
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hermis Iatrou | + 3898 word(s) | 3898 | 2020-12-04 09:27:36 | | | |
| 2 | Catherine Yang | Meta information modification | 3898 | 2020-12-08 02:13:55 | | |
Video Upload Options
Cardiovascular diseases (CVDs) are the leading cause of death globally, taking an estimated 17.9 million lives each year, representing one third of global mortality. As existing therapies still have limited success, due to the inability to control the biodistribution of the currently approved drugs, the quality of life of these patients is modest. The advent of nanomedicine has brought new insights in innovative treatment strategies.
1. Introduction
Cardiovascular diseases (CVDs) encompass all pathologies of the heart or circulatory system, including coronary artery disease (CAD), myocardial infarction (MI), heart failure and peripheral vascular disease. These diseases are the primary mortality cause worldwide and significantly impact patients’ quality of life. More than 17.9 million people died from CVDs in 2016, representing above of one third of global deaths. Of these deaths, 85% are due to heart attack and stroke [1]. In Europe, 45% of all deaths are due to CVDs, accounting for 3.9 million deaths per year [2]. In the economic sector, the direct healthcare expenditure related to CVD treatment is estimated to cost the EU economy over 100 billion euros every year. Unfortunately, these figures are expected to increase over the next years due to an increase in CVD risk factors, such as obesity and diabetes, as well as an expansion of the elderly population [3]. To date, available therapies such as heart transplantation, pharmacological interventions, coronary artery bypass graft surgery, and ventricular assistant devices have significantly prolonged patients’ longevity. However, cost effective and less invasive treatments with higher efficacy are vital for the future.
In recent decades, the continuous progress of nanotechnology has led to a dramatic expansion of potential biomedical applications of nanomaterials. Nanoparticles have been widely employed in the diagnosis, imaging and treatment of many diseases, such as cancer. Nanomedicine for curing cancer is the main field of research for decades and several nanomedical products (e.g., Doxil, Abraxane) have been approved by the US FDA and the EMA for their clinical use in humans and many others are in advanced stages of clinical trials [4]. On the contrary, the field of cardiovascular nanomedicine has recently started growing and is still in its infancy. Nonetheless, an increase in research publications with promising lab-scale results has been reported, especially in recent years.
2. Types of Nanoconstructs for CVDs
Recently, the interest in developing DDS for targeting the cardiovascular system has increased exponentially. Many types of nano-based structures have been developed and can be utilized for the transport of drugs for this purpose. Liposomes, polymer–drug conjugates, polymeric micelles, nanoshells, and magnetic or hybrid carriers are some of these and all can be used for drug delivery [5]. In this section, we report the emerging research progress and results on heart-targeted nanoscale DDS (Scheme 1).
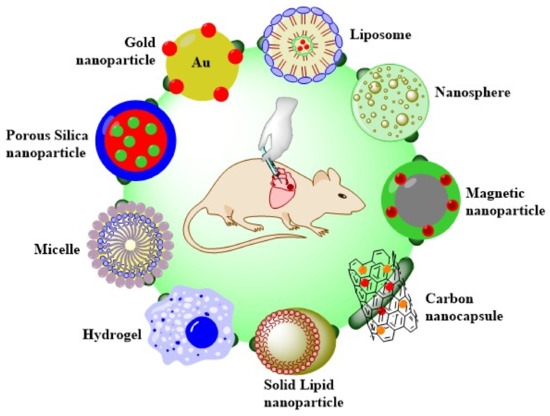
Scheme 1. Overview of the DDS types to treat heart diseases.
2.1. Liposomes
Among them, lipid-based nanoparticles are currently the most commonly used nanoconstructs for drug delivery. Many products based on liposomes have been available on the market for the past decade and some others are currently in clinical development. Liposomes are spherical structures that, most of the time, form vesicles. They are composed of a phospholipid bilayer membrane inside of which aqueous solutions can be entrapped. Their biocompatible and biodegradable composition, as well as their unique ability to encapsulate both hydrophilic and hydrophobic pharmaceutical agents, make liposomes excellent nanocarriers. Liposomes are often coated with biocompatible polymers, such as PEG, to prolong their circulation time and increase stability in aqueous environments. The polymer coating can also be modified to carry a functional group, which can be used as a surface marker [6]. Many experimental attempts to treat the infarcted heart include supplying growth factors, cytokines, drugs, and other biomolecules to the damaged cells in the infarcted tissue.
