Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bárbara Costa | -- | 2049 | 2022-11-10 10:59:10 | | | |
| 2 | Sirius Huang | Meta information modification | 2049 | 2022-11-11 02:19:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Costa, B.; Mourão, J.; Vale, N. Effect of Anesthetics on Cancer Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/33943 (accessed on 08 February 2026).
Costa B, Mourão J, Vale N. Effect of Anesthetics on Cancer Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/33943. Accessed February 08, 2026.
Costa, Bárbara, Joana Mourão, Nuno Vale. "Effect of Anesthetics on Cancer Treatment" Encyclopedia, https://encyclopedia.pub/entry/33943 (accessed February 08, 2026).
Costa, B., Mourão, J., & Vale, N. (2022, November 10). Effect of Anesthetics on Cancer Treatment. In Encyclopedia. https://encyclopedia.pub/entry/33943
Costa, Bárbara, et al. "Effect of Anesthetics on Cancer Treatment." Encyclopedia. Web. 10 November, 2022.
Copy Citation
Propofol, fentanyl, rocuronium, sugammadex, and dexamethasone are commonly used to induce anesthesia and prevent pain during surgery. The mechanisms of these drugs to induce the state of anesthesia are not yet fully understood, despite their use being considered safe. An association between anesthetic agents and cancer progression has been determined; therefore, it is essential to recognize the effects of all agents during cancer treatment and to evaluate whether the treatment provided to the patients could be more precise.
anesthesia
cancer recurrence
metastasis
drug interactions
1. Introduction
An area of ongoing attention is the determination of how anesthetic medications affect long-term oncological outcomes following cancer surgery. Surgery for cancer frequently makes use of anesthetics. Events that occur during surgery, such as being subjected to general or local anesthetic, may have an impact on the progression of cancer. Cancer patients undergoing surgery incur the risk of cancer recurrence and metastasis due to the triggering of the body’s natural stress response, which begins with increasing pro-inflammatory release, neuroendocrine signaling, and immunomodulation. However, depending on the type of cancer and how the therapy is administered, anesthesia may either inhibit cancer or serve as a catalyst for metastasis, which results in a detrimental effect on cancer growth [1][2].
