| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eva Paz Jimenez | + 3899 word(s) | 3899 | 2020-11-19 06:54:38 | | | |
| 2 | Rita Xu | -2152 word(s) | 1747 | 2020-12-09 10:29:44 | | |
Video Upload Options
This work explores the potential research opportunities and challenges of 3D printed biodegradable composite-based scaffolds containing carbon-based nanomaterials for bone tissue engineering applications. Bone possesses an inherent capacity to fix itself. However, when a defect larger than a critical size appears, external solutions must be applied. Traditionally, autograft has been the most used solution in these situations. However, it presents some issues such as donor-site morbidity. In this context, porous biodegradable scaffolds have emerged as an interesting solution. For adequate performance, these scaffolds must meet specific requirements: biocompatibility, interconnected porosity, mechanical properties, and biodegradability. The development of additive manufacturing methods has proposed a promising solution for this application since they allow the complete customization and control of scaffolds geometry and porosity. Furthermore, carbon-based nanomaterials present the potential to impart osteoconductivity and antimicrobial properties and reinforce the matrix from a mechanical perspective. These properties make them ideal for use as nanomaterials to improve the properties and performance of scaffolds for bone tissue engineering.
1. Bone Defect Healing
1.1. Natural Process of Bone Healing
Bone is composed of a mineralised organic matrix and cells. The matrix provides the bone with its mechanical properties and is comprised of organic and inorganic phases. In the organic phase, type I collagen is the major component and it is responsible for the tensile properties of the bone. Conversely, the inorganic phase comprises hydroxyapatite, which is responsible for exhibiting the compressive properties and for providing the building blocks for new bone formation. Cells are embedded in the matrix, which includes osteoblasts, osteoclasts, osteoprogenitor cells and mature osteocytes [1].
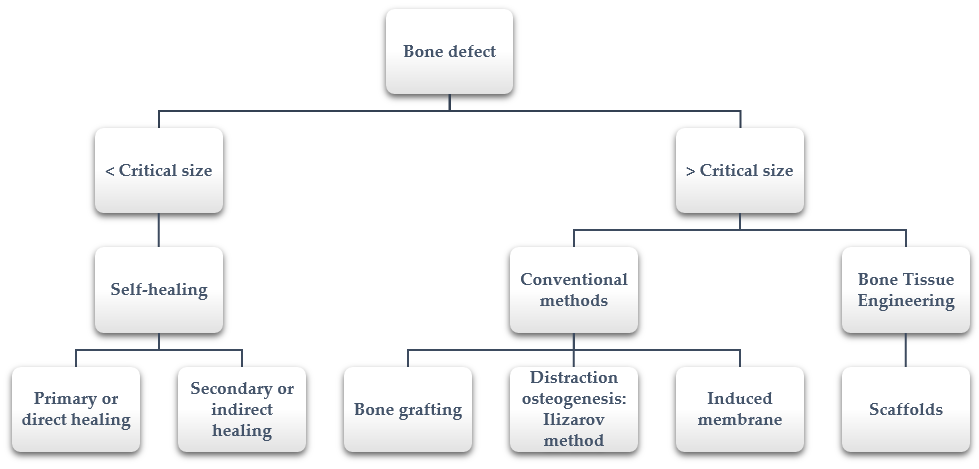
Bone fractures and segmental bone defects are often caused by traumatic injury, cancer or other diseases (e.g., osteoporosis or arthritis) [2]. When a defect appears, the spontaneous fracture healing process begins through two different mechanisms, depending on the mechanical environment. If the strain across the fracture site is less than 2%, primary or direct healing by internal remodeling occurs, whilst secondary or indirect healing by callus formation takes place when strain is between 2 and 10%. The latter type of healing is the process that most fractures follow and it depends on osteogenesis, osteoinductivity and osteoconductivity [3].
However, sometimes bones defect cannot heal spontaneously. This situation occurs especially in large segmental bone defects when the defect reaches a critical size [4]. The precise critical size depends on several factors (i.e., anatomic location, age of the patient, soft tissue environment); in the literature, it is suggested to include defect lengths greater than 1–2 cm and greater than 50% loss of the circumference of the bone [5]. In these cases, additional surgical interventions that help and allow bone healing are required. The available healing processes depending on the size defect are summarized in Figure 1.