Dvir and coworkers designed a nanoparticulate system that could specifically target cardiac cells. These particles are angiotensin II type 1 (AT1) nano-liposomes that contain a targeting amino acid sequence (Gly-Gly-Gly-Gly-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) for AT1 receptor binding. This peptide was conjugated to carboxylic groups on the PEG-HSPC liposomes. AT1 receptors found to be overexpressed in heart after MI or heart failure. The AT1 particles with 142 nm size exhibited great targeting capability in vitro and in vivo. The authors first found in vitro that there was a 3-fold increase in the expression of the AT1 receptor in cardiac cells subjected to hypoxia (ventricular myocytes) compared with cells growing under normal conditions, and 83% of total amount of NPs accumulated in these cells. Furthermore, in in vivo experiments, fluorescent liposomes were injected in mice 1, 4 and 7 days after MI and it was found that NPs amassed predominantly in the left ventricle of the infarcted heart. This finding shows the ability of these particles to specifically target the injured myocardium and may be exploited to deliver therapeutic agents to it [7].
The targeting efficiency of mAb 2G4 for the cardiac myosin was exploited by Liang and coworkers for the synthesis of ATP-containing immunoliposomes. In the ischemic heart, myocardial adenosine triphosphate (ATP) utilization is reduced due to inadequate contractile function, while the need for ATP remains high. This imbalance between supply and demand for ATP deteriorates left ventricular cardiac function. Free ATP cannot be administered because of its short circulation half-life and, because of its strong negatively charged nature, cannot enter into cells through their membrane. So, in this study, ATP was encapsulated in liposomes containing phosphatidylcholine, cholesterol, PEG-DSPE and 2G4-PEG-PE. Liposomes were produced via the freeze-thawing method and after ATP entrapment only minor ATP leakage was observed after 24 h at 37 °C. The specific binding of 2G4-immunoliposomes to myosin was evaluated using ELISA. The results showed strong binding to the myosin monolayer [8]. In vivo experiments using these NPs with or without the targeting antibody were conducted by Verma et al. in rabbits after an acute myocardial infarction. Infusion of ATP-NPs followed by 30 min of coronary artery occlusion and 3 h of reperfusion, resulted in a significant decrease (over 50%) in the size of irreversibly damaged myocardium within the total area at risk. These NPs accumulated in the heart due to the EPR effect and seem to have a cardioprotective effect by supplying the myocardium with the required energy [9]. Some myosin specific liposomes demonstrated even better cardioprotection by significantly restoring the systolic and diastolic function of the heart. At the end of reperfusion, the left ventricular pressure recovered to more than 80%, while the left ventricular end diastolic pressure reduced considerably [10]. In addition, the delivery of Coenzyme Q10 (CoQ10) with similar liposomes was investigated by the same team. Intracoronary infusion of CoQ10 loaded liposomes in rabbits with MI resulted in a 50% reduction in infarct size, which could in turn be sufficient for potential recovery from an infarction in a clinical situation [11].
In addition, an interesting study was reported by Liu for the therapy of arrhythmia to the ischemic heart. They synthesized antibody modified liposomes for the targeted delivery of anti-miR-1 antisense oligonucleotides AMO-1 to the ischemic myocardium. It is well established that microRNA-1 (miR-1) can be found in cardiac and skeletal muscle tissues and is overexpressed in the ischemic heart. The suppression of miR-1 by AMO-1, an anti-miR-1 antisense oligonucleotide, can lead to the therapy of cardiac arrhythmia. Furthermore, cardiac troponin I (cTn-I) is a specific marker which is expressed only in damaged heart tissues. Based on this, anti-cTn-I antibody was harnessed as a targeting moiety. The liposomes, containing egg phosphatidylcholine, cholesterol and DSPE-PEG, were fabricated via the thin film hydration method, and the antibody was conjugated using DSPE-PEG-MAL. The NPs had a slightly negative surface charge, a size close to 100 nm, high AMO-1 entrapment efficiency (>60%) and 23% in vitro release rate in 24 h. Time lapse live cell imaging and in vivo imaging utilized to monitor anti-cTn-I NPs and both demonstrated high accumulation in the heart and specifically in the cytoplasm of myocardial cells. After administration of AMO-I loaded NPs in rats, electrocardiograph recordings exhibited repression of arrhythmogenesis. To conclude, anti-cTnI liposomes not only delivered AMO-1 to infarcted myocardium in rats, but also relieved cardiac arrhythmia via the silencing of miR-1 in the ischemic heart [12].