Recent experimental results revealed that several of the anesthetics most frequently used in surgical oncology, whether general or local agents, can modify gene expression and epigenetic alterations [3][4]. Considering this, more studies should be developed to identify the best procedure (general or local/regional anesthesia) for any given patient population or disease. The use of different agents in anesthesia should be better understood to mitigate pro-inflammatory responses and regulate oncogenes, since different types of anesthesia assert different mechanism at play, see Figure 1.
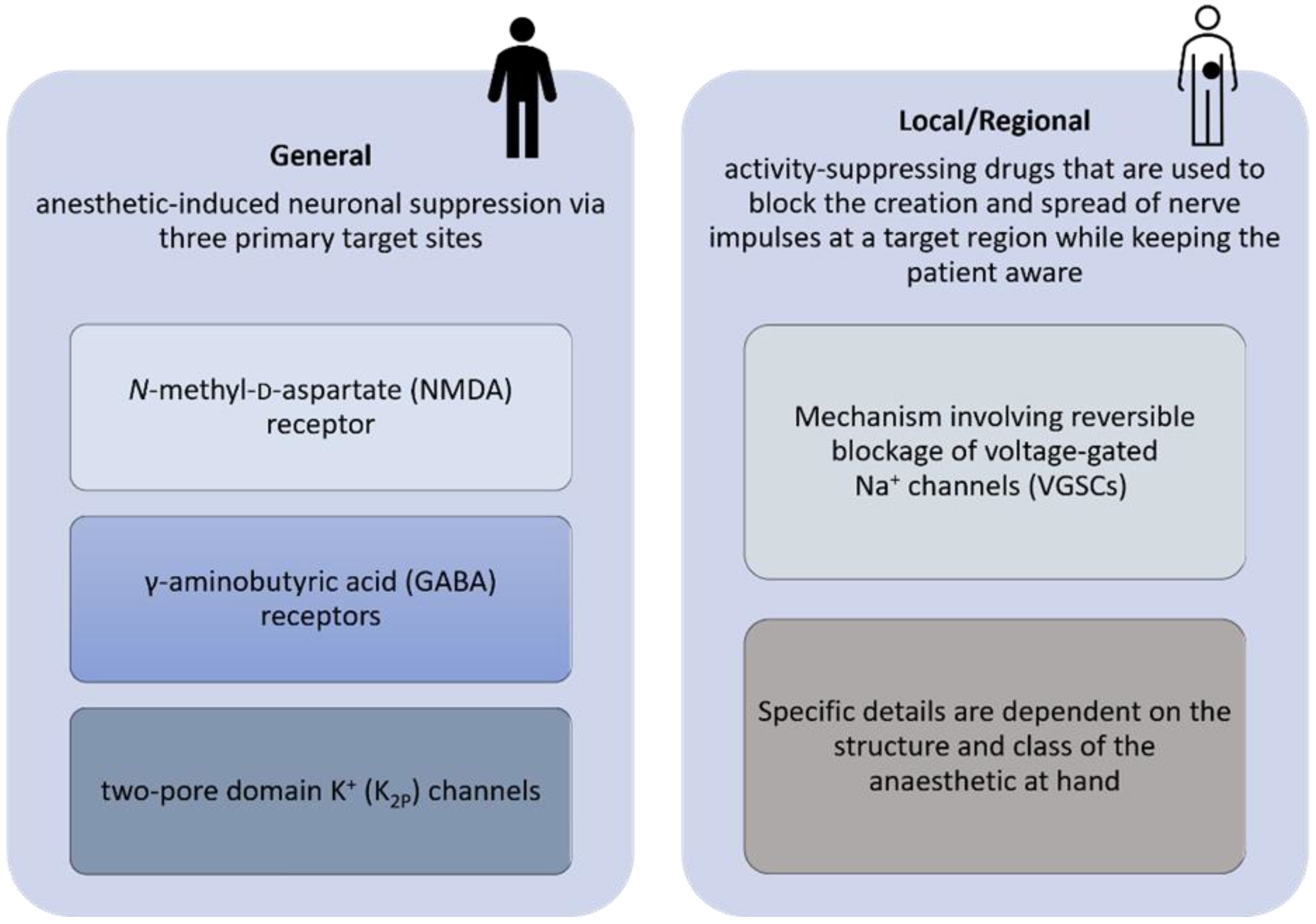
Figure 1. Diagram proposed for the pharmacology of general, local, and regional anesthetics.
2. Effect of Anesthetics on Cancer Treatment
Agents used to maintain and induce general anesthesia have anti-inflammatory and immunomodulatory effects [5]. According to the literature, regional anesthetics can protect cell-mediated immunity, reduce neuroendocrine stress, and may reduce the need for opioids [6]. On the other hand, local anesthetics can block the VGSC activity during or after surgery, resulting in a reduced ability of the cancer cells to escape from the preoperative area, and can thus increase chemo-sensitization, Figure 2 [7].
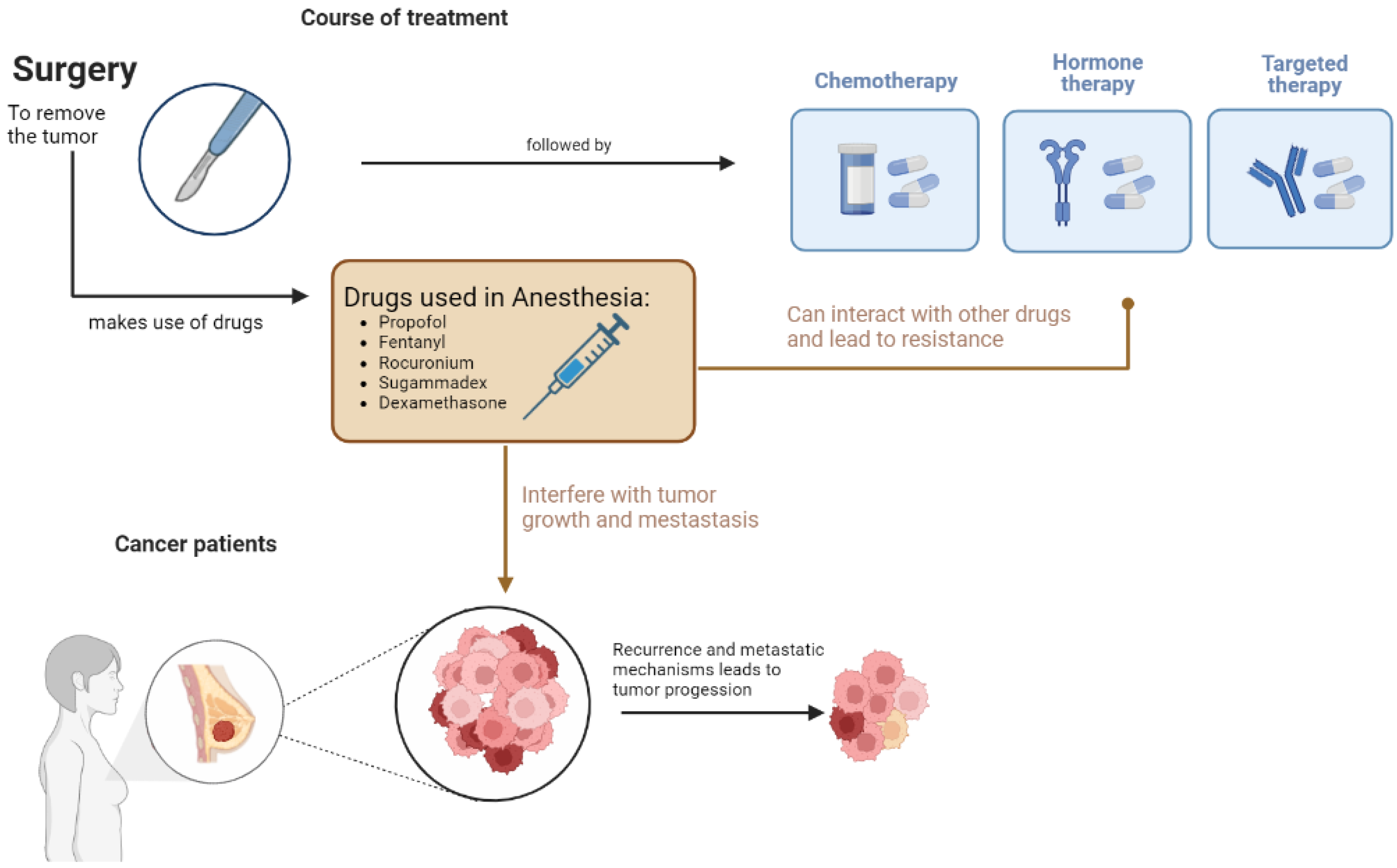
Figure 2. Schematization of the effect of drugs used in anesthesia in cancer progression and metastasis.
Propofol is a widely used anesthetic that can be correlated with anti-tumor and cancer-inducing effects by regulating different signaling pathways. A few studies have shown that propofol stimulated nuclear factor E2 and related factor 2 (Nrf2) at the transcriptional and translational levels, promoting cell proliferation and invasion in gallbladder cancer in a dose- and time-dependent manner [8]. Accordingly, in human breast cancer MDA-MB-231 cells, propofol increased cell migration via activating the