Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khaya Pearlman Shabangu | -- | 3533 | 2022-11-13 07:06:53 | | | |
| 2 | Jason Zhu | -1 word(s) | 3532 | 2022-11-14 03:37:33 | | | | |
| 3 | Jason Zhu | -24 word(s) | 3508 | 2022-11-18 03:55:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shabangu, K.P.; Bakare, B.F.; Bwapwa, J.K. Microbial Fuel Cells for Electrical Energy. Encyclopedia. Available online: https://encyclopedia.pub/entry/34239 (accessed on 07 February 2026).
Shabangu KP, Bakare BF, Bwapwa JK. Microbial Fuel Cells for Electrical Energy. Encyclopedia. Available at: https://encyclopedia.pub/entry/34239. Accessed February 07, 2026.
Shabangu, Khaya Pearlman, Babatunde Femi Bakare, Joseph Kapuku Bwapwa. "Microbial Fuel Cells for Electrical Energy" Encyclopedia, https://encyclopedia.pub/entry/34239 (accessed February 07, 2026).
Shabangu, K.P., Bakare, B.F., & Bwapwa, J.K. (2022, November 13). Microbial Fuel Cells for Electrical Energy. In Encyclopedia. https://encyclopedia.pub/entry/34239
Shabangu, Khaya Pearlman, et al. "Microbial Fuel Cells for Electrical Energy." Encyclopedia. Web. 13 November, 2022.
Copy Citation
Microbial fuel cell (MFC) technology turns chemical energy into bioelectricity in a clean and efficient manner, lowering carbon emissions and increasing bioenergy production. It is a multifaceted technique that has the potential to be a panacea for clean water scarcity and sustainable, renewable energy.
microbial fuel cell
renewable energy
wastewater treatment
scaling-up
commercial scale
cost-effective
operating factors
1. Introduction
The creation of a sustainable society will necessitate minimizing carbon footprints, which will reduce the amount of pollution produced and the excessive use of carbon sources. In a specialized area such as wastewater treatment, these two aspects should be addressed simultaneously. As it stands, the paradigm has shifted from disposing of wastewater/waste matter to turning its organic matter into electrical energy. METs have now gained popularity as a viable solution for dealing with this problem. MFCs and MECs are both fundamental disciplines of METs [1][2].
An MFC is a process unit that biodegrades complex substrates and produces bioenergy concurrently [2]. This technology henceforth produces multiple bioenergy products such as bioelectricity, hydrogen, methane, etc. Several rapidly biodegradable chemicals, such as glucose and acetate, as well as several types of wastewater, including residential, starching, and paper recycling plant effluent, have been adopted as a source of electrogenes in MFCs [2][3][4]. Most may remove a significant amount of chemical oxygen demand (COD) while also producing power. The United States (US) space program sparked the growth of MFCs in the 1960s as a way to dispose of garbage during space flights while simultaneously providing power [2][3]. MFC technology has been carefully studied, with a focus on current developments, practical applications, and a future roadmap. Due to their viable novelty and multifaceted approach to generating bioenergy concurrent to wastewater treatment, MFCs have since been studied immensely. Furthermore, some recent MFC modifications that used an anoxic cathode enhanced the external voltage at the cathode. Phototrophic MFCs and solar-powered MFCs are also noteworthy attempts at upscaling MFC technology for electricity generation [2]. MFC technology provides a flexible way to generate energy while also treating wastewater. MFCs are a renewable energy technology that can meet the needs for both clean, reliable energy and fresh water. More effort is needed to elevate MFC technology to a commercial level [4].
2. Microbial Electrochemical Technologies Blueprint
METs connect bacterial respiratory mechanisms to an electrochemical system [5]. The following portion of this entry provides an overview of some of the configurations and prospective applications of METs: the MET layout, with its subsidiary bio-electrochemical systems, MFCs and MECs, is clearly outlined to be a technical bioconversion of wastewater in the form of complex substrates into bioenergy, either in the form of bio-hydrogen, bioelectricity, etc. The primary product solely depends on the basic MET operating system objective and methodological approach.
