
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Homer Selton Black | -- | 1672 | 2022-11-11 19:26:52 | | | |
| 2 | Vivi Li | Meta information modification | 1672 | 2022-11-14 02:37:26 | | | | |
| 3 | Vivi Li | Meta information modification | 1672 | 2022-11-21 03:21:45 | | |
Video Upload Options
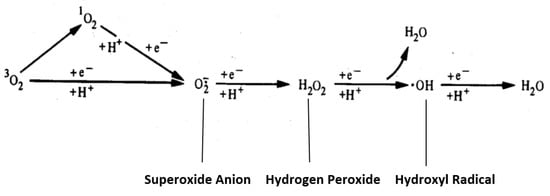
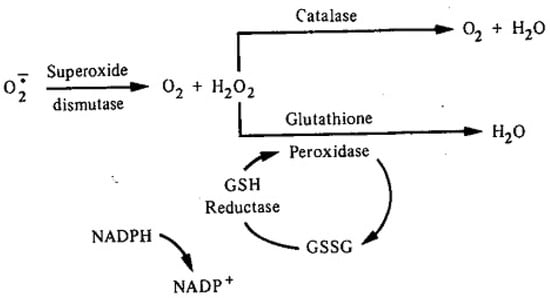
The Greek physician, Aretaios, coined the term “diabetes” in the 1st Century A.D. “Mellitus” arose from the observation that the urine exhibits a sweetness due to its elevated glucose levels. Diabetes mellitus (DM) accounted for 6.7 million deaths globally in 2021 with expenditures of USD 966 billion. Mortality is predicted to rise nearly 10-fold by 2030. Oxidative stress, an imbalance between the generation and removal of reactive oxygen species (ROS), is implicated in the pathophysiology of diabetes. Whereas ROS are generated in euglycemic, natural insulin-regulated glucose metabolism, levels are regulated by factors that regulate cellular respiration, e.g., the availability of NAD-linked substrates, succinate, and oxygen; and antioxidant enzymes that maintain the cellular redox balance. Only about 1–2% of total oxygen consumption results in the formation of superoxide anion and hydrogen peroxide under normal reduced conditions.
1. Introduction
2. Oxidative Stress, ROS, and Antioxidants
3. ROS Formation
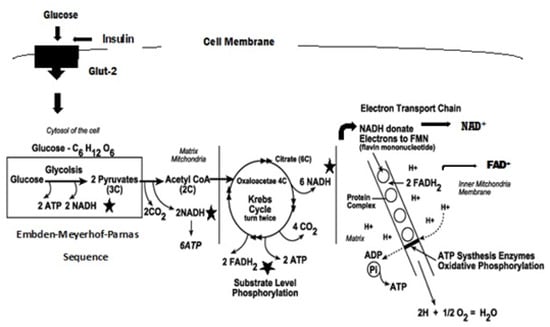
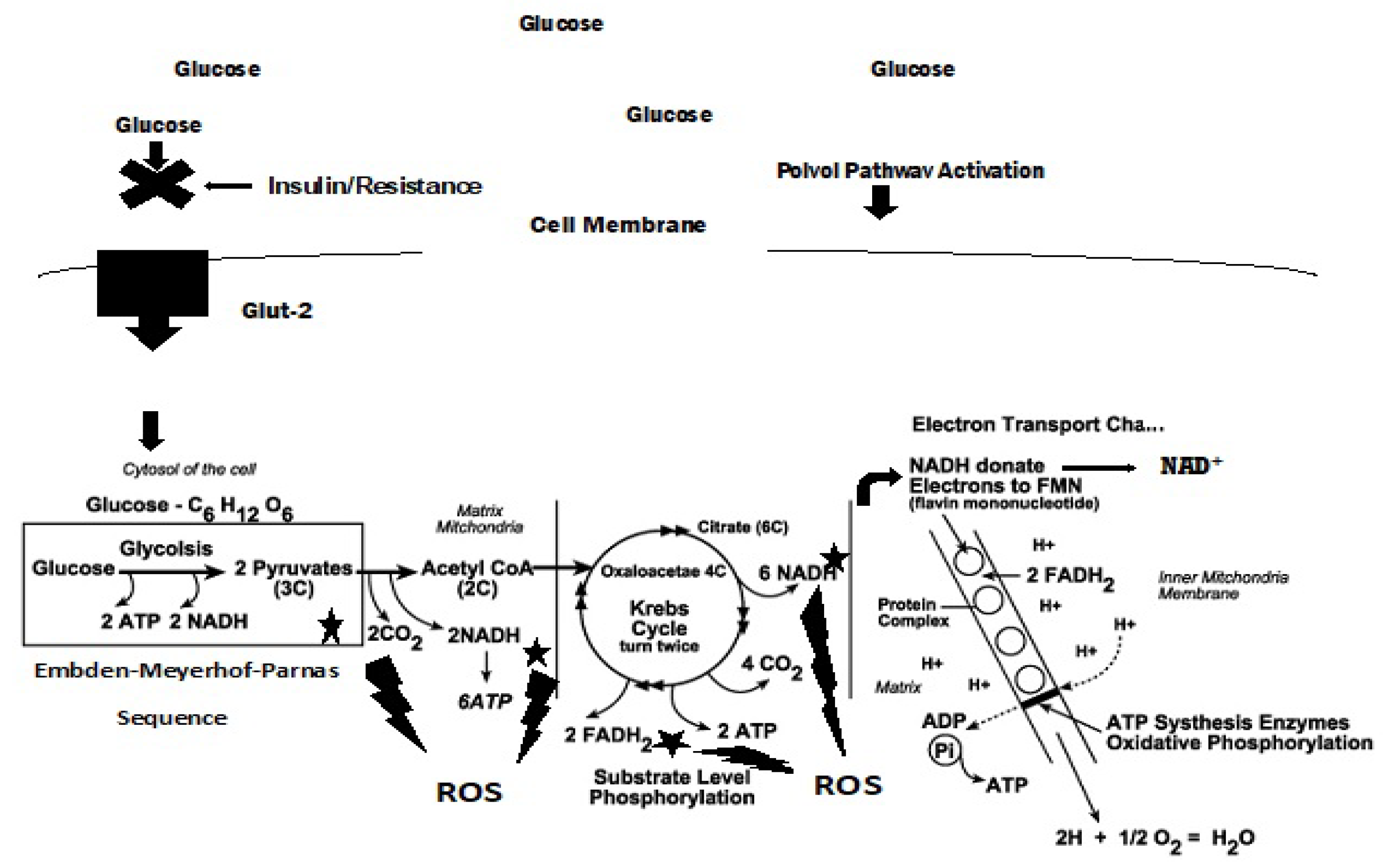
4. Electron Transport Chain, ROS Production, and Proton Pump Potential
References
- Ahmed, A.M. History of Diabetes Mellitus. Saudi Med. J. 2019, 23, 373–378.
- Polonsky, K.S. The Past 200 Years in Diabetes. N. Engl. J. 2012, 367, 1332–1340.
- Sarton, G. A History of Science. In Ancient Science through the Golden Age of Greece; Harvard University Press: Cambridge, MA, USA, 1952; p. 191.
- Dobson, M. Experiments and Observations on Urine in Diabetes; Medical Observations and Enquiries; T. Cadell: London, UK, 1776; pp. 298–316.
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 25 January 2022).
- Centers for Disease Control and Prevention. National Diabetes Statistics Report; Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services: Atlanta, GA, USA, 2020. Available online: https://cdc.gov/diabetes/data/statistics-report/index.html (accessed on 25 January 2022).
- American Diabetes Association. Diabetes Care. J. Clin. Appl. Res. Educ. 2022, 45 (Suppl. S1), S17–S36. Available online: https://www.diabetes.org/dibetescare (accessed on 11 February 2022).