2.2. Polymeric Nanoparticles
Biodegradable polymeric nanoconstructs have been extensively investigated as drug carriers. This type of nanocarrier includes copolymers consisting of two or more blocks with different hydrophobicity. In aqueous environments, these copolymers usually form micellar structures composed of a hydrophilic shell and a hydrophobic core. Inside the hydrophobic core, water-insoluble drugs can be hosted, while the hydrophilic corona can be additionally modified for the attachment of water-soluble drugs or targeting moieties. [13]. However, this is not always the case. To date, many other polymeric nanoconstructs have been synthesized with very different structures. For instance, polymers can form hydrogels [14][15][16], network-like scaffolds [17], microparticles [18], nanospheres or nanoshells that are suitable not only for the release of hydrophobic but also hydrophilic drugs, proteins, genes, nucleic acids or genes [19]. The release of encapsulated drugs occurs at a controlled rate in a time or environment dependent manner. The kinetics of the release of nanoparticle-bound drugs depend on the duration of nanoparticles residence in the organism and characteristics of their microenvironment. In general, biodegradable polymer formulations maintain the desired therapeutic drug concentration in the tissue of interest for a longer period of time than other nanocarriers. This feature makes them suitable platforms for the transport of highly toxic, poorly soluble, and unstable drugs.
Poly(d,l-lactic acid), poly(d,l-glycolic acid) (PLGA) is a copolymer that has been widely used for medical applications due to its low toxicity, high biocompatibility and biodegradability and because it is an FDA approved material. In this route, many research groups have focused their attempts on developing PLGA based nanomedicine materials. For example, Chang and coworkers presented the synthesis of insulin-like growth factor-1 (IGF-1) complexed PLGA NPs for early cardioprotection after acute MI. IGF-1 is crucial in regulating heart function and it has been found to promote cardiomyocyte proliferation. In humans, IGF-1 treatment enhances cardiac function after infarction. In order to maintain its biological function, anionic IGF-1 formed complexes with PLGA via electrostatic interactions. Because PLGA is also negatively charged, polyethylenimine conjugation was utilized to render PLGA’s surface cationic and to achieve PLGA-IGF-1 complexation. Various-sized NPs were synthesized (1 μm, 200 nm, 70 nm), with the smallest ones (70 nm) found to have the best binding capability and activated the most Akt phosphorylation in cultured cardiomyocytes. After a single intramyocardial injection in mice, IGF-1-PLGA NPs prolonged IGF-1 retention time to at least 24 h, inhibited cardiomyocyte apoptosis, reduced infarct size and prevented ventricular dilation and wall thinning 21 days after MI. These results highlight the potential of nanomedicine in treating cardiovascular diseases and encourage its translation into clinical applications [20].
Administration of statins at the time of reperfusion exhibited no therapeutic effects in animals and in patients with acute MI. Nevertheless, Nagaoka and his team found that nanoparticle delivered Pitavastatin presented cardioprotective effects. They synthesized Pitavastatin-PLGA NPs that accumulated in the infarcted region of the myocardium of a rat MI model due to its enhanced vascular permeability. After single intravenous injection at reperfusion, Pitavastatin NPs reduced the size of infarct after 24 h and improved left ventricular function. They also induced phosphorylation of Akt and GSK3β and inhibited inflammation and cardiomyocyte necrosis in the infarcted heart, while the free Pitavastatin drug failed to show any cardioprotection. Moreover, Pitavastatin NPs have successfully completed phase I clinical trials at a university hospital, suggesting that this nanoparticle-based technology can serve as novel therapeutic treatment for ischemic injury [21].
In coronary artery disease (CAD) and peripheral artery disease (PAD), therapeutic angiogenesis has appeared as a potential clinical approach for the recovery of ischemic tissues, including the direct administration of pro-angiogenic growth factors to promote the synthesis of growth factors by target tissues. In this context, adrenomedullin-2 (ADM-2) has been recently identified as a new angiogenic factor able to regulate blood flow and cardiovascular function. Due to its short biological half-life and low plasma stability, ADM-2 has, up to now, limited applications. For this reason, Quadros and coworkers tried to encapsulate this peptide into PLGA nanoformulations that could serve as delivery system for heart repair. PLGA-ADM-2 NPs, prepared by a double emulsion method, had a size of 300–350 nm, negative surface charge, good colloidal stability and did not present toxicity in cardiomyocyte cells. The entrapment efficiency of ADM-2 reached circa 70%, as identified by ELISA, and in vitro peptide release showed a 60% release in the first 3 days followed by a slower release rate in the next 3 weeks. In vitro experiments in EA.hy926 endothelial cells also exhibited enhanced cell proliferation (1.4 fold higher), rendering AMD-2-PLGA NPs a novel approach for therapeutic angiogenesis in CVDs [22].