There are numerous potential uses that are more specialized than the two main proposed paths for the industrial application of microbial electrochemical technologies, MFCs and MECs [6][7][8][9]. To list a few, bio-sensing, biocomputing, and fundamental studies of microbial metabolism. Because microbial electron transfer enables direct interaction between biological processes and electrical circuits, MET is helpful for bio-sensing because it can be integrated with conventional computing systems and produce short response times. Utilizing MET for biocomputing has the same drawback and furthermore has the ability to incorporate the intricate regulatory apparatus of bacterial cells into the biocomputing circuit [5]. The microbe–electrode interface is useful in fundamental metabolic research because it enables the direct, real-time assessment of a population of cells’ respiratory activity, a characteristic not present in any experimental system. New concepts and applications occasionally surface in the vast and dynamic field of MET research [10].
To create chemicals such as hydrogen, methane, ethanol, or hydrogen peroxide, MECs use electrode-respiring microorganisms [11][12]. Despite having a similar construction to MFCs, MECs use a resistor or power source to circumvent thermodynamic restrictions by introducing an external potential into the system. Due to the low cost of fossil fuels, the use of MFCs to produce energy in the real world is unlikely, but the increased value of MEC products (e.g., methane, hydrogen, hydrogen peroxide) makes MECs a more promising technology [4]. Biological, chemical, and physical restrictions that limit MFC scale-up also restrict the development of MECs; therefore, there are still numerous application-related obstacles to overcome [13]. The cathode’s accumulation of gaseous products, such as hydrogen and methane, also poses difficulties [4]. MEC technologies are promising, but they are still in the experimental stage, and they need to be shown to be overall efficient and reliable in the long term before they can be used in real-world applications [4].
MFCs use bacterial metabolism to produce a current. In order to transport electrons from a usable substrate (typically suggested as industrial effluent) through a circuit that eventually reduces a terminal electron acceptor, electrode-respiring bacteria must be able to donate electrons to negatively poised electrodes [5][10][13][14][15][16]. If a load is introduced to the circuit, the generated bacterial current can be used for work. Bacteria can breathe or accumulate charged electrodes in a variety of ways, including by direct respiration via extracellular or outer-membrane proteins, mediator-based respiration via endogenous or exogenous mediators, and mediator-based respiration via endogenous or exogenous mediators. This entry’s focus is on the investigation of the bioelectricity mechanisms in microbial fuel cells as well as their optimization for scalable and up-scaled bioelectricity production. In a microbial fuel cell, electricity is only produced if the whole process is thermodynamically favorable [15]. Gibbs free energy, which is measured in Joules, can be used to analyze the reaction [15]. This is a measure of the most work that can be carried out because of the reaction and is calculated as follows:
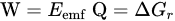
Given that Q = nF, n is the number of electrons per reaction mole, Q is the charge transferred in the reaction represented in Coulomb (C), and F is the Faraday constant, (9.64853 × 104 C/mol) [15]. Hence, there are:
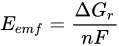
This model above simplifies to the following model, which is useful because it is positive for a favorable reaction and instantly generates a value of the Eemf for the reaction [15]. The MFC voltage is capped by this Emf, but due to various potential losses, the actual potential from the MFC will be lower:
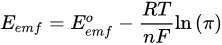
2.1. Microbial Fuel Cell (MFC) Principle and Anatomical Mechanisms
MFCs use bacterial metabolism to generate electricity. Electrode-breathing microorganisms that can donate electrons to negatively positioned electrodes transfer electrons from, typically, wastewater via a circuit that oxidizes the catholyte. If a load is presented to the circuit, the bacterial current can be used for work. Bacteria can breathe or populate with charged electrodes in a variety of ways, such as directly via extracellular or outer-membrane proteins, as well as via internal or external mediators.
