Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sang Jun Han | -- | 1993 | 2022-11-10 14:16:17 | | | |
| 2 | Dean Liu | Meta information modification | 1993 | 2022-11-11 02:41:41 | | | | |
| 3 | Dean Liu | Meta information modification | 1993 | 2022-11-18 14:40:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Park, Y.; Han, S.J. Roles of Interferons in Human Endometrial Function. Encyclopedia. Available online: https://encyclopedia.pub/entry/33953 (accessed on 04 March 2026).
Park Y, Han SJ. Roles of Interferons in Human Endometrial Function. Encyclopedia. Available at: https://encyclopedia.pub/entry/33953. Accessed March 04, 2026.
Park, Yuri, Sang Jun Han. "Roles of Interferons in Human Endometrial Function" Encyclopedia, https://encyclopedia.pub/entry/33953 (accessed March 04, 2026).
Park, Y., & Han, S.J. (2022, November 10). Roles of Interferons in Human Endometrial Function. In Encyclopedia. https://encyclopedia.pub/entry/33953
Park, Yuri and Sang Jun Han. "Roles of Interferons in Human Endometrial Function." Encyclopedia. Web. 10 November, 2022.
Copy Citation
Endometriosis is an estrogen-dependent inflammatory disease that develops in reproductive-aged women who experience pelvic pain and infertility. Even though endometriosis is not a new disease, its molecular etiology has not been clearly elucidated. Defects in the immune system might be one of the factors that promote endometriosis progression. For example, elevated levels of proinflammatory cytokines are associated with endometriosis. Interferon is one of the cytokines that is elevated in endometriotic tissues compared with normal endometrium.
endometriosis
endometrium
interferon
1. Introduction
Endometriosis is the growth of endometrial lesions outside the uterus, such as in the ovaries and peritoneal cavities [1]. Three different phenotypes of endometriosis are found in the pelvic cavity: superficial peritoneal endometriosis (SUP), ovarian endometrioma (OMA), and deeply infiltrating endometriosis (DIE) [2][3]. Red lesions are found on the surface of the peritoneum in SUP, while endometriotic tissues are invaginated into the ovary in OMA. In DIE, endometriotic tissue invades other organs, including the cervix and rectum [2]. Endometriotic lesions that are shown in red are early, active lesions with vascularization. The lesions become black after inflammatory reaction and scarification and eventually turn white [2]. Up to 5–10 percent of women of reproductive age experience symptoms of endometriosis [1]. Symptoms include mild to severe pelvic pain, infertility, painful urination or bowel movements, and abnormal menstrual flow. Surgical resection of the lesions is the primary treatment for endometriosis, but surgery cannot prevent disease relapse [4]. The 2-year and 5-year post-operative recurrence rates are about 21.5 percent and 40–50 percent, respectively [5]. The median time of post-operative recurrence is about 30 months [6]. Additionally, endometriosis is an estrogen-dependent disease [1][7]. Therefore, hormone-suppressive drugs that block the synthesis or activity of estrogens (E2), such as elagolix [8], progestin [9], and Danazol [10], have been applied to relieve endometriosis symptoms. Hormonal suppression treatment after conservative surgery significantly decreases the risk of endometriosis recurrence and its related symptoms [11]. However, hormone-blocking drugs cause severe adverse effects, such as postmenstrual symptoms, and off-target effects in other hormone-responsive organs, including bone and the brain, in endometriosis patients [4][12]. Therefore, alternative endometriosis treatments to replace hormonal therapy are in high demand.