In a pioneering work, Nguyen et al. presented a system for potential treatment of heart failure following a MI based on intravenous administration of enzyme responsive nanoparticles. These nanomaterials have the ability to respond to the enzymatic conditions present in the heart after an acute MI by changing their structure from discrete micellar nanostructures to network-like scaffolds. It is worth noting that these nanosystems (Figure 1), consisting of brush peptide–polymer amphiphiles based on a polynorbornene backbone with peptide targeting sequences specific for recognition by matrix metalloproteinases MMP-2 and MMP-9, have the ability to remain stable during blood circulation conditions until they reach the infarct region through the vascular leakage after MI. At this site, their structure is altered, and it has been found that they remain on the injured tissue for up to 28 days after injection. In previous work, super resolution fluorescence microscopy measurements showed that the responsive nanoparticles are MMP-9 activated and they are kept assembled into scaffolds at the injection site for 7 days, while the non-responsive particles were inactive in the presence of MMP-9 and were cleared within 1 h. Furthermore, the efficacy of the system was also studied in rats with MI and it was indicated that the responding particles were activated in the presence of MMPs and remained aggregated in network-like scaffolds for 6 days, in contrast with the reduced aggregation observed in non-responsive particles. Finally, after injecting responsive and non-responsive particles into the tail vein of healthy rats as well as in 24 h post-MI rats, a targeted and prolonged accumulation of responsive particles in the diseased heart tissue was demonstrated for 28 days. In conclusion, MMP-responsive nanoparticles exploit the EPR effect for initial passive targeting and present a very promising approach to deliver therapeutics to the heart after MI in an effective and controlled manner [17].
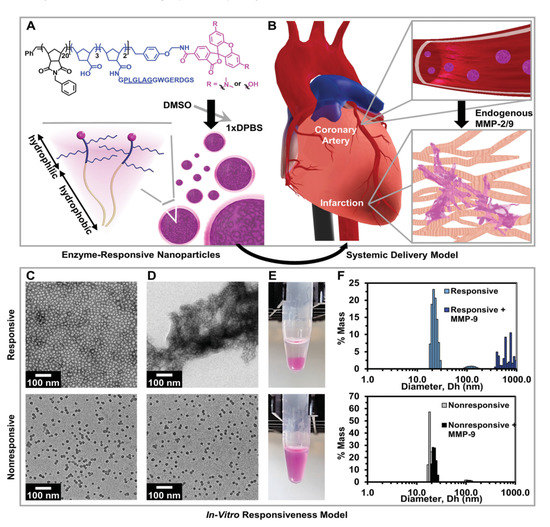
Figure 1. Responsive nanoparticles target, accumulate, and are retained within an acute MI due to enzyme-induced aggregation. (A) Diagram of a dye-labeled brush peptide–polymer amphiphile (PPA) containing an MMP-2 and MMP-9 specific recognition sequence, shown underlined. PPAs self-assemble into nanoparticles through hydrophobic–hydrophilic interactions when dialyzed into aqueous buffer. (B) Schematic of nanoparticles freely circulating in the bloodstream (not to scale) upon systemic delivery. Nanoparticles enter the infarct tissue through the leaky acute MI vasculature, and up-regulated MMPs within the infarct induce the formation of an aggregate-like scaffold. (C) In vitro, responsive (top) and non-responsive (bottom) nanoparticles are monodisperse micelles with diameters of 15–20 nm, and (D) upon activation, only responsive nanoparticles form an aggregate-like scaffold. (E) Corresponding images of nanoparticle solutions following activation. (F) Dynamic light scattering of nanoparticles before and after MMP activation. Reproduced with permission from [17], Wiley-VCH, 2015.
Diabetic cardiomyopathy (DCM) is a CVD caused by diabetes and it has many adverse effects in heart function, like systolic/diastolic cardiac changes and atherosclerotic heart disease that later leads to heart failure or ischemia. To tackle DCM, Tong and coworkers developed curcumin polymeric carriers consisting of a triblock copolymer poly(γ-benzyl l-glutamate)-poly(ethylene glycol)-poly(γ-benzyl l-glutamate) (PBLG-PEG-PBLG) (P). The copolymer was synthesized by the ring opening polymerization of the BLG-N-carboxy anhydride with H2N-PEG-NH2 as the macroinitiator. The curcumin incorporated particles had a diameter of 30 nm and the loading capacity of curcumin was over 30%. These particles showed very limited cell toxicity in vitro in high concentrations and curcumin release was achieved within 3 days. In previous studies, curcumin was found to have therapeutic effects on diabetes and to alleviate DCM. Indeed, it was found that curcumin loaded NPs reduced pathological morphological damage of cardiac tissue and increased H2S levels that play a vital role in the inhibition of apoptosis in cardiomyocytes [23].