In general, proton exchange membrane (PEM) and cation exchange membrane (CEM) refer to semi-permeable membranes. Other porous separators can also be considered to allow the permselectivity of protons and ions [17]. The most commonly used CEM or PEM is Nafion from DuPont (Wilmington, DE, USA). Perflourosulfonic acid membranes can also be considered but are limited due to their expensive nature in terms of the material of construction and its unfriendly environmental waste issue. The CEM or PEM exudes a certain permselectivity, which only allows passage for protons, in order to avoid the diffusion of trace amounts of oxygen into the anodic chamber. Frankly, the CEM or PEM serves as a barrier to undesired active species during the electrochemical and biodegradation process of the anolyte to produce bioenergy. In the anodic biofilm, the bacterial community mostly oxidizes the organic substrate’s organic compounds [17]. Due to the biodegradation process, electrons are discharged onto the anodic electrode and flow towards the cathodic compartment through a resistor load wire material [1][2][18][19][20][21]. Protons are released simultaneously with electrons in the anodic chamber, allowing them to pass through the semi-permeable film known as a PEM. This layer only enables protons to flow through and prevents oxygen from diffusing back into the anodic chamber. Protons migrate from the anode to the cathode chamber during a process known as electrogenesis, which is enabled by the PEM or CEM specialized membrane unit. As a result, the electrogenes are the viable active biomass cultures that release these electrons. This process underpins the basic principle of establishing green technology relevant to the MFC. MFC technology is based on the straightforward change of waste biological substances in the form of biological power into electrical energy. This biological conversion is ascertained in the presence of active electrogenes [2][4][22][23].
Electrodes, separators, and electrogenes are the three main components of a standard MFC. Some carbon-based, graphite-based, and metal-based electrodes have been recommended in previous studies. Carbon cloth, carbon paper, carbon felt [24][25], graphite granules, carbon mesh [26][27], platinum, platinum black, activated carbon single, tubular, or a design with many electrodes. Biocompatibility, stability, good electrical conductivity, and a broad surface area are all desirable characteristics for these electrodes [26][27][28][29][30]. The cathode can be exposed to air or other electron acceptors such as permanganate, chromium hexacynoferate, azo dye, and so on [11]. To maintain the chamber’s cleanliness, a cation exchange membrane or a salt bridge has been utilized as a separator [17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33]. While the microbial breakdown of waste materials provided as substrate generates bioelectricity, the electromotive force formed between the anode and cathode chambers pushes electrons to run on the circuit [26][27][28][29][30][31][32][33][34][35][36].
2.2. Analysis of the MFC Compartments
The major components of a typical MFC unit utilized in the treatment of wastewater while producing energy are itemized in Table 1. The essential aspects that have been studied are anticipated to highlight the most important factors to consider while designing, constructing, and utilizing MFC prototypes for any given industrial application. The most prevalent building materials, current market suppliers, and advantages and downsides of these components have all been thoroughly analyzed in Table 1.
Table 1. Perspectives on Key Design MFC Components.
| Component | Design Parameters and Material |
Most Common Electrode/Catalyst | Suppliers and Costs |
Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| ANODE- ELECTRODE |
Conductive Material. Bio-compatible. Chemically stable metal, non-corrosive; gold, silver, nickel, s-steel (304), graphite, and polycrystalline. |
Copper, aluminum, zinc, carbon, carbon paper, carbon brushes, graphene electrode, graphite: rods, plates, granules, fiber, etc. |
E-TEK and Electro-Synthesis United Sates of America (USA) GEE Graphite Ltd., (USA). Dewsbury; United Kingdom, (UK). Morgan Grinbergen; (Belgium). Alfer–Aeser (Germany). Generally in-expensive simple materials, e.g., graphite. |
Porous. Easy to handle. Large surface area. Allows efficient flow of electrons through the anode. Permits minimal oxygen into the chamber. |
Poor biofilm growth. Poor electron flow. Recurrent Biofouling on the film. |
[31][32][33][34][35][36][37] |
| CATHODE- ELECTRODE |
Bio-compatible. Chemically stable metal/alloy, non-corrosive. |
Copper, aluminum, zinc, carbon, carbon paper, carbon brushes, graphene electrode, graphite: rods, plates, granules, fiber, etc. |
Low-cost for O2 but expensive for catalyst catholyte, e.g., Pt, etc. | Lower potential. Greatly impacts power generation in MFC. |