Figure 1. Bone defect healing: from self-healing to bone tissue engineering.
1.2. Conventional Surgical Solutions
Among the conventional surgical solutions, it is important to highlight bone grafting, distraction osteogenesis and induced membrane techniques [4].
Autologous [6], allograft [7] and synthetic bone grafting [8] are extensively used for repairing bone defects. More specifically, autograft represents the gold standard for the treatment of critical-sized bone defects since it contains all the characteristics for new bone growth (osteoconductivity, osteogenicity and osteoinductivity). However, significant problems are associated with its use, from donor-site morbidity to a limited amount of donor bone [9][10][11]. Other associated issues, such as failed anastomosis, microvascular thrombosis and infection or progressive deformities, have also been reported [12][13].
Another technique extensively studied since the 1950s is distraction osteogenesis. Ilizarov successfully treated his first patient in 1954, reducing the healing time of tibial non-union [14]. This method is based on the capacity of regeneration under tension that the bone presents naturally. Despite the good results that the Ilizarov technique present [15][16], it also has some disadvantages, such as prolonged treatment times, pin site infection [17][18], pin breakage and the inconvenience and burden of prolonged external fixation, which includes muscle contractures, joint luxation and axial deviation [19][20][21][22].
Finally, the induced membrane technique is a two-stage procedure that combines the use of a temporary poly(methyl methacrylate) (PMMA) cement spacer, followed by bone grafting [23]. In 2000, the first cases using the induced membrane technique were reported [24]. Generally, this technique achieves its purpose; however, some complications have been reported. The most common complications include infection, amputation, malunion, fracture and reoperation and additional bone grafting due to non-union [24][25][26].
1.3. Scaffolds for Bone Regeneration
To avoid problems encountered when using conventional methods, the field of bone tissue engineering was developed in the early 1980s [27]. Researchers working in the field of bone tissue engineering have made an important effort for developing 3D porous matrices, known as scaffolds. They are based on guided bone regeneration, the aim of which is bone regeneration and growth along the surface of the scaffold [28].
Scaffolds act as a temporary matrix for the attachment, viability and growth of cells whilst maintaining the structure of the regenerated bone in vivo [29]. The main advantage of bone tissue engineering is the potential elimination of donor scarcity, pathogen transfer and immune rejection [30].
Ideally, to properly promote bone regeneration, scaffolds should meet specific requirements [29][31][32]:
- The material and its degradative by-products should be biocompatible and not evoke inflammation or toxicity when implanted in vivo.
- Three-dimensional structures should be manufactured in a reproducible manner.
- High surface area is needed for cell–polymer interactions, extracellular matrix regeneration and minimal diffusion constraints. It is achieved with a porosity of at least 90% and pore size of at least 100 μm [29]. Furthermore, it should have an interconnected porous structure, with a pore size suitable to allow cell adhesion, growth, vascularisation of the tissue and transportation of nutrients.
- Scaffolds should be capable of being resorbed once their function of providing a template for regenerating bone has completed. Permanent foreign materials inside the body could lead to a permanent risk of inflammation.
- The degradation or the resorption rate and the rate of bone formation should be similar. For this reason, the degradation rate of the scaffold should have the potential to be adjustable depending on the cell type.
- Scaffolds should also demonstrate mechanical properties similar to bone.
It is important to highlight that porosity and mechanical properties have an inverse relation. For this reason, a compromise must be found between these characteristics.
1.4. Limitation of Bone Tissue-Engineered Scaffolds
Despite the great research advancements in the design, manufacture and application of bone tissue-engineered scaffolds for bone repair and replacement, there still are some drawbacks and challenges that need to be addressed. The main drawbacks of synthetic scaffolds are: poor biodegradability, potential toxic degradation of by-products, poor osteoconductivity, poor mechanical properties, uncontrolled porosity or complicated reproducibility [33].
2. Nanomaterials for Scaffolds
2.1. Why Are Nanomaterials a Potential Solution?
The use of nanocomposite biomaterials in bone tissue engineering has emerged to improve the mechanical properties as well as physicochemical properties of the polymeric matrix, such as mechanical strength and Young’s modulus, hydrophilicity or biological response (e.g., cell adhesion, proliferation and differentiation, biocompatibility and antimicrobial effect). Nanocomposites for biomedical applications normally have two phases: a biocompatible matrix and a nanosized bioactive/resorbable filler [34][35][36]. One of the main advantages of nanomaterials is their large surface area, which results in a large volume fraction of interfacial material (even at low loadings).