Therefore, it was necessary to introduce unmodified PCD, in a ratio of 1:1 w/w with the PCM-PCD, in order to increase the positive charge and obtain stable nanoparticles with a diameter of less than 200 nm. These polymers can form complexes with polyanionic genes efficiently, like Fas siRNA. Transfection experiments and cellular uptake studies confirmed that the biodegradable PCM-modified polymer could bind specifically to H9C2 cells, revealing the cardiomyocyte-targeting ability of PCM-PCD nanoparticles. Flow cytometry measurements in H9C2 cells indicated the efficiency of Fas gene silencing by PCM-polymer/Fas siRNA complexes to inhibit apoptosis under hypoxic conditions. Quantitative real-time PCR analysis showed a 77% reduction in Fas gene expression in H9C2 cells treated with Fas siRNA, delivered using the PCM-PCD/PCD carrier. The PCM-modified polymer/Fas siRNA polyplexes facilitate Fas gene silencing and increase cardiomyocyte viability under hypoxic conditions. To conclude, PCM modification is a promising method for cardiomyocyte targeting, and siRNA delivery with PCM-PCD polymers inhibits cardiomyocyte apoptosis [24].
Another interesting aspect for treating MI is the restoration of mechanical and electrical function of the heart by exploiting the conductive properties of some nanoformulations. As an example, a soft conductive hydrogel was presented by Bao and his group as an electrical signal transmitter for the infarcted myocardium (Figure 2). They synthesized an injectable hydrogel consisting of a multi-armed PEGDA700-Melamine (PEG-MEL) crosslinker, stabilized through π–π interactions between the triazine rings of melamine, that was used to conjugate a thiol modified hyaluronic acid (HA). Moreover, the addition of graphene oxide (GO) has provided soft (G′ = 25 Pa) and conductive (G = 2.84 × 10−4 S cm−1) properties and enhanced mechanical stress stability. To improve the healing ability of the hydrogel, adipose tissue-derived stromal cells (ADSCs) were incorporated. After an injection in the MI area in a rat model, an overall enhancement of cardiac function was observed. In particular, the ejection fraction (EF) and fractional shortening (FS) greatly improved in a one month period (EF = 78% and FS = 53%), the size of infarction and fibrosis reduced and the vascular density increased. Eventually, the overexpression of Connexin 43 and α-Smooth Muscle Actin confirmed the efficient transmission of electrical and mechanical signals to the heart [14].
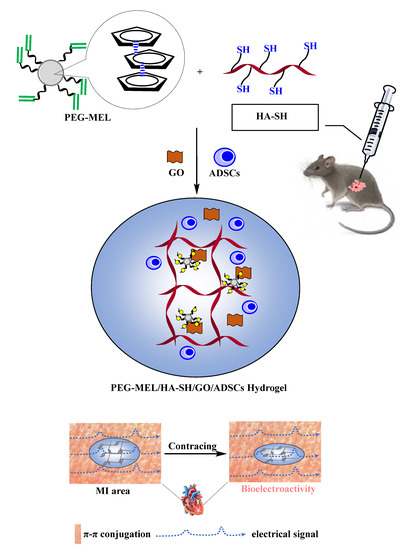
Figure 2. Schematic sketch illustrating the application of this soft and conductive PEG-MEL/HA-SH/GO hydrogel system encapsulating ADSCs for cardiac repair by injection into the MI area of Sprague-Dawley (SD) rats, with the objective of enhancing the transmission of mechanical and electrical signals to rebuild cardiac function. Adapted from [14], Elsevier 2017.
The insufficient propagation of electrical signals to the heart after MI can also cause cardiac arrhythmia. For this reason, Zhang and coworkers designed an injectable conductive hydrogel that ameliorates arrhythmia and preserves cardiac function (Figure 3).
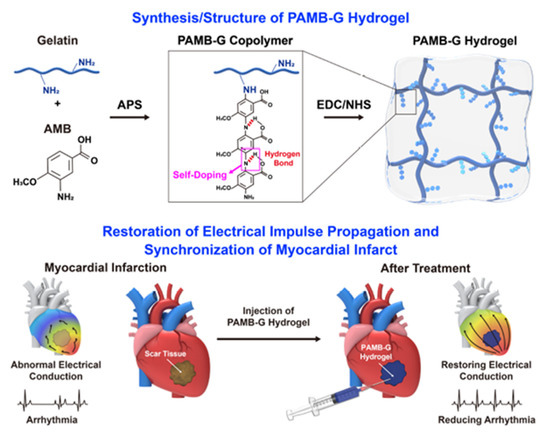
Figure 3. Synthesis and structure of PAMB hydrogel and mechanism of its restoring electrical impulse propagation and synchronizing myocardial contraction following a myocardial infarction. Reproduced with permission from [16], Elsevier, 2019.