Endometriosis is an estrogen-dependent proinflammatory disease [1][7][13]. Although the exact causes of endometriosis remain unknown, the theory of retrograde menstruation, which is an efflux of menstrual blood and cells via the fallopian tubes, is the well-accepted hypothesis by which endometriosis develops and progresses [1]. However, while 90% of reproductive-aged women experience retrograde menstruation, only 10% of them are diagnosed with endometriosis [14]. Therefore, in addition to retrograde menstruation, other factors are likely involved in the pathogenesis of endometriosis. Although the exact etiology of endometriosis has not yet been elucidated, the heritability of endometriosis has been estimated at 50 percent. In addition, a few twin studies have shown that the risk of endometriosis is increased in monozygotic twins compared with dizygotic twins, which indicates the possible contribution of specific genes or genetic alternations to endometriosis [15][16]. Meta-analyses of genome-wide association studies have identified single nucleotide polymorphisms (SNPs) associated with endometriosis risk. For example, SNPs in 11 different loci located in or near IL1A (interleukin 1 alpha), ETAA (ETAA1 activator of ATR kinase), RND3 (Rho family GTPase 3), NFE2L3 (nuclear factor, erythroid 2 like 3), WNT4 (Wnt family member 4), ID4 (inhibitor of DNA binding 4), CDKN2B-AS1 (cyclin-dependent kinase inhibitor 2B antisense RNA), VEZT (vezatin, adherens junctions transmembrane protein), GREB1 (growth regulating estrogen receptor binding 1), and FN1 (fibronectin 1) are correlated with the risk of endometriosis [17]. Among them, IL1A and NFE2L3 are the genes associated with inflammatory pathways [17]. In addition, eight loci in the IL1A gene are related to the risk of endometriosis in Japanese population, which supports the involvement of immune and inflammatory responses in the development of endometriosis [18][19]. While those genes have not yet been validated as potential targets for endometriosis treatment, a recent study has identified and validated a gene named Neuropeptide S receptor 1 (NPSR1) as a potential target through DNA sequencing [20]. This study showed that deleterious low-frequency coding variants in NPSR1 are overrepresented in patients with familial endometriosis, especially in moderate/severe stages. NPSR1 is strongly expressed in glandular epithelial cells in eutopic and ectopic endometrium, while its ligand NPS is mostly found in the stroma. Inhibition of NPSR1 with the small molecular inhibitor SHA 68R prevents the release of proinflammatory Tumor Necrosis Factor (TNF)α by monocytes in vitro. In addition, the administration of SHA 68R relieves inflammation and pain in mouse models of endometriosis. NPSR1 expression is increased in several inflammatory diseases, such as inflammatory bowel disease and asthma [21][22]. In addition, NPSR1 is found in macrophages and T lymphocytes [23]. Sundman et al. show that stimulating monocytic THP-1 cells with proinflammatory cytokines TNFα and Interferon (IFN)γ significantly increases NPSR1 isoform expression, suggesting that NPSR1 may be a key factor of proinflammatory cytokine signaling [24].
Genetic mutations in the exon-coding region of genes involved in cell adhesion and chromatin-remodeling complexes associated with endometriosis progression were identified [25]. There is no direct evidence showing how retrograde menstrual debris found outside the uterus is cleared in healthy women. However, the defective cytotoxic activity of Natural Killer (NK) cells and decreased phagocytic macrophage activity in endometriosis patients indirectly support the hypothesis that the impaired immunes surveillance system attributes to the pathogenesis of endometriosis [26][27]. In the peritoneal cavity of endometriosis patients, the immune cells are recruited and secrete excessive levels of proinflammatory cytokines (interleukin (IL)-1, IL-6, TNF, and IFNγ), which promote disease development and progression [1][28][29]. Additionally, dysregulation of growth factors is involved in endometriosis. For example, the serum levels of vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), insulin-like growth factor-I (IGF-I), granulocyte-macrophage colony-stimulating factor (GM-CSF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF) are higher in women with advanced endometriosis than in women without endometriosis [30][31][32][33]. In addition to genetic mutations, alterations in immune and growth factor signaling pathways are also causal factors for endometriosis progression.
During retrograde menstruation, refluxed apoptotic endometrial cell debris in the peritoneal area is recognized by dendritic cells. Dendritic cells presenting endometrial autoantigens express increased type I IFNs to promote monocyte differentiation, dendritic cell survival, and cytotoxic T-cell activity, which may attack endometrial cells that present autoantigens in healthy women [34][35]. However, type 1 IFN-mediated cell death signaling is dysregulated in endometriotic cells in endometriosis patients, and thus, these cells can evade the host immunosurveillance system. Type II IFN (IFNγ) fails to induce apoptosis in ectopic endometrial stromal cells, especially ovarian endometriotic cyst stromal cells, unlike in eutopic endometrial stromal cells with endometriosis and normal endometrial stromal cells [36]. While the underlying mechanisms of the IL family and TNF in endometriosis have been well studied, the IFN signaling pathways in endometriosis are not fully understood [1][35][37]. IFN signaling also plays a significant role in normal endometrium, especially during pregnancy [38].