In general, by controlling the volume fraction, arrangement and morphology of the filler phase within the matrix, it is possible to tailor the physicochemical and mechanical properties and the response to the host tissue [27]. In this section, some of the most interesting data relating to the application of nanocomposites in bone tissue engineering will be reported; the application of carbon-based nanomaterials will especially be considered.
2.2. Ceramic Nanomaterials
Ceramic nanomaterials, such as calcium phosphates or calcium silicates, may improve the biological response by releasing calcium and phosphate ions that are essential for bone growth. However, a detrimental effect on the mechanical properties has been reported when the amount of inorganic particles is high [37].
The most commonly used ceramic-based nanomaterial is nanohydroxyapatite (nHA). It demonstrates excellent biocompatibility and low toxicity. Several matrices have been filled with nHA (thermoplastic polyurethane (TPU)/polydimethylsiloxane (PDMS), poly ε-caprolactone (PCL), polylactic acid (PLA), etc.) and, in all cases, it has been observed that by comparing the nanocomposite with the pristine matrix, they show a reduction in hydrophobicity, as well as an increase in cell proliferation, mineralisation and differentiation [38][39][40][41].
Calcium phosphate nanoparticles have proved to improve mechanical resistance and the attachment and proliferation of osteoblasts and can demonstrate an antibacterial effect [42][43].
Other ceramics, such as nanosized aluminium oxide [44], titanium oxide [45] or silica [46], have been shown to augment different properties that make them very interesting as potential nanomaterials for bone tissue engineering applications.
2.3. Metallic Nanomaterials
In general, metallic nanomaterials are interesting in bone tissue engineering due to the antimicrobial and bactericidal activity that some of them demonstrate.
Silver nanoparticles are well-known for their antimicrobial activity against a broad spectrum of infectious agents [47]. Specifically, silver ions present a marked antibacterial effect since they cause a disruption of bacteria cell membranes and inhibit enzymatic activities and DNA replication [48]. In the same way, copper and bronze also present bactericidal nature [49].
Gold nanoparticles have also been used to create a polymer nanocomposite due to their inherent low toxicity and antiseptic and antibacterial activity, which prevent bacterial growth in the surgical wound [50][51].
Other metal ions, such as strontium or copper, have been widely used to dope bioactive glasses, improving their osteogenesis, angiogenesis and antibacterial activity [52][53].
Another interesting metal extensively used in bone tissue engineering is magnesium. It presents high mechanical properties, specific strength and elastic modulus, and has a good biodegradability and biocompatibility. However, its high degradation rate limits its application as a matrix material, but the presence of magnesium as a nanomaterial induces osteogenic differentiation [54][55].
2.4. Polymer Nanomaterials
Polymers are not used as nanomaterials in bone tissue engineering, but as a coating for other nanomaterials or to enable modification of the matrix. For instance, poly(acrylic acid) or poly(methacrylic acid) grafted to carbon nanotubes improves the potential for cell differentiation of scaffolds [56][57].
In the case of polymers added to the matrix, there are two different paths: co-polymers, formed by two or more monomeric species, and polymer–polymer blends, which involve a mixture of two polymers [27]. Among the co-polymers used in bone tissue engineering, poly(lactic-co-glycolic acid) (PLGA) [58][59] and poly(lactide-co-caprolactone) [60][61] are the most commonly used. Conversely, in the field of polymer blends, many studies are found on gelatin-polyvinyl pyrrolidone [62], gelatin-poly(lactide acid) [63], cellulose acetate-polycaprolactone [64], polyurethane/poly(lactic acid) [65], poly(lactide acid)/polycaprolactone [66][67], etc. Sometimes, they are incorporated into a ceramic matrix to improve their toughness and processability. Both synthetic [68][69] and natural [70] polymers are used for this purpose.
References
- Jones, M.S.; Waterson, B. Principles of management of long bone fractures and fracture healing. Surgery 2020, 38, 91–99, doi:10.1016/j.mpsur.2019.12.010.
- Jimi, E.; Hirata, S.; Osawa, K.; Terashita, M.; Kitamura, C.; Fukushima, H. The current and future therapies of bone regeneration to repair bone defects. J. Dent. 2012, 2012, 148261, doi:10.1155/2012/148261.