Insufficient re-oxidation. Expensive chemical acceptors. Catalyst catholyte activity drops over time. Problems with binder for catalyst. |
[5][38][39][40][41][42][43] |
| PEM/CEM | Ultrex CMI-7000 | Nafion 112/115/117 | DuPont USA Aldrich and Ion Power Highly Expensive |
Permeability to Protons | External Biofouling Internal Biofouling Costly Upscaling and practical application Problems |
[25][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46] |
| MFC Catalyst | Ferricyanide, Pt catalyst |
Pt catalyst. Ferricyanide. Permanganate Solution. |
Synthesis in local chemical suppliers. | Boost the conductivity of either anolyte or catholyte. Acts as a viable electrolyte. | Expensive for commercial-scale applications. Complex disposal procedures due to being environmentally unfriendly. | [43][44][45][46][47][48][49][50] |
2.2.1. Anode Chamber
The anode chamber acts as sort of the only anaerobic zone of any typical MFC bioreactor. The succinct biodegradation and flow of electrons from the complex sourced substrate to the external biofilm of the anodic electrode transpires here. For increased power generation, there is a need for properly cultured and well screened and selected active electrogenes, which are the source catalyst for the electrogenation process in this chamber. A properly operated and well-commissioned anode chamber will definitely result in an optimized overall potential difference in the MFC unit, hence upscaling the production of bioelectricity within this technique. In any case, one has to investigate the basic contributing factors towards the scaling-up of electricity production within an MFC in this chamber [17]. These factors may include the flow of electrons from the anode to the cathode chamber, hence resulting in smooth and improved potential difference, and the microbial activity rate mechanism of this chamber. The microbial respiratory principles are monitored and effective in the anode chamber. The pH medium and the effect of temperature have a much more impactful contribution in this chamber of the MFC unit. Precisely for efficient MFC unit scaling-up towards the production of increased power densities, the anode chamber has to be properly designed with effective materials, design layout, and mechanical configuration, and the solution chemistry for the microbes or electrogenes in this chamber has to be properly observed.
The sequence of existing augmentations with total inner surface area is as follows: (i) carbon felt, (ii) carbon foam, and (iii) graphite, according to [17][38][39][40]. There have not been any studies done on the long-term effects of biofilm formation or particles in the flow on any of the above surfaces. Manganese, Mn (IV) and Iron, Fe (III) were included, and covalently bonded neutral red was used to mediate electron transport to the anode [13][17][47]. In order for the anode to produce electrons, microbes are crucial. Due to the effectiveness of substrate oxidation and its impact on microbial activity rates, the structural and basic properties of the anode have a major impact on MFC efficiency [48][49][50]. One of the most important factors affecting MFC performance is anodic microbial electron flow, which improves the rate of microbial electron transfer utilizing a variety of practical techniques and modifies the electrode and cell design [17][48][49][50]. As a result, anodic electrodes are essential parts of an MFC and play a vital role in improving its effectiveness. Therefore, when establishing a standard anode in an MFC, appropriate anode materials should be considered. It should have a big surface area and be inexpensive, noncorrosive, and highly conductive, as shown in Table 1 [17].
The most typical anode material, as shown in Table 1, is carbon-based and includes metal electrodes as well as graphite rods, felt, brushes, and fibers. Due to their low cost, simple operation, and high pore stability, carbon-based electrode materials (plates or rods) are crucial components for anode electrodes [48][49]. Due to their large surface area and porosity, compact materials such as carbon-based electrodes are promising for the development of biocompatible and active microorganisms that can viably produce energy from complex substrates [17]. Carbon felt and carbon brushes have higher overall power outputs of 2437 and 2110 mW/m2 (90% COD elimination) [48][49][50]. Several studies on the suitability of anode materials have demonstrated and proved that improvement in MFC performance can be accomplished primarily through a highly porous structure. Despite the fact that carbon-based materials are commonly used as an anode, nitrogen-pre-treated carbon electrodes can achieve even higher power densities [48][49][50]. Corrosion resistance and cost-effectiveness are vital aspects of metal-based electrode materials [43]. Various types of anode materials are alternatives, especially carbon-structured electrode materials [17][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48].