- Himsworth, H. Diabetes mellitus: A differentiation into insulin-sensitive and insulin-insensitive types. Lancet 1936, 1, 127–130.
- Reaven, G.M. Role of Insulin Resistance in Human Disease. Diabetes 1988, 37, 15951607.
- Reaven, G.N. Syndrome X: A Short History. Ochsner J. 2001, 3, 124–125.
- Reaven, G.M. Role of Insulin Resistance in Human Disease (Syndrome X): An Expanded Definition. Annu. Rev. Med. 1993, 44, 121–131.
- Hung, P.L. A Comprehensive definition for Metabolic Syndrome. Dis. Models Mech. 2009, 2, 231–237.
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosos and Classification of Diabetes mellitus Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553.
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The Metabolic Syndrome—a New Worldwide definition. Lancet 2005, 366, 1059–1062.
- Kahn, R.; Buse, J.; Ferrannini, E.; Stern, M. The Metabolic Syndrome: Time for a Critical AppraisaL Joint Statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005, 28, 2289–2304.
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42, 510–515.
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; Conference Participants. Definition of Metabolic Syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology. J. Am. Heart Assoc. 2004, 24, e13–e18.
- Wright, E., Jr.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314.
- Turrens, J.F.; Freeman, B.A.; Crapo, J.D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Biochem. Biophys. 1982, 217, 411–421.
- Ceriello, A.; Quagliaro, L.; Catone, B.; Pascon, R.; Piazzola, M.; Bais, B.; Marra, G.; Tonutti, L.; Taboga, C.; Motz, E. Role of hyperglyceria in nitrotyrosine postprandial generation. Diabetes Care 2002, 25, 1439–1443.
- Proctor, P.H.; Reynolds, E.S. Free radicals and disease in man. Physiol. Chem. Phys. Med. NMR 1984, 16, 175–195.
- Black, H.S. Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem. Photobiol. 1987, 46, 213–221.
- Stringer, D.M.; Zahradka, P.; Taylor, C.G. Glucose transporters: Cellular links to hyperglycemia in insulin resistance and diabetes. Nutr. Rev. 2015, 73, 140–154.
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513.
- Hirst, J. Energy transduction by respiratory complex I—an evaluation of current knowledge. Biochem. Soc. Trans. 2005, 33, 525–529.
- Cardenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Bioenergetics 2018, 1859, 940–950.
- Trumpower, B.L. Cytochrome bc1 complex (Respirator Chain Complex III). In Encyclopedia of Biological Chemistry I; Elsevier Inc.: Amsterdam, The Netherlands, 2004; pp. 528–534.
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncb.n1m.nih.gov/books/NBK526105/ (accessed on 1 February 2022).