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404, doi:10.1016/j.injury.2005.07.019.
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. J. Orthop. Surg. Traumatol. 2018, 28, 351–362, doi:10.1007/s00590-017-2063-0.
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-size bone defects: Is there a consensus for diagnosis and treatment? Orthop. Trauma 2018, 32, S7–S11, doi:10.1097/BOT.0000000000001115.
- Sohn, J.M.; In, Y.; Jeon, S.H.; Nho, J.Y.; Kim, M.S. Autologous impaction bone grafting for bone defects of the medial tibia plateau during primary total knee arthroplasty: Propensity score matched analysis with a minimum of 7-year follow-up. Arthroplasty 2018, 33, 2465–2470, doi:10.1016/j.arth.2018.02.082.
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Podiatr. Med. Surg. 2015, 32, 21–34, doi:10.1016/j.cpm.2014.09.011.
- Kumar, V.; Ricks, M.; Aboul-Enin, S.; Dunlop, D.G. Long term results of impaction Bone grafting using a synthetic graft (Apapore) in revision hip surgery. Orthop. 2017, 14, 290–293, doi:10.1016/j.jor.2017.03.013.
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15, doi:10.1016/j.injury.2011.06.015.
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. Appl. Biomater. 1991, 2, 187–208, doi:10.1002/jab.770020307.
- Vail, T.P.; Urbaniak, J.R. Donor-site morbidity with use of vascularized autogenous fibular grafts. Bone Jt. Surg. 1996, 78, 204–211, doi:10.2106/00004623-199602000-00006.
- Arai, K.; Toh, S.; Tsubo, K.; Nishikawa, S.; Narita, S.; Miura, H. Complications of vascularized fibula graft for reconstruction of long bones. Reconstr. Surg. 2002, 109, 2301–2306, doi:10.1097/00006534-200206000-00021.
- Muramatsu, K.; Ihara, K.; Shigetomi, M.; Kawai, S. Femoral reconstruction by single, folded or double free vascularised fibular grafts. J. Plast. Surg. 2004, 57, 550–555, doi:10.1016/j.bjps.2003.08.021.
- Spiegelberg, B.; Parratt, T.; Dheerendra, S.K.; Khan, W.S.; Jennings, R.; Marsh, D.R. Ilizarov principles of deformity correction. R. Coll. Surg. Engl. 2010, 92, 101–105, doi:10.1308/003588410X12518836439326.
- Toon, D.H.; Khan, S.A.; Wong, K.H.Y. Lengthening of a below knee amputation stump with Ilizarov technique in a patient with a mangled leg. J. Traumatol.-Engl. Ed. 2019, 22, 364–367, doi:10.1016/j.cjtee.2019.07.001.
- Cai, G.; Liu, W.; Xiong, J.; Liu, L.; Wang, D.; Yang, J. Functional reconstruction of hindfoot with total calcaneus and talus loss by ilizarov technique: A case r J. Foot Ankle Surg. 2020, 59, 142–148, doi:10.1053/j.jfas.2019.03.022.
- Stuart,; Green, M.D. Skeletal defects. A comparison of bone grafting and bone transport for segmental skeletal defects. Clin. Orthop. Relat. Res. 1994, 301, 111–117.
- Blum, A.L.L.; Bongiovanni, J.C.; Morgan, S.J.; Flierl, M.A.; Dos Reis, F.B. Complications associated with distraction osteogenesis for infected nonunion of the femoral shaft in the presence of a bone defect: A retrospective series. Bone Joint Surg. Br. 2010, 92, 565–570, doi:10.1302/0301-620X.92B4.23475.
- Paley, D. Problems, obstacles, and complications of limb lengthening by Illizarov. Orthop. Relat. Res. 1990, 250, 81–104.
- Palatnik, Y.; Rozbruch, S.R. Femoral reconstruction using external fixation. Orthop. 2011, 2011, 967186, doi:10.4061/2011/967186.
- Paley, D.; Catagni, M.; Argnani, F.; Prevot, J.; Bell, D.; Armstrong, P. Treatment of congenital pseudoarthrosis of the tibia using the ilizarov techniqu Clin. Orthop. Relat. Res. 1992, 280, 81–93.