Nrf2 signaling system and stimulating cell proliferation, in part by downregulating the expression of p53 [9]. However, numerous studies have produced the opposite findings for different cancers. More recently, this dysfunction of metabolic reprogramming was documented on gastric cancer cells; however, in this case, the cell proliferation was suppressed through the Nrf2-mediated polyol pathway [10].
Furthermore, propofol in combination with chemotherapeutic drugs alters the effectiveness of chemotherapeutic treatment. For instance, through the EGFR/JAK2/STAT3 pathway, propofol increased the chemosensitivity of cervical cancer cells to cisplatin-induced death [11]. Another example is the result that treatment with propofol and cisplatin increased the FOXO1 pathway’s activity in ovarian cancer cells, promoting cell death [12]. Another study discovered that propofol increased the chemosensitivity of pancreatic cancer cells to gemcitabine by decreasing the activation of the NF-B signaling pathway induced by gemcitabine. Moreover, propofol can epigenetically regulate trastuzumab resistance in breast cancer [13]. Interestingly, in numerous clinical studies, it was discovered that propofol has no impact on the prognosis of various malignancies; according to several retrospective studies, propofol did not affect the prognosis for other cancer types after surgery [14]. In a retrospective cohort analysis, for instance, there was no difference in mortality or the rate of locoregional recurrence at 5 years following propofol therapy for breast cancer surgery [15]. These results were in accordance with those of earlier studies. A retrospective study of NSCLC patients found no difference in overall survival (OS) or relapse-free survival (RFS) between the propofol group and the inhalation group [16]. In a recent trial, the use of propofol intraoperative anesthesia had no effect on OS or progression-free survival in patients with high-grade gliomas undergoing cancer surgery (PFS) [14]. Similarly, there was no difference in cancer-related mortality among patients who underwent surgery for stomach cancer, whether or not propofol was used.
