| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiroshi Mitoma | + 2114 word(s) | 2114 | 2020-09-22 06:34:41 |
Video Upload Options
The terminology of cerebellar ataxias (CAs) embraces a ubiquitous deficit in 3 elemental domains: (a) limbs’ motor symptoms, (b) oculomotor symptoms, and (c) cognitive/emotional symptoms occurring in patients showing cerebellar dysfunction. Patients with CAs develop inaccuracy and clumsiness limb movements, characterized by dysmetria, tremor and adiadochokinesis. Posture is irregular and gait is unstable. Ocular movements lack accuracy. Cerebellar patients also show similar deficits in executive functions, linguistic processing, spatial cognition and affect regulation. Recent physiological studies have clarified that cerebellar outputs are modified by the release or facilitation of Purkinje cell-mediated inhibition on the cerebellar nucleus neurons. Thus, impairments in the formation of output signals elicit asthenia and adventitious movements, described by Holmes. These output signals are formed under controls of the internal forward model, embedded in the cerebellum. The deficits in the predictive computation for voluntary movements explains a range of characteristics accompanied by dysmetria. We argue that the impairments in the cerebellar outputs signals and the dysfunction in the internal forward model are fundamental mechanisms explaining the deficits in the 3 domains.
1. What are Cerebellar Ataxias?
Lesions of the cerebellum elicit inaccuracy and clumsiness in limb movements, instability in posture/gait and inaccurate ocular saccades, collectively termed as “ataxia”. The diseases affecting the cerebellum are gathered under the umbrella of cerebellar ataxias (CAs)[1]. The term "ataxia" originates from Bouillaud (1846), who used this term to describe a failure of coordination in voluntary movements[2].
The inaccuracy and clumsiness can be observed clearly in a test of fast alternative hand movements. When patients with CA are asked to alternate pronation and supination as fast as possible, their abilities to execute successive movements are reduced or abolished, termed as “adiadochokinésie” by Babinski[2]. Notably, the execution of elementary movements at average speed is still possible. Patients with CAs stand or walk with a large body-sway and a wide compensatory stance, and their stepings are irregular[1][2]. In addition to limbs’ motor control and oculomotor control, cerebellar dysfunction also generates similar deficits in executive functions, linguistic processing, spatial cognition, and affect regulation (cerebellar cognitive affective syndrome, also referred to as the Schmahmann syndrome)[3].
Among a wide range of manifestations of cerebellar ataxias, dysmetria is a core symptom not only in cerebellar motor ataxias[4] but also in cerebellar cognitive affective syndrome (dysmetria of thought)[5]. Babinski first reported with details motor dysmetria in 1899[6]. Its most common form is hypermetria, a movement overshooting the target. For example, in the finger-to-nose test, the index finger initially follows an intended direction but later exceeds the aimed nose and hits the cheek. Movement ends in a few corrective oscillations until the finger finally reaches the nose[2]. Hypermetria is larger when the inertia of the limb is increased and is velocity-dependent. Cerebellar patients can also show an undershoot or premature arrest before the target, called hypometria. Exaggerated and unstable movements are observed in the elbow, shoulder, trunk and head during the test. This motor dysmetria is distinct from kinetic tremor, which is reduced by addition of inertia to the moving limb.
2. Classical Pathophysiologies: Timing versus Synergy
What parameter does the cerebellum control? Two main models have been suggested to account for cerebelalr functions; timing and synergie[7].
Timing. Timing theory originates from studies by Luciani and Holmes. Luciani postulated that the cerebellum exerts a supportive influence on the rest of the nervous system, namely cerebellar fine-tuning of motor control[7][8]. This idea was pushed forward by Holmes who described that " the cerebellum reinforces or tunes up the cerebral motor apparatus, including subcortical structures with motor functions, so that they respond promptly to volitional stimuli and the impulses from them which excite muscular contractions are properly graded"[9]. He attributed decomposition of movements to the delay in initiation in single-joint movements. Accumulating evidence has confirmed the involvement of the cerebellum in precise timing control[10]. Patients with CAs show a disproportionate increase in temporal variability during a finger-tapping task compared to a circle drawing task, suggesting ataxic impairment in movement timing[11]. Timing of antagonist muscle commands is delayed. Patients also show disrupted timing in conditioned eyeblink responses in the eyeblink conditioning test[12].