- Zhai, J.; Weng, X.; Zhang, B.; Peng, H.; Bian, Y. Management of knee flexion contracture in haemophilia with the Ilizarov technique. Knee 2019, 26, 201–206, doi:10.1016/j.knee.2018.08.006.
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defect Orthop. Clin. N. Am. 2010, 41, 27–37, doi:10.1016/j.ocl.2009.07.011.
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Chir. Plast. Esthet. 2000, 45, 346–353.
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461, doi:10.1016/j.biomaterials.2014.03.055.
- Seebach, C.; Henrich, D.; Kähling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto β-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng.-Part A 2010, 16, 1961–1970, doi:10.1089/ten.tea.2009.0715.
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Rev. Biomed. Eng. 2012, 40, 363–408, doi:10.1615/CritRevBiomedEng.v40.i5.10.
- Kellomäki, M.; Niiranen, H.; Puumanen, K.; Ashammakhi, N.; Waris, T.; Törmälä, P. Bioabsorbable scaffolds for guided bone regeneration and generation. Biomaterials 2000, 21, 2495–2505, doi:10.1016/S0142-9612(00)00117-4.
- Meskinfam, M. 17—Polymer scaffolds for bone regeneration. In Characterization of Polymeric Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 441–475, ISBN 9780081007372.
- Chapekar, M.S. Tissue engineering: Challenges and opportunities. Biomed. Mater. Res. 2000, 53, 617–620, doi:10.1002/1097-4636(2000)53:6<617::AID-JBM1>3.0.CO;2-C.
- Freed, L.E.; Vunjak-Novakoric, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Biotechnology 1994, 12, 689–693.
- Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Int. 2019, 45, 21051–21061, doi:10.1016/j.ceramint.2019.07.096.
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 2016, 71, 353–366, doi:10.1515/biolog-2016-0056.
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Mater. 2015, 27, 1143–1169, doi:10.1002/adma.201403354.
- Bonfield, W.; Grynpas, M.D.; Tully, A.E.; Bowman, J.; Abram, J. Hydroxyapatite reinforced polyethylene - a mechanically compatible implant material for bone replacement. Biomaterials 1981, 2, 185–186, doi:10.1016/0142-9612(81)90050-8.
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Sci. Eng. C 2020, 110, 110698, doi:10.1016/j.msec.2020.110698.
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly porous polycaprolactone scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for bone regeneration. Sci. Eng. C 2019, 102, 341–361, doi:10.1016/j.msec.2019.04.040.
- Drupitha, M.P.; Das, B.; Parameswaran, R.; Dhara, S.; Nando, G.B.; Naskar, K. Hybrid electrospun fibers based on TPU-PDMS and spherical nanohydroxyapatite for bone tissue engineering. Today Commun. 2018, 16, 264–273, doi:10.1016/j.mtcomm.2018.06.013.
- Moeini, S.; Mohammadi, M.R.; Simchi, A. In-situ solvothermal processing of polycaprolactone/hydroxyapatite nanocomposites with enhanced mechanical and biological performance for bone tissue engineering. Mater. 2017, 2, 146–155, doi:10.1016/j.bioactmat.2017.04.004.
- Morelli, S.; Salerno, S.; Holopainen, J.; Ritala, M.; De Bartolo, L. Osteogenic and osteoclastogenic differentiation of co-cultured cells in polylactic acid-nanohydroxyapatite fiber scaffolds. Biotechnol. 2015, 204, 53–62, doi:10.1016/j.jbiotec.2015.03.023.
- Kim, M.H.; Yun, C.; Chalisserry, E.P.; Lee, Y.W.; Kang, H.W.; Park, S.H.; Jung, W.K.; Oh, J.; Nam, S.Y. Quantitative analysis of the role of nanohydroxyapatite (nHA) on 3D-printed PCL/nHA composite scaffolds. Lett. 2018, 220, 112–115, doi:10.1016/j.matlet.2018.03.025.
- Ba Linh, N.T.; Lee, K.H.; Lee, B.T. Functional nanofiber mat of polyvinyl alcohol/gelatin containing nanoparticles of biphasic calcium phosphate for bone regeneration in rat calvaria defects. Biomed. Mater. Res.-Part A 2013, 101A, 2412–2423, doi:10.1002/jbm.a.34533.