Although numerous in vitro and in vivo studies point towards a beneficial use of propofol for certain cancers, clinical retrospective and prospective studies assessing the connection between propofol and prognosis have thus far present contradicting conclusions: either propofol has no influence on survival outcomes, or it can increase survival in many cancer patients. For example, propofol treatment for colon cancer resulted in better OS and disease-free survival (DFS) and reduced postoperative metastases [17]. Nonetheless, these findings have some important limitations. Interestingly, many investigations have revealed that the initial site of cancer may have an impact on the various effects of propofol on oncogenic outcomes. Moreover, most of the research continues to demonstrate that propofol improves prognosis. Cancers that seem to have a better prognosis after utilizing propofol during the surgery encourage its use in favor of the patient. Prospective multicenter studies, with bigger sample sizes, are currently being conducted for malignancies in which propofol did not affect the prognosis [14]. To better understand the impact that anesthesia has on the development of cancer and to influence the selection of anesthetics used during cancer surgery, additional animal experiments and prospective clinical studies are required.
Opioids are powerful immunomodulators affecting NK cells activity and cytokine production, being pro-angiogenic through the activation of VEGF-receptors [18]. Fentanyl can have an effect on NK-cell activity and lymphocyte proliferation [19]. Moreover, the use of opioids for the treatment of pain in cancer patients is very common post-operatively. Fentanyl suppresses the growth and invasion of lung cancer by upregulating miR-331-3p and suppressing HDAC5 [20]. The results of the study demonstrated that fentanyl treatment reduced the number of cancer cells and cancer stem cells in the PANC-1 cell population, decreased the expression of stem cell markers, and increased the expression of genes related to apoptosis. These findings suggest that fentanyl, which is frequently used to relieve pancreatic cancer pain, may be an alternative for pancreatic cancer treatment.
The effect of fentanyl on chemotherapeutic drugs after cancer surgery has been well explored; however, no evidence emerges relating reduced sensitivity of cancer cells to chemotherapy. In a cell proliferation study, the effect of fentanyl and 5-fluorouracil was assessed in human colon cancer cell lines, and there was no evidence of affected sensitivity [21]. Moreover, in patients with ovarian cancer treated with anthracycline, fentanyl was considered safe for use [22]. However, strong CYP3A4 iso-enzyme inhibitors, such as itraconazole, can increase the plasma concentrations and pharmacologic effects of fentanyl, due to this CYP3A4 inhibitor’s potential reduction in the metabolic clearance of this opioid. More examples may follow, as bicalutamide and dexamethasone, which also affect the CYP3A4 enzyme, can increase or decrease (respectively) the level or effect of fentanyl [23]. Therefore, it is necessary to watch for the cumulative narcotic effects of fentanyl when this drug is administered with another chemotherapeutics.
Rocuronium can be correlated with the invasion, adhesion, and migration of cancer cells. Jiang et al. evaluated these effects on breast cancer cells (MDA-231) and gastric cancer cells (SGC7901, and BCG 823) [24][25]. Additionally, another in vitro study on MRC-5 cells with rocuronium demonstrated a reduced expression of stromal cell-derived factor-1 (SDF-1). This study indicates the effect of anesthetics on fibroblasts, a component of the tumor microenvironment [26]. These results alone only shed light on how cancer surgery must be optimized to lower the risk of recurrence and metastasis; however, more research is needed to confirm these results (in vitro, in silico, and clinical trials).
Sugammadex is considered to enhance recovery after surgery in cancer patients, and a link between its effect on chemotherapy is only documented for breast cancer patients. Sugammadex is related to growth hormone activities [27], also binding to steroid-structure molecules [28], such as estrogen and tamoxifen. Therefore, different agents should be given to patients on tamoxifen to reverse NMB. Otherwise, sugammadex may not be as effective in removing rocuronium [29]. The same applies for the drug toremifene, which is structurally similar to tamoxifen. The researchers could not find any articles reporting the effect of these drugs on cancer cells, and this may be due to the size of this drug, as sugammadex does not enter the cells [30].