Synergy. “Synergie” theory originates from a series of classic work published more than a century ago[13]. Babinski[14] defined synergy as "an association of movements that constitute a single act". Thach[13] emphasized that "the cerebellum combines and integrates the many factors to initiate coordinated movements of the many body parts, and it thus enables to play the unique role of initiating coordinated movements". The cerebellar role in coordinating movements becomes more critical in multi-joint movements. Consistently, inactivation or ablation of the cerebellar nucleus did not affect simple movements. On the other hand, compound movements were impaired and eliminated[15]. A recent study showed that a PC inhibited and disinhibited the activity of a selective muscle, but not in surrounding muscles, allowing execution of movement performed with a small number of muscles[16].
3. Disorganized Disinhibition and Inhibition in Cerebellar Outputs Signals: Pathomecanisms underlying Asthenia and Adventitious Movements
Holmes proposed the term ataxia, which included decomposition of movement, asynergia, dysmetria, and tremor[17]. These deficits are the major clinical signs of cerebellar disorders. He also described other curious complaints by the patients: "they had not nearly so much power in affected limbs". Then he described "a delay in initiating muscle contractions and a slowness in attaining the exertion of full power", which was defined clinically as asthenia. He also introduced a simple method for examination of asthenia; "when the observer’s hands are placed in the patient’s, and he is asked to grasp them firmly at a given signal; the slowness of the affected limb in starting the action and the developing full power is often unmistakable" (asthenia was already coined by Luciani). In addition, he described adventitious movements in a chapter on adiadochokinesis. In addition to patients’ inability to execute alternate movements quickly and correctly, i.e., adiadochokinesis, he pointed out adventitious movements at elbow and shoulder, which led to instability of proximal joints[17]. Asthenia represents insufficient activities of muscles to be recruited, while adventitious movement is unintentional activation of muscle to be suppressed. Thus, asthenia and adventitiousness are opposite to each other in terms of pathophysiology.
Indeed, recent physiological findings from our lab[18] explained asthenia and adventitiousness systematically. We found that during wrist movement of monkeys, a great majority of Purkinje cells (PCs), with somatosensory receptive fields (RFs) in the distal arm, was strongly suppressed before movement onset, while a great majority of dentate cells (DNs) with the same RFs demonstrated concurrent burst of activity. In contrast, PCs with RFs in the proximal arm demonstrated marked and simultaneous increase in activity, while DNs with the same RFs were strongly suppressed[18]. Our observation suggests that activation of DNs generated by reduced inhibition from PCs, i.e., disinhibition, facilitates the execution of wrist movement, while suppression of the DNs by increased PC activity contributes to the stabilization of proximal muscles to improve task performance. Namely, deficits of disinhibition and inhibition of DNs could be the physiological counterparts of asthenia and adventitiousness, respectively.
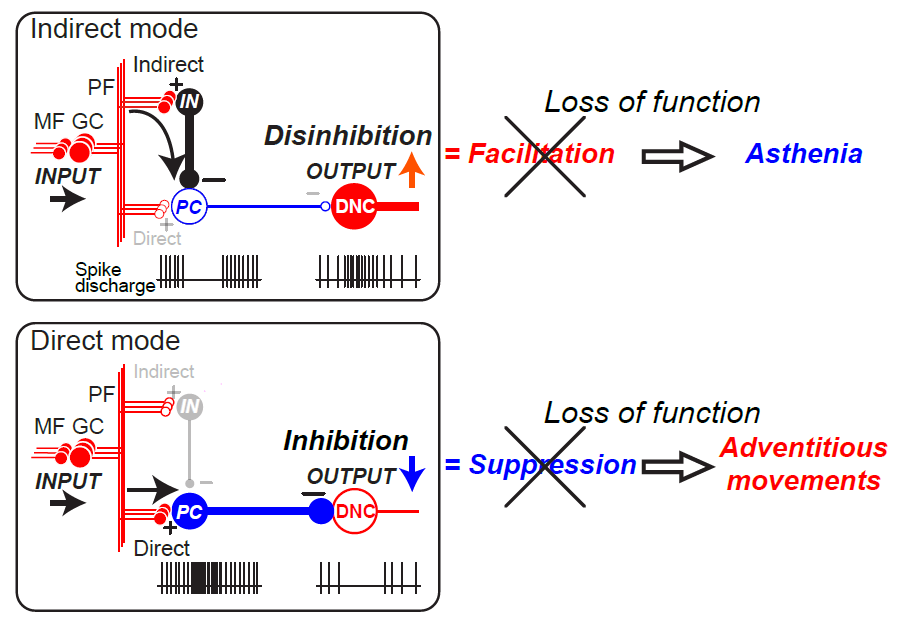
In conclusion, asthenia and adventitiousness appear to reflect deficits in the control of disinhibition and inhibition that determines the cerebellar outputs. We propose that these Holmes’ overlooked minor clinical signs could be essential building blocks of major signs of ataxia such as decomposition of movement, asynergia, dysmetria, and tremor[19] (Figure 1).