2. Interferons in Human Endometrial Function
2.1. Type I IFNs
The endometrium is the innermost lining of the uterus and changes during the menstrual cycle in response to ovarian hormones. The endometrium consists of columnar epithelial cells, the underlying stroma containing numerous embedded glands, immune cells, and vascular endothelial cells. This lining becomes thickened during the proliferative phase (i.e., before ovulation) and prepares for a possible pregnancy during the secretory phase (i.e., after ovulation). The thickened endometrium is shed during menstruation when progesterone and estrogen levels fall [39]. In conjunction with ovarian hormones, IFNs modulate pregnancy [38][40].
Type I IFNs are highly expressed in the preimplantation blastocyst, trophoblast, and decidua trophectoderm during decidualization in mice [41]. In addition, preimplantation embryos secrete IFNα in vitro [42]. Therefore, type I IFNs might be crucial in embryo implantation and decidualization progression (Figure 1).
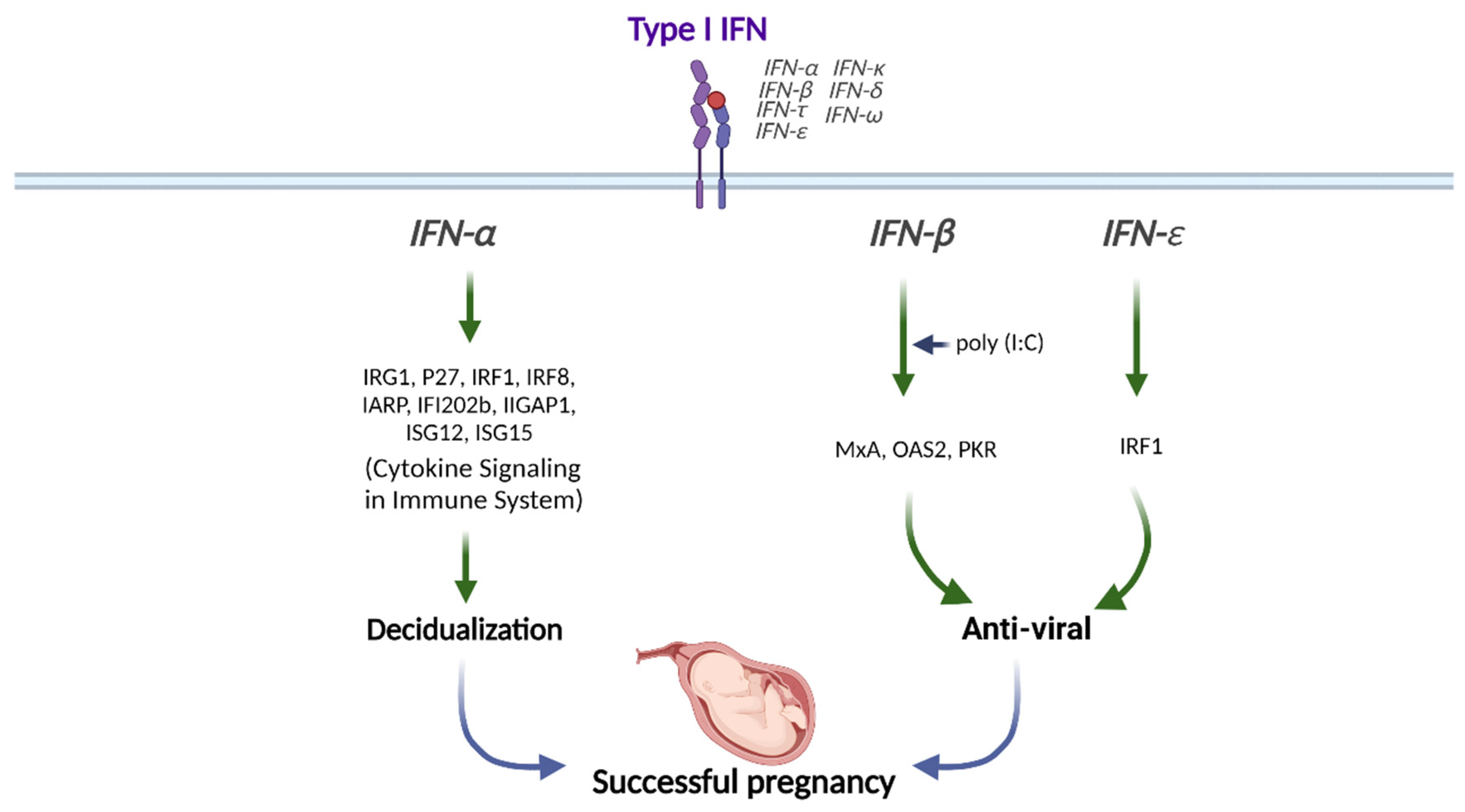
Figure 1. Role of Type I IFN in human pregnancy. Type I IFNs (IFNα, IFNβ, and IFNε) differentially modulate the downstream signals for successful pregnancy. This figure was created with BioRender.com.
IFNα stimulation synergically induces the expression of IFN-regulated gene 1 (IRG1) in humans at peri-implantation along with E2, although the underlying mechanisms are still unknown [43]. The transcription factor IFN regulatory factor 1 (IRF1) promotes implantation and is expressed in the human endometrium throughout the menstrual cycle and during peri-implantation in response to type I IFN exposure [44][45]. The levels of IFNAR1 and IFNAR2 are increased in the menstrual stage than in the proliferative phase in human endometrium [46]. IFNAR1 and IFNAR2 expressions are mostly found in the glandular epithelial cells but not in endometrial stromal cells, suggesting that glandular cells are the main cell type mediating type I IFN signaling in human endometrium [46]. In addition, ISGs, including interferon regulatory factor 8 (IRF8), interferon α-responsive protein (IARP), interferon-activated gene 202B (IFI202b), interferon γ-inducible GTPase 1 (IIGP1), interferon-stimulated gene 12 (ISG12), and ISG15, are upregulated in decidual tissues to enhance the decidualization of endometrial stromal cells in mice (Figure 1) [47].