Dexamethasone is already used in combination with other drugs as a treatment for certain types of cancer, such as lymphoma, leukemia, or multiple myeloma, and it is widely used, either alone or with other drugs, to prevent certain conditions related to cancer (e.g., drug hypersensitivity). In multiple myeloma, dexamethasone in combination with lenalidomide improves the success of treatment by extending time-to-progression and survival. Dexamethasone reduces inflammation and suppresses the immune response by inhibiting CD28-mediated cell cycle entrance and differentiation, while upregulating CTLA-4 in the CD4 and CD8T cells. These data imply that corticosteroids hinder the response in immunotherapy. Giles et al. observed that with the injection of CTLA-4 for inhibition, T cells might be somewhat shielded or saved from the immunosuppressive effects of dexamethasone. Additionally, after an effective anti-tumor immune response, harmful corticosteroid effects are reduced. This research indicates that the effectiveness of immunotherapy is greatly influenced by the timing of dexamethasone treatment in relation to the emergence of anti-tumor immunity [31].
Moreover, before chemotherapy, dexamethasone is used in breast cancer patients to prevent adverse chemo effects. Several studies have shown that this pre-treatment improves the chemotherapy effects against breast cancer and may prevent metastasis in breast cancer by diminishing cell adhesion and migration. Mohammadi et al. observed a decrease in viability in T47D breast cancer cells. However, a different study stated that dexamethasone could promote the lung metastasis of breast cancer via the PI3K-SGK1-CTGF pathway, both in vitro and in vivo [32]. Dexamethasone pre-treatment prior to paclitaxel is a routine method used in the treatment of breast cancer, and metastasis caused by chemotherapy drugs has been documented in the past. Dexamethasone provides a stronger pro-metastatic ability [32]. These findings should prompt serious reservations about the therapeutic application of paclitaxel or other treatments in breast cancer patients. In chemotherapy, dexamethasone is still a commonly utilized pre-treatment drug due to its durable effects and generally affordable. Keep in mind that dexamethasone is still frequently used as a pre-treatment medication in the treatment of tumors because it successfully lessens the symptoms regarding nervous system compression and vomiting caused by chemotherapy, as well as prevents radiotherapy-related toxicity and other side effects.
Interestingly, in the MCF-7 and MDA-MB-231 xenograft mouse models, the administration of low-dose dexamethasone decreased tumor growth and distant metastasis, but treatment with high-dose dexamethasone increased tumor growth and metastasis, respectively. Breast cancer cells subjected to dexamethasone treatment showed a dose-dependent inhibition of cell adhesion, migration, and invasion. Part of the manner in which dexamethasone inhibits cell adhesion, migration, and invasion in MDA-MB-231 cells is via causing the induction of microRNA-708 and the subsequent mediation of Rap1B-signaling. On the other hand, the dexamethasone suppression of cell migration in MCF-7 cells does not depend on microRNA-708-mediated signaling. Overall, dexamethasone has a double role in the progression and metastasis of breast cancer: While larger amounts might unintentionally advance breast cancer, lower quantities prevent the growth and metastasis of breast cancer tumors [33], highlighting the importance of using in silico studies and further developing precision medicine to guarantee the proper treatment.
In conclusion, it is interesting to acknowledge that individuals could benefit or suffer, depending on the drugs used to induce and maintain general anesthesia during surgical intervention. There is inadequate evidence to support the use of certain anesthetic agents or techniques to lower the risk of cancer recurrence in individuals who undergo cancer surgery, despite laboratory, animal, and retrospective human data suggesting that anesthetic agents may alter cancer recurrence (Figure 3). The use of in silico studies is a tool that we can use in our favor to gain more information and make more powerful and informed decisions during treatment.