Figure 1. Relationship between breakdown of two modes of dentate nucleus cell (DNC) output and asthenia (top) and adventitious movements (bottom) (Ishikawa et al., 2015). Asthenia (top) is the consequence of breakdown of the Indirect mode while Adventitious movements (bottom) is the result of breakdown of the Direct mode. The diagram provides a summary of the functional organization of the cerebellum (Ishikawa et al. 2014). In the cerebellar cortex, mossy fiber (MF) inputs (INPUT) are relayed by granule cells (GCs) and processed through two parallel but different pathways, an indirect pathway (Indirect) and a direct pathway (Direct). In the indirect pathway (top), parallel fiber (PF) inputs activate interneurons (INs) that suppress PCs. Because PC activity provides tonic suppression of DNCs, suppression of PC activity facilitates DNCs through disinhibition (OUTPUT↑). Breakdown of this output mode leads to a decrease in facilitatory output, resulting in Asthenia. In the direct pathway (bottom), PF inputs excite PCs directly. Because PCs are inhibitory, their activation suppresses the DNCs (OUTPUT↓). Breakdown of this output mode leads to a decrease in suppression, resulting in Adventitious movements. CF: climbing fiber. (+): excitatory synapses, (-): inhibitory synapses (Ishikawa et al., 2015).
4. Deficits in the Internal forward Model: Pathomechanisms underlying Dysmetria
A challenge in biological motor control is the temporal delay in afferent sensory signals in reaching the central nervous system from peripheral sensory organs[20]. The feedback delay originates mainly from the nerve conduction and ranges from 10 ms in small animals and 100 ms in large animals[21]. In control engineering, feedback control based on such delayed sensory signals results in oscillatory and unstable movements. In reaching, for example, a feedback controller based on delayed sensory signals pushes a plant toward a target even when the plant already arrives at the target, leading to an overshoot of the target. An internal model solves the delayed feedback problem by simulating the musculoskeletal dynamics internally in the brain[22]. There are two broad classes of an internal model. An internal forward model performs the predictive computation of a current state of the body from a previous state and an efference copy of control signals. A state estimate by an internal model allows fast and stable control of the body, termed as internal feedback control. An internal inverse model, in contrast, computes control signals from a movement goal or a desired movement. The control signals produced by an internal inverse model drive the musculoskeletal system and then realizes the desired action without depending on delayed sensory signals. In summary, internal forward and inverse models are a state predictor and a controller, respectively, as a possible solution for the delayed feedback problem.
Recent psychophysical, neuroimaging and computational studies support that the cerebellum performs the predictive computation of an internal forward model[23]. The cerebrocerebellum receives projections from both the cerebral cortex and peripheral sensory pathways, satisfying an anatomical requirement for an internal forward model[24]. We here explain two of our recent studies that support the internal-forward-model hypothesis of the cerebellum. One study compared behavioral data of healthy controls and ataxic patients in a wrist-tracking task[25]. We found that the predictive component in the movement showed increased error and delay compared to that of the controls. The control subjects could smoothly track the target motion, whereas the ataxic patients moved their wrists lagged against the target, resulting in irregular trajectories with intermittent corrections. The predictive component in the control subjects lagged the target motion only by 66 ms, whereas the cerebellar patients lagged significantly as much as 172 ms. Therefore, the behavioral analysis revealed a selective deficit in the predictive tracking movement in the ataxic patients. The other study analyzed single-unit activities of the cerebellar cells in a behaving monkey during a step-tracking movement of the wrist joint[26]. The theory of an internal forward model provides a straightforward and experimentally confirmable prediction; the current output from an internal forward model should predict the future input to the model. If the cerebellum is to function as a forward model, the theory requires that the current cerebellar output (activities of dentate cells) should contain predictive information about the future cerebellar input (activities of mossy fibers). Supporting this prediction, we found that the cerebellar output at time t could predict the cerebellar output at time t+t1. These studies support the internal-forward-model hypothesis of the cerebellum.