2.2.2. Cathode Chamber
This compartment sort of acts as the final stage of the MFC electricity generation stepwise process. It is the last unit operation section of the MFC technique. This chamber, as aforementioned in the above section, aids the completion of the flow of electrons and completes the reduction of the treated wastewater towards basic cleaner effluent, free of high organic or biodegradable pollutants and perhaps particulate or non-biodegradable pollutants with the aid of exogenous mediums, e.g., permanganate solution. Although cathode electrodes have made significant strides, they still have drawbacks such as high cost, surface toxicity of microbes, and insufficient re-oxidation, necessitating the routine maintenance of the catholyte [17]. These factors have led to research into more appealing materials to increase MFC power output. Among these are materials with a carbon basis, metal oxides and complexes, and others. This solution improves the electron reception of this chamber while treating inorganic contents from the complex substrate such as phosphates and nitrates. A good cathodic chamber with optimized operation factors and proper configuration would assist in upscaling the production of bioelectricity in the MFC unit.
The practice of oxygen cathodes is restricted for MFC designs that can stand low performance due to the comparatively low oxygen acceptance rates of plain carbon and the associated significant overpotential. In saltwater, microbial assistance for oxygen reduction on carbon cathodes has been shown [9][18][25]. Stainless steel cathodes, which rapidly decrease oxygen when aided by a bacterial biofilm, have also been reported to undergo microbial-supported decline. Platinum (Pt) catalysts are commonly used for dissolved oxygen [30][31][32][33][34][35][36][37][38][39][40][41][42][43] or open-air cathodes, enhancing oxygen reduction rates. The platinum load for the MFC can be retained as low as 0.1 mg/cm2 to reduce costs [49]. Platinum’s long-term strength needs to be further examined, and new types of low-cost catalysts are still needed. MFC cathodes made of pyrolyzed iron (II) phthalocyanine have recently been recommended as noble-metal-free catalysts [17][49].
In numerous studies, carbon-based cathode electrodes have assisted in= significantly advancing high catalytic activity and performance. One investigation on heteroatom-doped carbon, for instance, showed significant cathodic performance (1328.9 mW/m2) that was comparable to the traditional catalyst Pt/C utilized in cathode cells (1337.7 mW/m2) [17]. It has been suggested that cathodes made from carbon fiber cloth, polyvinylidene fluoride, and the catalysts Mn, O, Fe, and C could serve as cathode-viable electrodes for wastewater treatment, with low operating costs and appropriate removal efficiency. According to research, 90% of the COD, 80% of the ammonium, and 65% of the total phosphorous were successfully eliminated [19]. A study carried out in a stacked MFC with a fed-batch operating time of 48 h successfully removed 97% COD from the wastewater while achieving a maximum power density of 1.7 W/m3. Granular activated carbon (GAC) compact electrodes were utilized in the experiment [17]. Remarkable MFC performance was attained when graphite plates were used in both electrodes, resulting in a power density of 1771 mW/m2 [17]. Additionally, 90% oxidative removal of tetracycline hydrochloride by reactive oxygen species was achieved using incapacitated GAC on the cathode. There has been a lot of attention on carbon and metal nanoparticles with novel combinations and varied dimensional structures [17]. A thorough study was conducted on the utilization of a bio-cathode to remove carbon and nitrogen while also generating electricity [17].
2.2.3. Cation Exchange Membrane (CEM)/Proton Exchange Membrane (PET)
This section acts as the rudimentary passage to the precise protons that are released during the reduction of the wastewater as the suitable substrate biodegrades and produces the viable electrons and protons in the process of electrogenation. A CEM is required in mainstream MFC designs to partition the anode and cathode chambers. Natural separation systems, such as sediment MFCs [2][3][4][51] and, particularly, fabricated single-compartment MFCs [14][52][53], are exceptions. The concept and implementation of ion exchange membranes are gradually rising, demanding more broad and vital studies to measure the membrane’s impact on performance and lasting steadiness [11][17][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54]. Rossi et al. [55][56][57][58][59][60] studied the effect of an anion exchange membrane (AEM) utilized to create a membrane electrode assembly (MEA) in an MFC, with the anode, AEM, and cathode placed closely together to improve the movement of hydroxide ions from the cathode to the anode, eliminate pH imbalances, and shorten electrode distance. The MFC produced 5.7 W/m2 using a flow-through felt anode. Due to the effects of localized pH on the performance of the electrode, MFCs may be restricted to low power densities. The anodic biofilm’s acidification reduces the amount of current the bacteria can produce, and the oxygen reduction reaction’s increase in cathode pH lowers the potential of the entire cell. In light of the aforementioned facts about some aspects of ion exchange membrane technology, it can be concluded that most MFC applications can achieve practical scale-up power by using this technology, which is better suited for high power densities and more compatible with the thermodynamics of bioenergy production by active bacterial species with few restrictions.