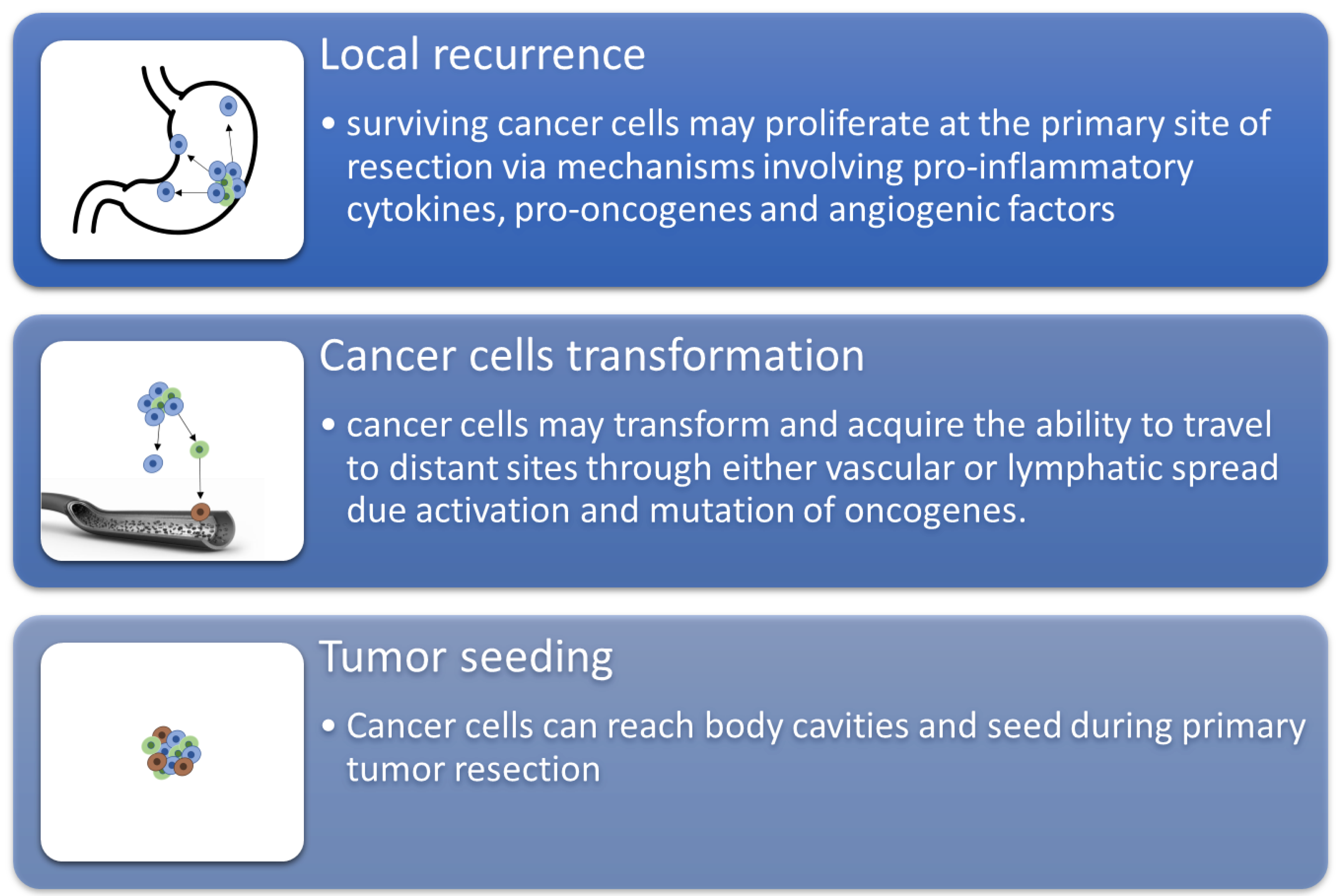
Figure 3. The schematization of recurrence and metastasis through three main mechanisms.
References
- Perry, N.J.S.; Buggy, D.; Ma, D. Can Anesthesia Influence Cancer Outcomes After Surgery? JAMA Surg. 2019, 154, 279–280.
- Niwa, H.; Rowbotham, D.J.; Lambert, D.G.; Buggy, D.J. Can Anesthetic Techniques or Drugs Affect Cancer Recurrence in Patients Undergoing Cancer Surgery? J. Anesth. 2013, 27, 731–741.
- Huitink, J.M.; Heimerikxs, M.; Nieuwland, M.; Loer, S.A.; Brugman, W.; Velds, A.; Sie, D.; Kerkhoven, R.M. Volatile Anesthetics Modulate Gene Expression in Breast and Brain Tumor Cells. Anesth Analg. 2010, 111, 1411–1415.
- Montejano, J.; Jevtovic-Todorovic, V. Anesthesia and Cancer, Friend or Foe? A Narrative Review. Front. Oncol. 2021, 11, 5525.
- Cruz, F.F.; Rocco, P.R.M.; Pelosi, P. Anti-Inflammatory Properties of Anesthetic Agents. Crit. Care 2017, 21, 17.