Motor dysmetria in cerebellar ataxia stems from deficits of the predictive computation of the internal forward model in the cerebellum[27]. When the predictive computation does not function properly, the brain depends on the delayed sensory feedback, leading to oscillatory and unstable movements, as in the reaching example explained above. The predictive computation also plays a possible role in coordination among multiple degrees of freedom and properly controlled timing in muscle activity onsets. All parts of the body are not independent of each other but interact dynamically, so controlling one degree of freedom requires the prediction of dynamical interactions from other degrees of freedom. In multi-joint actions, a movement in one joint influences changes in other joints through interaction torques such as Coriolis and centrifugal forces. Coordinated and appropriately timed activities in multiple muscles necessitate the dynamic prediction of body states.In this way, the two perspectives in classic pathophysiology, the timing and the synergy theories, fit with the framework of the predictive forward model. We propose that motor dysmetria occurs as a result of the impaired predictive computation of the internal forward model in the cerebellum.
References
- Mario Manto; James M. Bower; Adriana Bastos Conforto; José M. Delgado-García; Suzete Nascimento Farias Da Guarda; Marcus Gerwig; Christophe Habas; Nobuhiro Hagura; Richard B. Ivry; Peter Mariën; et al.Marco MolinariEiichi NaitoDennis A. NowakNordeyn Oulad Ben TaibDenis PelissonClaudia D. TescheCaroline TiliketeDagmar Timmann Consensus Paper: Roles of the Cerebellum in Motor Control—The Diversity of Ideas on Cerebellar Involvement in Movement. The Cerebellum 2011, 11, 457-487, 10.1007/s12311-011-0331-9.
- Garcin, R. The ataxias. In Handbook of Clinical neurology. volume 1 Disturbances of nervous function; Vinken, P.J., Bruyn, G.W., Ed.; North Holland Publishing Company: Amsterdam, Nertherland, 1969; pp. 309-335.
- Jeremy D. Schmahmann; David Caplan; Cognition, emotion and the cerebellum. Brain 2006, 129, 290-292, 10.1093/brain/awh729.
- Mario Manto; Mechanisms of human cerebellar dysmetria: experimental evidence and current conceptual bases. Journal of NeuroEngineering and Rehabilitation 2009, 6, 10-10, 10.1186/1743-0003-6-10.
- Jeremy D. Schmahmann; The cerebellum and cognition. Neuroscience Letters 2019, 688, 62-75, 10.1016/j.neulet.2018.07.005.
- Babinski, J. Exposé des travaux scientifiques; Masson: Paris, France, 1913.
- W T Thach; H P Goodkin; J G Keating; The Cerebellum and the Adaptive Coordination of Movement. Annual Review of Neuroscience 1992, 15, 403-442, 10.1146/annurev.ne.15.030192.002155.
- Ermanno Manni; Laura Petrosini; Luciani's work on the cerebellum a century later. Trends in Neurosciences 1997, 20, 112-116, 10.1016/s0166-2236(96)10077-1.
- Holmes, G.; The cerebellum of man. Brain 1939, 62, 1-30.
- Martin Bareš; Richard Apps; Laura Avanzino; Assaf Breska; Egidio D’Angelo; Pavel Filip; Marcus Gerwig; Richard B Ivry; Charlotte L. Lawrenson; Elan D. Louis; et al.Nicholas A. LuskMario MantoWarren H. MeckH. MitomaElijah A. Petter Consensus paper: Decoding the Contributions of the Cerebellum as a Time Machine. From Neurons to Clinical Applications. The Cerebellum 2018, 18, 266-286, 10.1007/s12311-018-0979-5.
- J. E. Schlerf; R. M. C. Spencer; H. N. Zelaznik; R. B. Ivry; Timing of rhythmic movements in patients with cerebellar degeneration. The Cerebellum 2007, 6, 221-231, 10.1080/14734220701370643.