2.3. Summary of Challenges and Improvements of Electrode Materials of Construction towards Scaling Up
The procedure, application, and general design of the CEM/PEM membranes and electrodes largely affect the overall performance and cost-effectiveness of the MFC system. The performance of MFC can be enhanced by using efficient electrode materials. This is such that activation polarization losses in a fuel cell might vary depending on the anode materials employed. Evaluating an effective electrode material is one of the biggest difficulties for the MFC to operate as an affordable and accessible technology [17]. As such, both in anode and cathode configurations, Pt and Pt electrodes outperform carbon and graphite-based electrodes, despite being much more expensive [17][49]. Studies reveal that the least resistant electrode materials are the most effective, so it is vital to measure their resistance power in order to select the most effective electrode materials [17]. The ideal electrode material, applicable to both anode and cathode, should be non-flammable, conductive, non-fouling, and affordable. In large-scale applications, adopting a high-efficiency electrode material such as platinum is not economically viable and perhaps impractical, according to the literature and aforementioned sections [18. As a result, current MFC research has shifted focus towards improving the overall fabrication, installation, and operational cost-effectiveness of this system with commercially viable electrodes and membranes in overall a more feasible and realistic operation methodology of this technique. Wei et al. [17][18][19] concentrated on the significance of the high conductivity and mechanical strength required for effective electron transport in the realm of material properties. To increase bacterial adherence, the electrode’s surface area needs to be augmented and alienated into several different conformations [19]. The literature claims that a nanoparticle-modified electrode produces more power than a regular electrode [17][49].
References
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Jafari, T.; Haghparast, F. Microbial Fuel Cell a Reliable Source for Recovery of Electrical Power from Synthetic Wastewater. Linnaeus Eco-Tech. 2010, 627–635.
- Tamboli, E.; Eswari, J.S. Microbial fuel cell configurations: An overview. In Microbial Electrochemical Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 407–435.
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807.
- Shah, S.; Venkatramanan, V.; Prasad, R. Microbial fuel cell: Sustainable green technology for bioelectricity generation and wastewater treatment. In Sustainable Green Technologies for Environmental Management; Springer: Berlin/Heidelberg, Germany, 2019.
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Jafary, T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 2011, 88, 3999–4004.
- Min, B.; Kim, J.R.; Oh, S.E.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968.
- Noori, M.T.; Ganta, A.; Tiwari, B.R. Recent Advances in the Design and Architecture of Bioelectrochemical Systems to Treat Wastewater and to Produce Choice-Based Byproducts. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020023.
- Pant, D.; Singh, A.; Van Bogaert, G.; Gallego, Y.A.; Diels, L.; Vanbroekhoven, K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: Relevance and key aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313.
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer-can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106.
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475.
- Kumar, G.; Saratale, R.G.; Kadier, A.; Sivagurunathan, P.; Zhen, G.; Kim, S.H.; Saratale, G.D. A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production. Chemosphere 2017, 177, 84–92.
- Tabassum, N.; Islam, N.; Ahmed, S. Progress in microbial fuel cells for sustainable management of industrial effluents. Process Biochem. 2021, 106, 20–41.
- Rabaey, K.; Rodríguez, J.; Blackall, L.L.; Keller, J.; Gross, P.; Batstone, D.; Verstraete, W.; Nealson, K.H. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007, 1, 9–18.
- Gudiukaite, R.; Nadda, A.K.; Gricajeva, A.; Shanmugam, S.; Nguyen, D.D.; Lam, S.S. Bioprocesses for the recovery of bioenergy and value-added products from wastewater: A review. J. Environ. Manag. 2021, 300, 113831.
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482.
- Lay, C.H.; Kokko, M.E.; Puhakka, J.A. Power generation in fed-batch and continuous up-flow microbial fuel cell from synthetic wastewater. Energy 2015, 91, 235–241.