Stimulation of human uterine epithelial cells with poly (I:C), a viral double-stranded RNA (dsRNA) mimic, increases IFNβ and ISG expressions [48]. In addition, inhibition of IFNβ signaling either with IFNβ-neutralizing antibody or IFNAR2-blocking antibody partially prevents the increases of ISG expressions, including MxA, OAS2, and PKR upon poly (I:C) stimulation, indicating that epithelial IFNβ exerts antiviral responses (Figure 1) [48].
While IFNε expression is widely found in the female reproductive tract, its expression in the endometrium is hormonally regulated during the menstrual cycle [49]. Unlike the vagina and ectocervix, in which IFNε is expressed in the basal and parabasal layers of the epithelium, IFNε is also detected in the surface of the endometrial luminal epithelium, suggesting that IFNε may play a role in immune protection from infection in the endometrium [49]. In female mice, IFNε-gene-deleted mice (IFNε−/−) are more susceptible to Herpes Simplex Virus (HSV)-2 and Chlamydia muridarum bacteria than wildtype mice [50].
In addition, vaginal concentrations of IFNε are lower in genital HSV-infected pregnant women than in healthy pregnant women [51]. The amniotic fluid concentration of IFNε is elevated in women undergoing spontaneous preterm labor with intra-amniotic infection than in women with no infection or sterile intra-amniotic inflammation [52]. The IFNε expression level is significantly elevated in the endometrium during the luteal phase than during the follicular phase. Progesterone receptor (PR) and its ligand progesterone regulate IFNε expression, in which progesterone stimulation interferes with the activation of IFNε promoter in endometrial epithelial cells in vitro and ex vivo [49]. In contrast, uterine IFNε expression is increased in estrogen-treated ovariectomized mice, which suggests hormonal regulation in IFNε expression [50]. The excessive expression of IFNε in the endometrium and its change during the menstrual cycle suggests that IFNε plays an essential role during pregnancy [49]. The vaginal concentration of IFNε is increased during pregnancy [51]. IFNε is gradually expressed in the myometrium from mid- to late gestation [52]. Therefore, IFNε exerts antiviral and anti-bacterial effects in the endometrium and during pregnancy (Figure 1).