- Marcus Gerwig; Karim Hajjar; Albena Dimitrova; Matthias Maschke; Florian P. Kolb; Markus Frings; Alfred F. Thilmann; Michael Forsting; Hans-Christoph Diener; Dagmar Timmann; et al. Timing of Conditioned Eyeblink Responses Is Impaired in Cerebellar Patients. The Journal of Neuroscience 2005, 25, 3919-3931, 10.1523/jneurosci.0266-05.2005.
- W. T. Thach; Does the Cerebellum Initiate Movement?. The Cerebellum 2013, 13, 139-150, 10.1007/s12311-013-0506-7.
- Babinski, J.; De l'asynergie cérébelleuse. Rev. Neurol. 1899, 7, 806–816.
- H. P. Goodkin; W. T. Thach; Cerebellar Control of Constrained and Unconstrained Movements. II. EMG and Nuclear Activity. Journal of Neurophysiology 2003, 89, 896-908, 10.1152/jn.00115.2002.
- Pattamon Panyakaew; Hyun Joo Cho; Prachaya Srivanitchapoom; Traian Popa; Tianxia Wu; Mark Hallett; Cerebellar brain inhibition in the target and surround muscles during voluntary tonic activation. European Journal of Neuroscience 2016, 43, 1075-1081, 10.1111/ejn.13211.
- Gordon Holmes; THE SYMPTOMS OF ACUTE CEREBELLAR INJURIES DUE TO GUNSHOT INJURIES. Brain 1917, 40, 461-535, 10.1093/brain/40.4.461.
- Takahiro Ishikawa; Saeka Tomatsu; Yoshiaki Tsunoda; Jongho Lee; Donna S. Hoffman; Shinji Kakei; Releasing Dentate Nucleus Cells from Purkinje Cell Inhibition Generates Output from the Cerebrocerebellum. PLOS ONE 2014, 9, e108774, 10.1371/journal.pone.0108774.
- Takahiro Ishikawa; Shinji Kakei; H. Mitoma; Overlooked Holmes' clinical signs: reevaluation by recent physiological findings.. Cerebellum & Ataxias 2015, 2, 13, 10.1186/s40673-015-0033-z.
- R. Chris Miall; D.M. Wolpert; Forward Models for Physiological Motor Control. Neural Networks 1996, 9, 1265-1279, 10.1016/s0893-6080(96)00035-4.
- Heather L. More; John R. Hutchinson; David F. Collins; Douglas J. Weber; Steven K. H. Aung; J. Maxwell Donelan; Scaling of sensorimotor control in terrestrial mammals. Proceedings of the Royal Society B: Biological Sciences 2010, 277, 3563-3568, 10.1098/rspb.2010.0898.
- Daniel M. Wolpert; R.Chris Miall; Mitsuo Kawato; Internal models in the cerebellum. Trends in Cognitive Sciences 1998, 2, 338-347, 10.1016/s1364-6613(98)01221-2.
- Diedrichsen, J.; Bastian, A. Cerebellar Function. In The Cognitive Neurosciences; Michael, S.G., Mangun, G.R.; The MIT Press: Cambridge, England, 2014; pp. 451-460.
- Hirokazu Tanaka; Takahiro Ishikawa; Jongho Lee; Shinji Kakei; The Cerebro-Cerebellum as a Locus of Forward Model: A Review. Frontiers in Systems Neuroscience 2020, 14, 19, 10.3389/fnsys.2020.00019.
- Shinji Kakei; Jongho Lee; Hiroshi Mitoma; Hirokazu Tanaka; Mario Manto; Christiane S. Hampe; Contribution of the Cerebellum to Predictive Motor Control and Its Evaluation in Ataxic Patients. Frontiers in Human Neuroscience 2019, 13, 216, 10.3389/fnhum.2019.00216.
- Hirokazu Tanaka; Takahiro Ishikawa; Shinji Kakei; Neural Evidence of the Cerebellum as a State Predictor. The Cerebellum 2019, 18, 349-371, 10.1007/s12311-018-0996-4.
- Cabaraux Pierre; Jordi Gandini; Shinji Kakei; Mario Manto; H. Mitoma; Hirokazu Tanaka; Dysmetria and Errors in Predictions: The Role of Internal Forward Model. International Journal of Molecular Sciences 2020, 21, 6900, 10.3390/ijms21186900.