- Ahmed, S.F.; Mofijur, M.; Islam, N.; Parisa, T.A.; Rafa, N.; Bokhari, A.; Klemeš, J.J.; Mahlia, T.M.I. Insights into the development of microbial fuel cells for generating biohydrogen, bioelectricity, and treating wastewater. Energy 2022, 254, 124163.
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232.
- Ogugbue, C.J.; Ebode, E.E.; Leera, S. Electricity generation from swine wastewater using microbial fuel cell. J. Ecol Eng. 2015, 16, 26–33.
- Wang, H.; Park, J.; Do, R.Z.J. Practical energy harvesting for microbial fuel cells: A review. Environ. Sci. Technol. 2015, 49, 3267–3277.
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334.
- Ren, L.; Ahn, Y.; Logan, B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014, 48, 4199–4206.
- Fan, Y.; Hu, H.; Liu, H. Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 2007, 171, 348–354.
- Sharif, H.M.A.; Farooq, M.; Hussain, I.; Ali, M.; Mujtaba, M.A.; Sultan, M.; Yang, B. Recent innovations for scaling up microbial fuel cell systems: Significance of physicochemical factors for electrodes and membranes materials. J. Taiwan Inst. Chem. Eng. 2021, 129, 207–226.
- Zhuang, L.; Zheng, Y.; Zhou, S.; Yuan, Y.; Yuan, H.; Chen, Y. Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment. Bioresour. Technol. 2012, 106, 82–88.
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496.
- Wang, C.-T.; Huang, R.-Y.; Lee, Y.-C.; Zhang, C.-D. Electrode Material of Carbon Nanotube/Polyaniline Carbon Paper Applied in Microbial Fuel Cells. J. Clean Energy Technol. 2013, 1, 206–210.
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435.
- Pattanayak, P.; Pramanik, N.; Kumar, P.; Kundu, P.P. Fabrication of cost-effective non-noble metal supported on conducting polymer composite such as copper/polypyrrole graphene oxide (Cu2O/PPy–GO) as an anode catalyst for methanol oxidation in DMFC. Int. J. Hydrog. Energy 2018, 43, 11505–11519.
- Zhang, F.; Brastad, K.S.; He, Z. Integrating forward osmosis into microbial fuel cells for wastewater treatment, water extraction and bioelectricity generation. Environ. Sci. Technol. 2011, 45, 6690–6696.
- Cabrera, J.; Irfan, M.; Dai, Y.; Zhang, P.; Zong, Y.; Liu, X. Bioelectrochemical system as an innovative technology for treatment of produced water from oil and gas industry: A review. Chemosphere 2021, 285, 131428.
- Kumar, G.; Bakonyi, P.; Zhen, G.; Sivagurunathan, P.; Koók, L.; Kim, S.H.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. Microbial electrochemical systems for sustainable biohydrogen production: Surveying the experiences from a start-up viewpoint. Renew. Sustain. Energy Rev. 2017, 70, 589–597.
- Mathuriya, A.S. Eco-affectionate face of microbial fuel cells. Crit. Rev. Environ. Sci. Technol. 2014, 44, 97–153.
- Lai, M.F.; Lou, C.W.; Lin, J.H. Improve 3D electrode materials performance on electricity generation from livestock wastewater in microbial fuel cell. Int. J. Hydrog. Energy 2018, 43, 11520–11529.
- Kalathil, S.; Lee, J.; Cho, M.H. Granular activated carbon based microbial fuel cell for simultaneous decolorization of real dye wastewater and electricity generation. N. Biotechnol. 2011, 29, 32–37.
- Kim, B.H.; Chang, I.S.; Gadd, G.M. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 2007, 76, 485–494.
- Chung, K.; Okabe, S. Continuous power generation and microbial community structure of the anode biofilms in a three-stage microbial fuel cell system. Appl. Microbiol. Biotechnol. 2009, 83, 965–977.
- Scott, K.; Rimbu, G.A.; Katuri, K.P.; Prasad, K.K.; Head, I.M. Application of modified carbon anodes in microbial fuel cells. Process Saf. Environ. Prot. 2007, 85, 481–488.