2.3. Other Types
Apart from polymeric and lipid NPs, other types of nanoconstructs have also been developed for drug delivery to the myocardium. Some of them may contain inorganic components such as silica, gold or carbon nanocapsules, and others may contain biomaterials, such as alginate salts or chitosan.
Silica nanoparticles can be used for heart-targeted drug delivery in both passive and active ways. In particular, Galagudza and coworkers designed adenosine loaded silica nanoparticles for the passive delivery of adenosine to the ischemic-reperfused cardiac tissue. As mentioned before, adenosine is a cardioprotective agent and its incorporation to the NPs was achieved through adsorption on their surface. The NPs mean diameter was 6–13 nm, presenting no toxic effects in vitro and in vivo. However, their long-term toxicity remains unclear due to their capture by the liver. After intravenous administration of adenosine-silica NPs in rats with ischemic-reperfusion injured hearts, augmented accumulation in the infarcted area was observed. Adenosine bounded to NPs restored blood pressure and notably reduced the infarct size in comparison with free adenosine administration. It must be emphasized that in order to maximize the therapeutic effects of the DDS, it must be administered right after ischemia or at the beginning of reperfusion [25].
In contrast, Ferreira and her team developed multifunctional silica NPs loaded with a cardioprotective compound for active targeting delivery to the endocardial layer of the injured heart (Figure 4).
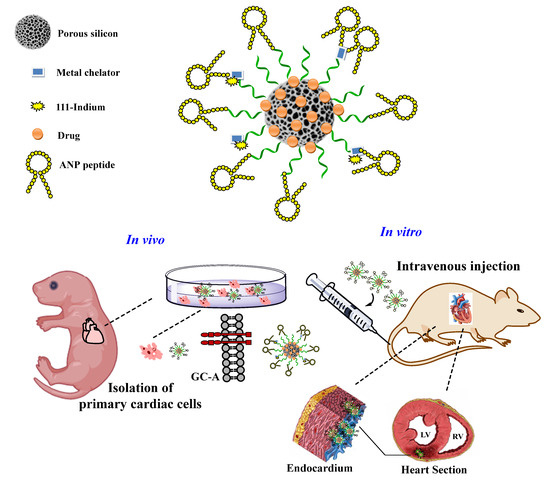
Figure 4. This schematic representation of the engineered multifunctional nanoparticle and its components, as well as an overview of the conducted in vitro and in vivo studies and the main findings of this study. Adapted from [26], Wiley-VCH, 2019.
Gold NPs are already known for their various applications in many fields of material science, as they have unique optical and physical properties. They are also an emerging type of DDS that can be easily functionalized and serve as vehicles for various therapeutic agents, as well as for imaging and diagnosis of diseases. Due to their potential biomedical applications, such as in photothermal therapy [27], many researchers are trying to develop gold-based nanosystems for curing different diseases. Recently, Somasuntharam and coworkers presented a very promising system for the treatment of inflammatory response caused during acute MI (Figure 5). They synthesized 80 nm sized (as determined by dynamic light scattering) gold NPs functionalized on their surface with deoxyribozyme (Dz) (Dz-AuNPs). DNAzymes are DNA oligonucleotides that are capable of inhibiting the activation of tumor necrosis factor α (TNF-α), a key factor implicated in many pathological processes, such as cellular inflammation, causing damage to the infarcted myocardium. Dz-AuNPs possess enhanced stability, cytocompatibility and in vitro studies showed that they are able to internalize in cardiomyocytes and in macrophages. Ex vivo fluorescent imaging revealed predominant accumulation in heart and secondary accumulation in liver and kidney. In vivo experiments in a rat model of acute MI exhibited a 50% reduction in TNF-α, followed by several beneficial effects, such as reduction in pro-inflammatory cytokines that regulate cell survival or apoptosis and trigger oxidative stress. Furthermore, echocardiography and pressure–volume catheter analysis followed by hemodynamic measurements showed a 40% improvement in cardiac function, while many cardiac indices, such as ejection fraction, end-diastolic/systolic pressure, end systolic volume, all improved. All these features indicate that Dz-AuNPs seem to be a potent vehicle for MI treatment by reducing local inflammatory responses and improving cardiac cell survival [28].