- Kim, R. Effects of Surgery and Anesthetic Choice on Immunosuppression and Cancer Recurrence. J. Transl. Med. 2018, 16, 8.
- Zhang, Y.; Jing, Y.; Pan, R.; Ding, K.; Chen, R.; Meng, Q. Mechanisms of Cancer Inhibition by Local Anesthetics. Front. Pharmacol. 2021, 12, 770694.
- Zhang, L.; Wang, N.; Zhou, S.; Ye, W.; Jing, G.; Zhang, M. Propofol Induces Proliferation and Invasion of Gallbladder Cancer Cells through Activation of Nrf2. J. Exp. Clin. Cancer Res. 2012, 31, 66.
- Chao, M.; Linlin, S.; Juan, W.; Li, D.; Liu, Y.; Cui, X. Propofol Induces Proliferation Partially via Downregulation of P53 Protein and Promotes Migration via Activation of the Nrf2 Pathway in Human Breast Cancer Cell Line MDA-MB-231. Oncol. Rep. 2017, 37, 841–848.
- Cao, Y.; Fan, L.; Li, L.; Zhou, J. Propofol Suppresses Cell Proliferation in Gastric Cancer Cells through NRF2-Mediated Polyol Pathway. Clin. Exp. Pharmacol. Physiol. 2022, 49, 264–274.
- Li, H.; Lu, Y.; Pang, Y.; Li, M.; Cheng, X.; Chen, J. Propofol Enhances the Cisplatin-Induced Apoptosis on Cervical Cancer Cells via EGFR/JAK2/STAT3 Pathway. Biomed. Pharmacother. 2017, 86, 324–333.
- Sun, Y.; Peng, Y.B.; Ye, L.L.; Ma, L.X.; Zou, M.Y.; Cheng, Z.G. Propofol Inhibits Proliferation and Cisplatin Resistance in Ovarian Cancer Cells through Regulating the MicroRNA-374a/Forkhead Box O1 Signaling Axis. Mol. Med. Rep. 2020, 21, 1471.
- Tian, D.; Tian, M.; Ma, Z.M.; Zhang, L.; Cui, Y.F.; Li, J. long Anesthetic Propofol Epigenetically Regulates Breast Cancer Trastuzumab Resistance through IL-6/MiR-149-5p Axis. Sci. Rep. 2020, 10, 8858.
- Xu, Y.; Pan, S.; Jiang, W.; Xue, F.; Zhu, X. Effects of Propofol on the Development of Cancer in Humans. Cell Prolif. 2020, 53, e12867.
- Huang, Y.H.; Lee, M.S.; Lou, Y.S.; Lai, H.C.; Yu, J.C.; Lu, C.H.; Wong, C.S.; Wu, Z.F. Propofol-Based Total Intravenous Anesthesia Did Not Improve Survival Compared to Desflurane Anesthesia in Breast Cancer Surgery. PLoS ONE 2019, 14, e0224728.
- Oh, T.K.; Kim, K.; Jheon, S.; Lee, J.; Do, S.H.; Hwang, J.W.; Song, I.A. Long-Term Oncologic Outcomes for Patients Undergoing Volatile VersusIntravenous Anesthesia for Non-Small Cell Lung Cancer Surgery: A Retrospective Propensity Matching Analysis. Cancer Control. 2018, 25, 1073274818775360.
- Wu, Z.F.; Lee, M.S.; Wong, C.S.; Lu, C.H.; Huang, Y.S.; Lin, K.T.; Lou, Y.S.; Lin, C.; Chang, Y.C.; Lai, H.C. Propofol-Based Total Intravenous Anesthesia Is Associated with Better Survival Than Desflurane Anesthesia in Colon Cancer Surgery. Anesthesiology 2018, 129, 932–941.
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904.
- Boland, J.W.; Pockley, A.G. Influence of Opioids on Immune Function in Patients with Cancer Pain: From Bench to Bedside. Br. J. Pharmacol. 2018, 175, 2726.
- Gong, S.; Ying, L.; Fan, Y.; Sun, Z. Fentanyl Inhibits Lung Cancer Viability and Invasion via Upregulation of MiR-331-3p and Repression of HDAC5. Onco. Targets. Ther. 2020, 13, 13131–13141.