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/gelatin/chitosan/β-TCP electrospun composite for guided bone regeneration. Biomater. 2018, 7, 225–237, doi:10.1007/s40204-018-0098-x.
- Chern, M.J.; Yang, L.Y.; Shen, Y.K.; Hung, J.H. 3D scaffold with PCL combined biomedical ceramic materials for bone tissue regeneration. J. Precis. Eng. Manuf. 2013, 14, 2201–2207, doi:10.1007/s12541-013-0298-1.
- Arumugam, R.; Subramanyam, V.; Chinnadurai, R.K.; Srinadhu, E.S.; Subramanian, B.; Nallani, S. Development of novel mechanically stable porous nanocomposite (PVDF-PMMA/HAp/TiO2) film scaffold with nanowhiskers surface morphology for bone repair applications. Lett. 2019, 236, 694–696, doi:10.1016/j.matlet.2018.11.023.
- Guo, W.; Xu, L.; Feng, P.; Gu, Y.; Shuai, C. In-situ growth of silica nano-protrusions on halloysite nanotubes for interfacial reinforcement in polymer/halloysite scaffolds. Surf. Sci. 2020, 513, 145772, doi:10.1016/j.apsusc.2020.145772.
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. J. Biol. Macromol. 2018, 111, 923–934, doi:10.1016/j.ijbiomac.2018.01.089.
- Marsich, E.; Bellomo, F.; Turco, G.; Travan, A.; Donati, I.; Paoletti, S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: Preparation, characterization and biological properties. Mater. Sci. Mater. Med. 2013, 24, 1799–1807, doi:10.1007/s10856-013-4923-4.
- Alam, F.; Shukla, V.R.; Varadarajan, K.M.; Kumar, S. Microarchitected 3D printed polylactic acid (PLA) nanocomposite scaffolds for biomedical applications. Mech. Behav. Biomed. Mater. 2020, 103, 103576, doi:10.1016/j.jmbbm.2019.103576.
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K. International Journal of Biological Macromolecules Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. J. Biol. Macromol. 2020, 154, 62–71, doi:10.1016/j.ijbiomac.2020.03.095.
- Abdelrasoul, G.N.; Farkas, B.; Romano, I.; Diaspro, A.; Beke, S. Nanocomposite scaffold fabrication by incorporating gold nanoparticles into biodegradable polymer matrix: Synthesis, characterization, and photothermal effect. Sci. Eng. C 2015, 56, 305–310, doi:10.1016/j.msec.2015.06.037.
- Erol, M.; Özyuĝuran, A.; Özarpat, Ö.; Küçükbayrak, S. 3D Composite scaffolds using strontium containing bioactive glasses. Eur. Ceram. Soc. 2012, 32, 2747–2755, doi:10.1016/j.jeurceramsoc.2012.01.015.
- Gönen, S.Ö.; Taygun, M.; Küçükbayrak, S. Fabrication of bioactive glass containing nanocomposite fiber mats for bone tissue engineering applications. Compos. Struct. 2016, 138, 96–106, doi:10.1016/j.compstruct.2015.11.033.
- Golzar, H.; Mohammadrezaei, D.; Yadegari, A.; Rasoulianboroujeni, M.; Hashemi, M.; Omidi, M.; Yazdian, F.; Shalbaf, M.; Tayebi, L. Incorporation of functionalized reduced graphene oxide/magnesium nanohybrid to enhance the osteoinductivity capability of 3D printed calcium phosphate-based scaffolds. Part B Eng. 2020, 185, 107749, doi:10.1016/j.compositesb.2020.107749.
- Shen, J.; Wang, W.; Zhai, X.; Chen, B.; Qiao, W.; Li, W.; Li, P.; Zhao, Y.; Meng, Y.; Qian, S.; et al. 3D-printed nanocomposite scaffolds with tunable magnesium ionic microenvironment induce in situ bone tissue regeneration. Mater. Today 2019, 16, 493–507, doi:10.1016/j.apmt.2019.07.012.
- Chao, T.I.; Xiang, S.; Chen, C.S.; Chin, W.C.; Nelson, A.J.; Wang, C.; Lu, J. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biophys. Res. Commun. 2009, 384, 426–430, doi:10.1016/j.bbrc.2009.04.157.