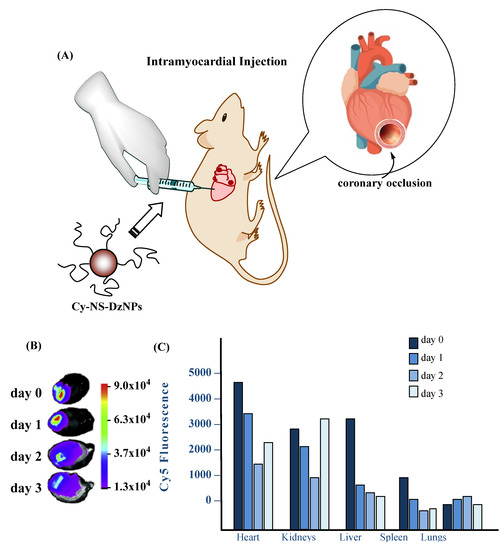
Figure 5. Ex-vivo fluorescence imaging of organs from animals injected with Cyanine 5-Non-Specific (Cy5-NS)-deoxyribozyme Nanoparticles (DzNP). (A) Schematic of ligation of the coronary artery to cause permanent ligation MI in rats followed by particle injection. (B) Fluorescence images of hearts from each day following injection. The heat map indicates the Cy5 emission intensity in arbitrary units. (C) Fluorescence intensity of all organs imaged from days 0, 1, 2 and 3. The Cy5 fluorescence intensity is expressed in arbitrary units. A total of four rats were used where each time point consists of fluorescence from an individual rat. Adapted from [29], NRC, 2018.
These particles, loaded with a cell-penetrating mimetic peptide (R7W-MP, DQRPDREAPRS), can be translocated from the pulmonary tree to the bloodstream and then to the heart. CaPs-R7W NPs can be internalized in cardiomyocytes by passing through their cell membrane, as in vitro experiments showed, and release their cargo. In vivo biodistribution studies revealed that NPs were rapidly deposited to the myocardium, as confirmed by fluorescence molecular tomography imaging and transmission electron microscopy. Notably, echocardiography showed that after administration of CaPs-R7W NPs in rats suffering from diabetic cardiomyopathy, complete restoration of heart contractility and function was achieved. These data underscore the importance of this DDS, as its non-invasive method and its efficacy open avenues for the treatment of CVDs [30].
References
- World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 25 May 2019).
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579.
- Heidenreich, P. Heart Failure Prevention and Team-based Interventions. Heart Fail. Clin. 2015, 11, 349–358.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387.
- Wang, A.Z.; Gu, F.; Zhang, L.; Chan, J.M.; Radovic-Moreno, A.; Shaikh, M.R.; Farokhzad, O.C. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin. Biol. Ther. 2008, 8, 1063–1070.
- Nekkanti, V.; Kalepu, S. Recent advances in liposomal drug delivery: A review. Pharm. Nanotech. 2015, 3, 35–55.
- Dvir, T.; Bauer, M.; Schroeder, A.; Tsui, J.H.; Anderson, D.G.; Langer, R.; Liao, R.; Kohane, D.S. Nanoparticles targeting the infarcted heart. Nano Lett. 2011, 11, 4411–4414.
- Liang, W.; Levchenko, T.; Khaw, B.A.; Torchilin, V. ATP-containing immunoliposomes specific for cardiac myosin. Curr. Drug Deliv. 2004, 1, 1–7.
- Verma, D.D.; Hartner, W.C.; Levchenko, T.S.; Bernstein, E.A.; Torchilin, V.P. ATP-loaded liposomes effectively protect the myocardium in rabbits with an acute experimental myocardial infarction. Pharm. Res. 2005, 22, 2115–2120.
- Verma, D.D.; Levchenko, T.S.; Bernstein, E.A.; Mongayt, D.; Torchilin, V.P. ATP-loaded immunoliposomes specific for cardiac myosin provide improved protection of the mechanical functions of myocardium from global ischemia in an isolated rat heart model. J. Drug Target. 2006, 14, 273–280.
- Verma, D.D.; Hartner, W.C.; Thakkar, V.; Levchenko, T.S.; Torchilin, V.P. Protective effect of coenzyme Q10-loaded liposomes on the myocardium in rabbits with an acute experimental myocardial infarction. Pharm. Res. 2007, 24, 2131–2137.
- Liu, M.; Li, M.; Sun, S.; Li, B.; Du, D.; Sun, J.; Cao, F.; Li, H.; Jia, F.; Wang, T.; et al. The use of antibody modified liposomes loaded with AMO-1 to deliver oligonucleotides to ischemic myocardium for arrhythmia therapy. Biomaterials 2014, 35, 3697–3707.
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16.