2.2. Type II IFNs
IFNγ, the only type II IFN, is highly expressed in human trophoblast cells in the first trimester, while its receptor IFNGR is found in the placenta throughout the pregnancy [53]. Decidualized human endometrial stromal cells treated with conditioned media from human trophoblasts upregulate IFN-related genes, including IFNGR1 and JAK2 [54]. IFNγ is expressed in luminal and glandular uterine epithelial cells during the estrus stage in mice [55]. Additionally, IFNγ expression is highly increased within implantation sites in mice, which suggests the possible involvement of IFNγ in the implantation process [56]. Human uterine NK cells secrete cytokines, including IFNγ, and IFNGR is found in endometrial epithelial cells [57][58]. Ablation of NK cells, IFNγ, IFNGR, or STAT1 in mice causes defects in the decidual remodeling of spiral arteries that supply blood to the placenta. Moreover, the administration of IFNγ to NK-cell knockout (KO) mice led to the recovery of decidual artery remodeling [59]. Therefore, IFNγ may play a critical role in decidualization during the implantation process in humans.
2.3. Type III IFNs
In addition to type I and II IFNs, type III IFNs also modulate normal endometrial functions. For example, IFNλ1 has an essential role in the immune defense of the placenta against viral pathogens, as human uterine epithelial cells and fibroblasts secrete IFNλ1 after exposure to the synthetic dsRNA viral ligand poly (I:C) [60]. Sex hormones also modulate IFNλ1 signaling because estrogen suppresses, but progesterone stimulates, IFNλ1-induced ISG expression in uterine epithelial cells [60]. Therefore, IFNλ1 might differentially modulate endometrial function based on menstrual cycle stage, pregnancy, and menopausal status.
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256.
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596.
- Cornillie, F.J.; Oosterlynck, D.; Lauweryns, J.M.; Koninckx, P.R. Deeply infiltrating pelvic endometriosis: Histology and clinical significance. Fertil. Steril. 1990, 53, 978–983.
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824.
- Guo, S.W. Recurrence of endometriosis and its control. Hum. Reprod. Update 2009, 15, 441–461.
- Nirgianakis, K.; Ma, L.; McKinnon, B.; Mueller, M.D. Recurrence Patterns after Surgery in Patients with Different Endometriosis Subtypes: A Long-Term Hospital-Based Cohort Study. J. Clin. Med. 2020, 9, 496.
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815.
- Struthers, R.S.; Nicholls, A.J.; Grundy, J.; Chen, T.; Jimenez, R.; Yen, S.S.; Bozigian, H.P. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J. Clin. Endocrinol. Metab. 2009, 94, 545–551.
- Ambacher, K.; Secter, M.; Sanders, A.P. The use of progestin subdermal implants in the management of endometriosis-related pain symptoms and quality of life: A systematic review. Curr. Med. Res. Opin. 2022, 38, 479–486.
- Ashfaq, S.; Can, A.S. Danazol. In StatPearls; Copyright © 2022, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022.
- Zakhari, A.; Delpero, E.; McKeown, S.; Tomlinson, G.; Bougie, O.; Murji, A. Endometriosis recurrence following post-operative hormonal suppression: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 96–107.
- Tosti, C.; Biscione, A.; Morgante, G.; Bifulco, G.; Luisi, S.; Petraglia, F. Hormonal therapy for endometriosis: From molecular research to bedside. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 61–66.
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279.
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154.
- Treloar, S.A.; O’Connor, D.T.; O’Connor, V.M.; Martin, N.G. Genetic influences on endometriosis in an Australian twin sample. Fertil. Steril. 1999, 71, 701–710.