- Chao, T.I.; Xiang, S.; Lipstate, J.F.; Wang, C.; Lu, J. Poly(methacrylic acid)-grafted carbon nanotube scaffolds enhance differentiation of hESCs into neuronal cells. Mater. 2010, 22, 3542–3547, doi:10.1002/adma.201000262.
- Xie, X.; Wang, W.; Cheng, J.; Liang, H.; Lin, Z.; Zhang, T.; Lu, Y.; Li, Q. Bilayer pifithrin-α loaded extracellular matrix/PLGA scaffolds for enhanced vascularized bone formation. Colloids Surfaces B Biointerfaces 2020, 190, 110903, doi:10.1016/j.colsurfb.2020.110903.
- Rasoulianboroujeni, M.; Fahimipour, F.; Shah, P.; Khoshroo, K.; Tahriri, M.; Eslami, H.; Yadegari, A.; Dashtimoghadam, E.; Tayebi, L. Development of 3D-printed PLGA/TiO2 nanocomposite scaffolds for bone tissue engineering applications. Sci. Eng. C 2019, 96, 105–113, doi:10.1016/j.msec.2018.10.077.
- Honda, M.; Morikawa, N.; Hata, K.; Yada, T.; Morita, S.; Ueda, M.; Kimata, K. Rat costochondral cell characteristics on poly (L-lactide-co-ε-caprolactone) scaffolds. Biomaterials 2003, 24, 3511–3519, doi:10.1016/S0142-9612(03)00210-2.
- Walejewska, E.; Idaszek, J.; Heljak, M.; Chlanda, A.; Choinska, E.; Hasirci, V.; Swieszkowski, W. The effect of introduction of filament shift on degradation behaviour of PLGA- and PLCL-based scaffolds fabricated via additive manufacturing. Degrad. Stab. 2020, 171, 109030, doi:10.1016/j.polymdegradstab.2019.109030.
- Mishra, R.; Varshney, R.; Das, N.; Sircar, D.; Roy, P. Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Polym. J. 2019, 119, 155–168, doi:10.1016/j.eurpolymj.2019.07.007.
- Chen, W.; Ma, J.; Zhu, L.; Morsi, Y.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Superelastic, superabsorbent and 3D nanofiber-assembled scaffold for tissue engineering. Colloids Surfaces B Biointerfaces 2016, 142, 165–172, doi:10.1016/j.colsurfb.2016.02.050.
- Xu, T.; Liang, Z.; Ding, B.; Feng, Q.; Fong, H. Polymer blend nanofibers containing polycaprolactone as biocompatible and biodegradable binding agent to fabricate electrospun three-dimensional scaffolds/structures. Polymer 2018, 151, 299–306, doi:10.1016/j.polymer.2018.07.074.
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D printing biocompatible polyurethane/poly(lactic acid)/graphene oxide nanocomposites: Anisotropic properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023, doi:10.1021/acsami.6b11793.
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Sci. Eng. C 2019, 104, 109960, doi:10.1016/j.msec.2019.109960.
- Shahrezaee, M.; Salehi, M.; Keshtkari, S.; Oryan, A.; Kamali, A.; Shekarchi, B. In vitro and in vivo investigation of PLA/PCL scaffold coated with metformin-loaded gelatin nanocarriers in regeneration of critical-sized bone defects. Nanomed. Nanotechnol. Med. 2018, 14, 2061–2073, doi:10.1016/j.nano.2018.06.007.
- Lin, Y.H.; Chuang, T.Y.; Chiang, W.H.; Chen, I.W.P.; Wang, K.; Shie, M.Y.; Chen, Y.W. The synergistic effects of graphene-contained 3D-printed calcium silicate/poly-ε-caprolactone scaffolds promote FGFR-induced osteogenic/angiogenic differentiation of mesenchymal stem cells. Sci. Eng. C 2019, 104, 109887, doi:10.1016/j.msec.2019.109887.
- Wu, C.; Xia, L.; Han, P.; Xu, M.; Fang, B.; Wang, J.; Chang, J.; Xiao, Y. Graphene-oxide-modified β-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon N. Y. 2015, 93, 116–129, doi:10.1016/j.carbon.2015.04.048.
- Cabral, C.S.D.; Miguel, S.P.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. Green reduced graphene oxide functionalized 3D printed scaffolds for bone tissue regeneration. Carbon N. Y. 2019, 146, 513–523, doi:10.1016/j.carbon.2019.01.100.