- Bao, R.; Tan, B.; Liang, S.; Zhang, N.; Wang, W.; Liu, W. A pi-pi conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials 2017, 122, 63–71.
- Garbern, J.C.; Minami, E.; Stayton, P.S.; Murry, C.E. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 2011, 32, 2407–2416.
- Zhang, C.; Hsieh, M.H.; Wu, S.Y.; Li, S.H.; Wu, J.; Liu, S.M.; Wei, H.J.; Weisel, R.D.; Sung, H.W.; Li, R.K. A self-doping conductive polymer hydrogel that can restore electrical impulse propagation at myocardial infarct to prevent cardiac arrhythmia and preserve ventricular function. Biomaterials 2020, 231, 119672.
- Nguyen, M.M.; Carlini, A.S.; Chien, M.P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv. Mater. 2015, 27, 5547–5552.
- Sy, J.C.; Seshadri, G.; Yang, S.C.; Brown, M.; Oh, T.; Dikalov, S.; Murthy, N.; Davis, M.E. Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nat. Mater. 2008, 7, 863–868.
- Perez, C.; Sanchez, A.; Putnam, D.; Ting, D.; Langer, R.; Alonso, M.J. Poly(lactic acid)-poly(ethylene glycol) nanoparticles as new carriers for the delivery of plasmid DNA. J. Control. Release 2001, 75, 211–224.
- Chang, M.Y.; Yang, Y.J.; Chang, C.H.; Tang, A.C.; Liao, W.Y.; Cheng, F.Y.; Yeh, C.S.; Lai, J.J.; Stayton, P.S.; Hsieh, P.C. Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J. Control. Release 2013, 170, 287–294.
- Nagaoka, K.; Matoba, T.; Mao, Y.; Nakano, Y.; Ikeda, G.; Egusa, S.; Tokutome, M.; Nagahama, R.; Nakano, K.; Sunagawa, K.; et al. A New Therapeutic Modality for Acute Myocardial Infarction: Nanoparticle-Mediated Delivery of Pitavastatin Induces Cardioprotection from Ischemia-Reperfusion Injury via Activation of PI3K/Akt Pathway and Anti-Inflammation in a Rat Model. PLoS ONE 2015, 10, e0132451.
- Quadros, H.C.; Santos, L.M.F.; Meira, C.S.; Khouri, M.I.; Mattei, B.; Soares, M.B.P.; de Castro-Borges, W.; Farias, L.P.; Formiga, F.R. Development and in vitro characterization of polymeric nanoparticles containing recombinant adrenomedullin-2 intended for therapeutic angiogenesis. Int J. Pharm 2020, 576, 118997.
- Tong, F.; Chai, R.; Jiang, H.; Dong, B. In vitro/vivo drug release and anti-diabetic cardiomyopathy properties of curcumin/PBLG-PEG-PBLG nanoparticles. Int. J. Nanomed. 2018, 13, 1945–1962.
- Nam, H.Y.; McGinn, A.; Kim, P.H.; Kim, S.W.; Bull, D.A. Primary cardiomyocyte-targeted bioreducible polymer for efficient gene delivery to the myocardium. Biomaterials 2010, 31, 8081–8087.
- Galagudza, M.; Korolev, D.; Postnov, V.; Naumisheva, E.; Grigorova, Y.; Uskov, I.; Shlyakhto, E. Passive targeting of ischemic-reperfused myocardium with adenosine-loaded silica nanoparticles. Int. J. Nanomed. 2012, 7, 1671–1678.
- Ferreira, M.P.A.; Ranjan, S.; Kinnunen, S.; Correia, A.; Talman, V.; Makila, E.; Barrios-Lopez, B.; Kemell, M.; Balasubramanian, V.; Salonen, J.; et al. Drug-Loaded Multifunctional Nanoparticles Targeted to the Endocardial Layer of the Injured Heart Modulate Hypertrophic Signaling. Small 2017, 13.
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28.
- Somasuntharam, I.; Yehl, K.; Carroll, S.L.; Maxwell, J.T.; Martinez, M.D.; Che, P.L.; Brown, M.E.; Salaita, K.; Davis, M.E. Knockdown of TNF-alpha by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials 2016, 83, 12–22.
- Tian, A.; Yang, C.; Zhu, B.; Wang, W.; Liu, K.; Jiang, Y.; Qiao, Y.; Fu, H.; Li, Z. Polyethylene-glycol-coated gold nanoparticles improve cardiac function after myocardial infarction in mice. Can. J. Physiol. Pharmacol. 2018, 96, 1318–1327.
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodriguez, G.B.; Patricio, T.; Esposti, L.D.; et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 2018, 10.