- Saha, R.; Pettersson, H.J.; Svedberg, P.; Olovsson, M.; Bergqvist, A.; Marions, L.; Tornvall, P.; Kuja-Halkola, R. Heritability of endometriosis. Fertil. Steril. 2015, 104, 947–952.
- Cardoso, J.V.; Perini, J.A.; Machado, D.E.; Pinto, R.; Medeiros, R. Systematic review of genome-wide association studies on susceptibility to endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 74–82.
- Hata, Y.; Nakaoka, H.; Yoshihara, K.; Adachi, S.; Haino, K.; Yamaguchi, M.; Nishikawa, N.; Kashima, K.; Yahata, T.; Tajima, A.; et al. A nonsynonymous variant of IL1A is associated with endometriosis in Japanese population. J. Hum. Genet. 2013, 58, 517–520.
- Adachi, S.; Tajima, A.; Quan, J.; Haino, K.; Yoshihara, K.; Masuzaki, H.; Katabuchi, H.; Ikuma, K.; Suginami, H.; Nishida, N.; et al. Meta-analysis of genome-wide association scans for genetic susceptibility to endometriosis in Japanese population. J. Hum. Genet. 2010, 55, 816–821.
- Tapmeier, T.T.; Rahmioglu, N.; Lin, J.; De Leo, B.; Obendorf, M.; Raveendran, M.; Fischer, O.M.; Bafligil, C.; Guo, M.; Harris, R.A.; et al. Neuropeptide S receptor 1 is a nonhormonal treatment target in endometriosis. Sci. Transl. Med. 2021, 13, eabd6469.
- Laitinen, T.; Polvi, A.; Rydman, P.; Vendelin, J.; Pulkkinen, V.; Salmikangas, P.; Makela, S.; Rehn, M.; Pirskanen, A.; Rautanen, A.; et al. Characterization of a common susceptibility locus for asthma-related traits. Science 2004, 304, 300–304.
- D’Amato, M.; Bruce, S.; Bresso, F.; Zucchelli, M.; Ezer, S.; Pulkkinen, V.; Lindgren, C.; Astegiano, M.; Rizzetto, M.; Gionchetti, P.; et al. Neuropeptide s receptor 1 gene polymorphism is associated with susceptibility to inflammatory bowel disease. Gastroenterology 2007, 133, 808–817.
- Pulkkinen, V.; Majuri, M.L.; Wang, G.; Holopainen, P.; Obase, Y.; Vendelin, J.; Wolff, H.; Rytila, P.; Laitinen, L.A.; Haahtela, T.; et al. Neuropeptide S and G protein-coupled receptor 154 modulate macrophage immune responses. Hum. Mol. Genet. 2006, 15, 1667–1679.
- Sundman, L.; Saarialho-Kere, U.; Vendelin, J.; Lindfors, K.; Assadi, G.; Kaukinen, K.; Westerholm-Ormio, M.; Savilahti, E.; Maki, M.; Alenius, H.; et al. Neuropeptide S receptor 1 expression in the intestine and skin—Putative role in peptide hormone secretion. Neurogastroenterol. Motil. 2010, 22, 79–87.
- Li, X.; Zhang, Y.; Zhao, L.; Wang, L.; Wu, Z.; Mei, Q.; Nie, J.; Li, X.; Li, Y.; Fu, X.; et al. Whole-exome sequencing of endometriosis identifies frequent alterations in genes involved in cell adhesion and chromatin-remodeling complexes. Hum. Mol. Genet. 2014, 23, 6008–6021.
- Oosterlynck, D.J.; Cornillie, F.J.; Waer, M.; Vandeputte, M.; Koninckx, P.R. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil. Steril. 1991, 56, 45–51.
- Xie, Q.; He, H.; Wu, Y.H.; Zou, L.J.; She, X.L.; Xia, X.M.; Wu, X.Q. Eutopic endometrium from patients with endometriosis modulates the expression of CD36 and SIRP-alpha in peritoneal macrophages. J. Obs. Gynaecol. Res. 2019, 45, 1045–1057.
- Lin, Y.H.; Chen, Y.H.; Chang, H.Y.; Au, H.K.; Tzeng, C.R.; Huang, Y.H. Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies. Int. J. Mol. Sci. 2018, 19, 2385.