- Cheng, S.; Liu, H.; Logan, B.E. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ. Sci. Technol. 2006, 40, 2426–2432.
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346.
- Huggins, T.M.; Pietron, J.J.; Wang, H.; Ren, Z.J.; Biffinger, J.C. Graphitic biochar as a cathode electrocatalyst support for microbial fuel cells. Bioresour. Technol. 2015, 195, 147–153.
- Ter Heijne, A.; Liu, F.; Van Rijnsoever, L.S.; Saakes, M.; Hamelers, H.V.M.; Buisman, C.J.N. Performance of a scaled-up Microbial Fuel Cell with iron reduction as the cathode reaction. J. Power Sources 2011, 196, 7572–7577.
- HaoYu, E.; Cheng, S.; Scott, K.; Logan, B. Microbial fuel cell performance with non-Pt cathode catalysts. J. Power Sources 2007, 171, 275–281.
- Salahuddin, M.; Uddin, M.N.; Hwang, G.; Asmatulu, R. Superhydrophobic PAN nanofibers for gas diffusion layers of proton exchange membrane fuel cells for cathodic water management. Int. J. Hydrog. Energy 2018, 43, 11530–11538.
- Gu, H.Y.; Zhang, X.W.; Li, Z.J.; Lei, L.C. Studies on treatment of chlorophenol-containing wastewater by microbial fuel cell. Chin. Sci. Bull. 2007, 52, 3448–3451.
- Xiao, B.; Yang, F.; Liu, J. Enhancing simultaneous electricity production and reduction of sewage sludge in two-chamber MFC by aerobic sludge digestion and sludge pretreatments. J. Hazard. Mater. 2011, 189, 444–449.
- Jung, R.K.; Dec, J.; Bruns, M.A.; Logan, B.E. Removal of odors from swine wastewater by using microbial fuel cells. Appl. Environ. Microbiol. 2008, 74, 2540–2543.
- Anwar, A.; Siddique, M.; Eyup Dogan Sharif, A. The moderating role of renewable and non-renewable energy in environment-income nexus for ASEAN countries: Evidence from Method of Moments Quantile Regression. Renew. Energy 2021, 164, 956–967.
- Muchtar, A.; Tambunan, A.H.; Machfud, M. Preliminary Analysis of Single-Flash Geothermal Power Plant by Using Exergy Method: A Case Study from Ulubelu Geothermal Power Plant in Indonesia Bioethanol from Brown Seaweed View project Production System Design of Banana based Energy Bar Industry View Project. 2018. Available online: https://www.researchgate.net/publication/335175433 (accessed on 21 January 2022).
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119.
- Wang, B.; Han, J.I. A single chamber stackable microbial fuel cell with air cathode. Biotechnol. Lett. 2009, 31, 387–393.
- Kaku, N.; Yonezawa, N.; Kodama, Y.; Watanabe, K. Plant/microbe cooperation for electricity generation in a rice paddy field. Appl. Microbiol. Biotechnol. 2008, 79, 43–49.
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880.
- Zuo, Y.; Cheng, S.; Call, D.; Logan, B.E. Tubular membrane cathodes for scalable power generation in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3347–3353.
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518.
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular microbial fuel cells for efficient electricity generation. Environ. Sci. Technol. 2005, 39, 8077–8082.
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A novel UASB-MFC-BAF integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour. Technol. 2009, 100, 5687–5693.
- Sivasankaran, A.; Sangeetha, D.; Ahn, Y.H. Nanocomposite membranes based on sulfonated polystyrene ethylene butylene polystyrene (SSEBS) and sulfonated SiO2 for microbial fuel cell application. Chem. Eng. J. 2016, 289, 442–451.
- Sil, A.; Ijeri, V.S.; Srivastava, A.K. Coated-wire iron(III) ion-selective electrode based on iron complex of 1,4,8,11-tetraazacyclotetradecane. Sens. Actuators B Chem. 2005, 106, 648–653.
- Rossi, R.; Wang, X.; Logan, B.E. High performance flow through microbial fuel cells with anion exchange membrane. J. Power Sources 2020, 475, 228633.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
18 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




