.; Wang, T.; Liu, Q.; Snyder, M. Dynamic Human Environmental Exposome Revealed by Longitudinal Personal Monitoring. Cell 2018, 175, 277–291.e31.
- Vrijheid, M.; Slama, R.; Robinson, O.; Chatzi, L.; Coen, M.; Hazel, P.V.D.; Thomsen, C.; Wright, J.; Athersuch, T.J.; Avellana, N.; et al. The Human Early-Life Exposome (HELIX): Project Rationale and Design. Environ. Health Perspect. 2014, 122, 535–544.
- Vineis, P.; Chadeau-Hyam, M.; Gmuender, H.; Gulliver, J.; Herceg, Z.; Kleinjans, J.; Kogevinas, M.; Kyrtopoulos, S.; Nieuwenhuijsen, M.J.; Phillips, D.; et al. The exposome in practice: Design of the EXPOsOMICS project. Int. J. Hyg. Environ. Health 2017, 220, 142–151.
- Schutsky, E.K.; DeNizio, J.E.; Hu, P.; Liu, M.Y.; Nabel, C.S.; Fabyanic, E.B.; Hwang, Y.; Bushman, F.D.; Wu, H.; Kohli, R.M. Nondestructive, base-resolution sequencing of 5-hydroxymethylcytosine using a DNA deaminase. Nat. Biotechnol. 2018, 36, 1083–1090.
- Meadow, J.F.; Altrichter, A.E.; Bateman, A.C.; Stenson, J.; Brown, G.Z.; Green, J.L.; Bohannan, B.J. Humans differ in their personal microbial cloud. PeerJ 2015, 3, e1258.
- Chung, M.K.; Kannan, K.; Louis, G.M.; Patel, C.J. Toward Capturing the Exposome: Exposure Biomarker Variability and Coexposure Patterns in the Shared Environment. Environ. Sci. Technol. 2018, 52, 8801–8810.
- Laker, R.C.; Garde, C.; Camera, D.M.; Smiles, W.J.; Zierath, J.R.; Hawley, J.A.; Barrès, R. Transcriptomic and epigenetic responses to short-term nutrient-exercise stress in humans. Sci. Rep. 2017, 7, 15134.
- Schloissnig, S.; Arumugam, M.; Sunagawa, S.; Mitreva, M.; Tap, J.; Zhu, A.; Waller, A.S.; Mende, D.R.; Kultima, J.R.; Martin, J.; et al. Genomic variation landscape of the human gut microbiome. Nature 2013, 493, 45–50.
- Lax, S.; Smith, D.P.; Hampton-Marcell, J.; Owens, S.M.; Handley, K.M.; Scott, N.M.; Gibbons, S.M.; Larsen, P.; Shogan, B.D.; Weiss, S.; et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014, 345, 1048–1052.
- Pfeifer, G.P. Environmental exposures and mutational patterns of cancer genomes. Genome Med. 2010, 2, 54.
- Yuan, J.; Liu, Y. Progress of Genomics in Atherosclerosis-Coronary Heart Disease and Myocardial Infarction. Appl. Clin. Bioinform. 2018, 16, 219–240.
- McCreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Järup, L.; Harrington, R.; Svartengren, M.; Han, I.-K.; Ohman-Strickland, P.; et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007, 357, 2348–2358.
- Frellstedt, L.; Waldschmidt, I.; Gosset, P.; Desmet, C.; Pirottin, D.; Bureau, F.; Farnir, F.; Franck, T.; Dupuis-Tricaud, M.-C.; Lekeux, P.; et al. Training Modifies Innate Immune Responses in Blood Monocytes and in Pulmonary Alveolar Macrophages. Am. J. Respir. Cell Mol. Biol. 2014, 51, 135–142.
- Fujimura, K.E.; Demoor, T.; Rauch, M.; Faruqi, A.A.; Jang, S.; Johnson, C.C.; Boushey, H.A.; Zoratti, E.; Ownby, D.; Lukacs, N.W.; et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc. Natl. Acad. Sci. USA 2013, 111, 805–810.
- Sørup, S.; Benn, C.S.; Poulsen, A.; Krause, T.G.; Aaby, P.; Ravn, H. Live Vaccine Against Measles, Mumps, and Rubella and the Risk of Hospital Admissions for Nontargeted Infections. JAMA 2014, 311, 826.
- Bekkering, S.; Arts, R.J.; Novakovic, B.; Kourtzelis, I.; Van Der Heijden, C.D.; Li, Y.; Popa, C.D.; Ter Horst, R.; Van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e9.
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632.