- Malutan, A.M.; Drugan, T.; Costin, N.; Ciortea, R.; Bucuri, C.; Rada, M.P.; Mihu, D. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent. Eur. J. Immunol. 2015, 40, 96–102.
- Li, Y.Z.; Wang, L.J.; Li, X.; Li, S.L.; Wang, J.L.; Wu, Z.H.; Gong, L.; Zhang, X.D. Vascular endothelial growth factor gene polymorphisms contribute to the risk of endometriosis: An updated systematic review and meta-analysis of 14 case-control studies. Genet. Mol. Res. GMR 2013, 12, 1035–1044.
- Hayrabedyan, S.; Kyurkchiev, S.; Kehayov, I. FGF-1 and S100A13 possibly contribute to angiogenesis in endometriosis. J. Reprod. Immunol. 2005, 67, 87–101.
- KhoshdelRad, N.; Salehi, Z.; Mashayekhi, F.; Abbasi, O.; Mirzajani, E. Soluble c-Met expression in the peritoneal fluid and serum of patients with different stages of endometriosis. Arch. Gynecol. Obstet. 2014, 289, 1107–1112.
- May, K.E.; Conduit-Hulbert, S.A.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Peripheral biomarkers of endometriosis: A systematic review. Hum. Reprod. Update 2010, 16, 651–674.
- Psarras, A.; Emery, P.; Vital, E.M. Type I interferon–mediated autoimmune diseases: Pathogenesis, diagnosis and targeted therapy. Rheumatology 2017, 56, 1662–1675.
- Martensen, P.; Vestergaard, A.; Knudsen, U. Virus Infection and Type I Interferon in Endometriosis. Endometr.-Basic Concepts Curr. Res. Trends 2012, 245–262.
- Nishida, M.; Nasu, K.; Ueda, T.; Fukuda, J.; Takai, N.; Miyakawa, I. Endometriotic cells are resistant to interferon-gamma-induced cell growth inhibition and apoptosis: A possible mechanism involved in the pathogenesis of endometriosis. Mol. Hum. Reprod. 2005, 11, 29–34.
- Herington, J.L.; Bruner-Tran, K.L.; Lucas, J.A.; Osteen, K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011, 7, 611–626.
- Casazza, R.L.; Lazear, H.M.; Miner, J.J. Protective and Pathogenic Effects of Interferon Signaling During Pregnancy. Viral Immunol. 2020, 33, 3–11.
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179.
- Murphy, S.P.; Tayade, C.; Ashkar, A.A.; Hatta, K.; Zhang, J.; Croy, B.A. Interferon gamma in successful pregnancies. Biol. Reprod. 2009, 80, 848–859.
- Yamamoto, Y.; Kurohmaru, M.; Hayashi, Y. Localization of type I interferon in murine trophoblast and decidua during decidual formation. J. Reprod. Fertil. 1992, 95, 559–565.
- Jones, K.P.; Warnock, S.H.; Urry, R.L.; Edwin, S.S.; Mitchell, M.D. Immunosuppressive activity and alpha interferon concentrations in human embryo culture media as an index of potential for successful implantation. Fertil. Steril. 1992, 57, 637–640.
- Li, Q.; Zhang, M.; Kumar, S.; Zhu, L.J.; Chen, D.; Bagchi, M.K.; Bagchi, I.C. Identification and implantation stage-specific expression of an interferon-alpha-regulated gene in human and rat endometrium. Endocrinology 2001, 142, 2390–2400.
- Kitaya, K.; Yasuda, J.; Fushiki, S.; Honjo, H. Localization of interferon regulatory factor-1 in human endometrium throughout the menstrual cycle. Fertil. Steril. 2001, 75, 992–996.
- Jabbour, H.N.; Critchley, H.O.; Yu-Lee, L.Y.; Boddy, S.C. Localization of interferon regulatory factor-1 (IRF-1) in nonpregnant human endometrium: Expression of IRF-1 is up-regulated by prolactin during the secretory phase of the menstrual cycle. J. Clin. Endocrinol. Metab. 1999, 84, 4260–4265.