- Nomura, Y.; Kawaraguchi, Y.; Sugimoto, H.; Furuya, H.; Kawaguchi, M. Effects of Morphine and Fentanyl on 5-Fluorouracil Sensitivity in Human Colon Cancer HCT116 Cells. J. Anesth. 2014, 28, 298–301.
- Thorne, A.C.; Orazem, J.P.; Shah, N.K.; Matarazzo, D.; Dwyer, D.; Pierri, M.K.; Hoskins, W.J.; Rubin, S.C.; Bedford, R.F. Isoflurane versus Fentanyl: Hemodynamic Effects in Cancer Patients Treated with Anthracyclines. J. Cardiothorac. Vasc. Anesth. 1993, 7, 307–311.
- Korucu, F.C.; Senyigit, E.; Köstek, O.; Demircan, N.C.; Erdogan, B.; Uzunoglu, S.; Cicin, I. A Retrospective Study on Potential Drug Interactions: A Single Center Experience. J. Oncol. Sci. 2018, 4, 80–84.
- Jiang, A.; Zhao, H.; Cai, J.; Jiang, W.G. Possible Effect of Muscle-Relaxant Anaesthetics on Invasion, Adhesion and Migration of Breast Cancer Cells. Anticancer. Res. 2016, 36, 1259–1265. Available online: https://ar.iiarjournals.org/content/36/3/1259.long (accessed on 12 July 2022).
- Jiang, A.; Zhao, H.; Liu, X.; Yu, M.; Chen, J.; Jiang, W.G. Comparison of Different Muscle-Relaxant Anesthetics on Growth, Migration and Invasion of Gastric Cancer Cells. Anticancer. Res. 2017, 37, 4371–4378. Available online: https://ar.iiarjournals.org/content/37/8/4371.long (accessed on 12 July 2022).
- Gong, W.; Martin, T.A.; Sanders, A.J.; Hargest, R.; Jiang, A.; Sun, P.; Jiang, W.G. Influence of Anaesthetics on the Production of Cancer Cell Motogens, Stromal Cell-Derived Factor-1 and Hepatocyte Growth Factor by Fibroblasts. Oncol. Lett. 2021, 21, 140.
- Lyu, X.; Xie, F.; Tao, Y.; Bai, J. Sugammadex Affects GH/GHR’s Signaling Transduction on Muscle Cells by Regulating the Membrane-Localized GHR Level. Turkish J. Biochem. 2022, 47, 333–339.
- Gunduz Gul, G.; Ozer, A.B.; Demirel, I.; Aksu, A.; Erhan, O.L. The Effect of Sugammadex on Steroid Hormones: A Randomized Clinical Study. J. Clin. Anesth. 2016, 34, 62–67.
- Sekar, V. Center for Drug Evaluation and Research Application Number: 022225Orig1s000 Clinical Pharmacology and Biopharmaceutics Review(s). Available online: https://www.semanticscholar.org/paper/CENTER-FOR-DRUG-EVALUATION-AND-RESEARCH-APPLICATION-Sekar/2d13d0cfdfb159c5a741dbfa5a4f5af36da96a0a (accessed on 12 July 2022).
- Kleijn, H.J.; Zollinger, D.P.; van den Heuvel, M.W.; Kerbusch, T. Population Pharmacokinetic–Pharmacodynamic Analysis for Sugammadex-Mediated Reversal of Rocuronium-Induced Neuromuscular Blockade. Br. J. Clin. Pharmacol. 2011, 72, 415–433.
- Giles, A.J.; Hutchinson, M.K.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-Induced Immunosuppression: Mechanisms and Implications for Immunotherapy. J. Immunother. Cancer 2018, 6, 51.
- Zhang, Y.; Shi, G.; Zhang, H.; Xiong, Q.; Cheng, F.; Wang, H.; Luo, J.; Zhang, Y.; Shi, P.; Xu, J.; et al. Dexamethasone Enhances the Lung Metastasis of Breast Cancer via a PI3K-SGK1-CTGF Pathway. Oncogene 2021, 40, 5367–5378.
- Pang, J.M.; Huang, Y.C.; Sun, S.P.; Pan, Y.R.; Shen, C.Y.; Kao, M.C.; Wang, R.H.; Wang, L.H.; Lin, K.T. Effects of Synthetic Glucocorticoids on Breast Cancer Progression. Steroids 2020, 164, 108738.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
11 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