- Ozaki, T.; Takahashi, K.; Kanasaki, H.; Iida, K.; Miyazaki, K. Expression of the type I interferon receptor and the interferon-induced Mx protein in human endometrium during the menstrual cycle. Fertil. Steril. 2005, 83, 163–170.
- Kashiwagi, A.; DiGirolamo, C.M.; Kanda, Y.; Niikura, Y.; Esmon, C.T.; Hansen, T.R.; Shioda, T.; Pru, J.K. The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology 2007, 148, 4173–4184.
- Patel, M.V.; Ghosh, M.; Fahey, J.V.; Wira, C.R. Uterine epithelial cells specifically induce interferon-stimulated genes in response to polyinosinic-polycytidylic acid independently of estradiol. PloS One 2012, 7, e35654.
- Bourke, N.M.; Achilles, S.L.; Huang, S.U.; Cumming, H.E.; Lim, S.S.; Papageorgiou, I.; Gearing, L.J.; Chapman, R.; Thakore, S.; Mangan, N.E.; et al. Spatiotemporal regulation of human IFNepsilon and innate immunity in the female reproductive tract. JCI Insight 2022, 7, e135407.
- Fung, K.Y.; Mangan, N.E.; Cumming, H.; Horvat, J.C.; Mayall, J.R.; Stifter, S.A.; De Weerd, N.; Roisman, L.C.; Rossjohn, J.; Robertson, S.A.; et al. Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science 2013, 339, 1088–1092.
- Nickodem, C.; Criscitiello, M.F.; Bazer, F.; Abiodun-Ojo, O.; Taylor, B.D. Interferon epsilon in the reproductive tract of healthy and genital herpes simplex virus-infected pregnant women: Results of a pilot study. Am. J. Reprod. Immunol. 2018, 80, e12995.
- Miller, D.; Romero, R.; Kacerovsky, M.; Musilova, I.; Galaz, J.; Garcia-Flores, V.; Xu, Y.; Pusod, E.; Demery-Poulos, C.; Gutierrez-Contreras, P.; et al. Defining a role for Interferon Epsilon in normal and complicated pregnancies. Heliyon 2022, 8, e09952.
- Paulesu, L.; Romagnoli, R.; Cintorino, M.; Ricci, M.G.; Garotta, G. First trimester human trophoblast expresses both interferon-gamma and interferon-gamma-receptor. J. Reprod. Immunol. 1994, 27, 37–48.
- Hess, A.P.; Hamilton, A.E.; Talbi, S.; Dosiou, C.; Nyegaard, M.; Nayak, N.; Genbecev-Krtolica, O.; Mavrogianis, P.; Ferrer, K.; Kruessel, J.; et al. Decidual stromal cell response to paracrine signals from the trophoblast: Amplification of immune and angiogenic modulators. Biol. Reprod. 2007, 76, 102–117.
- Platt, J.S.; Hunt, J.S. Interferon-gamma gene expression in cycling and pregnant mouse uterus: Temporal aspects and cellular localization. J. Leukoc. Biol. 1998, 64, 393–400.
- Seaward, A.V.; Burke, S.D.; Croy, B.A. Interferon gamma contributes to preimplantation embryonic development and to implantation site structure in NOD mice. Hum. Reprod. (Oxf. Engl.) 2010, 25, 2829–2839.
- Saito, S.; Nishikawa, K.; Morii, T.; Enomoto, M.; Narita, N.; Motoyoshi, K.; Ichijo, M. Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int. Immunol. 1993, 5, 559–563.
- Tabibzadeh, S. Evidence of T-cell activation and potential cytokine action in human endometrium. J. Clin. Endocrinol. Metab. 1990, 71, 645–649.
- Ashkar, A.A.; Di Santo, J.P.; Croy, B.A. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 2000, 192, 259–270.
- Patel, M.V.; Hopkins, D.C.; Barr, F.D.; Wira, C.R. Sex Hormones and Aging Modulate Interferon Lambda 1 Production and Signaling by Human Uterine Epithelial Cells and Fibroblasts. Front. Immunol. 2021, 12, 718380.
More
Information
Subjects:
Reproductive Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
18 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





