Sarcopenia is a disease that becomes more prevalent as the population ages, since it is directly linked to the process of senility, which courses with muscle atrophy and loss of muscle strength. Over time, sarcopenia is linked to obesity, being known as sarcopenic obesity, and leads to other metabolic changes. At the molecular level, organokines act on different tissues and can improve or harm sarcopenia. It all depends on their production process, which is associated with factors such as physical exercise, the aging process, and metabolic diseases. Because of the seriousness of these repercussions, herein, it is to conduct a review on the relationship between organokines, sarcopenia, diabetes, and other metabolic repercussions, as well the role of physical exercise.
1. Muscular Tissue, Sarcopenia, and Physical Exercises
Muscle contraction is one of the functions of the muscular tissue, which is given by the principle of conducting chemical synapses through electrical impulses from the motor neuron to the muscle fibers innervated in the neuromuscular junction (NMJ) region. The NMJ has some central regions: presynaptic (including Schwann cells surrounding the nerve terminal with a neurotransmitter); the synaptic space lined by the basement membrane; and the postsynaptic area (with the junctional sarcoplasm) and the postsynaptic membrane containing receptors for the neurotransmitter
[1][2].
As pointed out above, during the aging process, there is a reduction in the number and a decrease in the size of skeletal muscle fibers. Mitchell et al.
[3] found a reduction in skeletal muscle mass of about 0.37 and 0.47%/year in women and men, respectively. Other clinical studies involving aging humans indicated that muscle strength decreases in a higher way in older adults than muscle mass. With aging, changes in structural proteins may occur, causing reduced muscle performance and alteration of proteins, such as skeletal muscle cells’ calcium handling proteins. In addition, aging can also promote a decline in muscle performance due to the imbalance of excitation–contraction duet, an increase in intermuscular fat tissue, and the quantity of extracellular water associated with the muscle volume
[4][5].
Sarcopenia is the condition in which the muscles present a reduced number of myofibers and hypotrophic myofibers (mainly type II myofibers), infiltration with adipose tissue, and, in later stages, fibrotic and a decrease in the number of satellite cells (SCs). The etiology of the onset of sarcopenia is multifactorial, involving neurological factors related to the loss of motor neurons, endocrine changes due to reduction or loss of hormone expression, such as testosterone or growth hormone (GH), loss of muscle motor units, and also by nutritional and lifestyle changes closely linked to adherence to sedentary habits
[6][7][8].
The pathogenesis of sarcopenia can lead to several molecular dysregulations. Forkhead box O3 (FoxO3)-dependent transcription and reduced protein production positively regulated by insulin-like growth factor-1 (IGF-1) and via phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) lead to reduced muscle size due to smaller cell size (loss of cell content) resulting from catabolism of the protein through amplified proteasomal and lysosomal functions. This process also reduces energy production, leading to fatigue. FoxO3a leads to iron accumulation, as it leads to increased expression of human ferritin and positively regulates iron transport, increasing motor neuron vulnerability to degeneration and negatively affecting myokine production. Thus, there may be a two-way pathway, as atrophy and reduced physical performance may be due to myokine signaling that is modified during sarcopenia, and at the same time, the reduction and expression of myokine generation are due to muscle failure
[9][10][11].
Myokines and interleukin IL-15 are capable of stimulating myofiber hypertrophy, IL-6 increases glucose uptake and fatty acid oxidation, IL-8 can lead to the stimulation of angiogenesis, and they have a relationship with age-related alterations in muscle function and strength. TNF-α activates endothelial cells and the synthesis of nitric oxide (NO), increasing vascular permeability, promoting the release of pro-inflammatory cells, and triggering the inflammatory process. As a result, it is an important marker of muscle loss and muscle strength during the latent inflammatory process. The activation of apoptosis in muscle cells can also be stimulated by this organokine. The association of IL-6 and TNF-α results in the liver production of acute-phase reactive proteins (C-reactive protein (CRP) or α1-antichymotrypsin (ACT)). The increased concentration of IL-6 and CRP results in a decrease of physical performance and disability. On the other hand, macrophages modulate the regenerative muscle response associated with a decrease/inhibition of the regenerative muscle capacity, also due to the greater degree of neutrophilia and the creation of a pro-inflammatory environment
[12][13][14].
Furthermore, sarcopenia involves an upregulation of myogenin, an essential transcription factor in regulating myoblast differentiation. Myogenin has been found to induce muscle wasting under different conditions, including denervation, spinal muscle atrophy, starvation, and tumor necrosis factor α-induced atrophy (TNF-α). In conjunction with increased myogenin, higher levels of Heat Shock 70kDa protein 1 (HSPA1A) and lower production of nuclear factures erythroid 2-related factor 2 (Nrf2) may contribute to intracellular accumulation of ROS, negatively impacting tropism and cell function. ROS imbalance may determine the unusual activity of p38 mitogen-activated protein kinase (MAPK) and dysregulated expression of the cell proliferation inhibitor, p16INK4a, disrupting the Janus kinase/signal transducer and activator of transcription (JAK-STAT) signaling and defective autophagy detected in aged SCs, responsible for their altered proliferation and differentiation capacities
[15][16][17][18][19].
Finally, some neuronal factors are also involved. Brain-derived neurotrophic factor (BDNF) is an example. It is a neurotrophin associated with synaptic plasticity and cell survival observed with the aging process. BDNF is stimulated by the contraction of skeletal muscles resulting in augmented fat oxidation and muscle fiber actions that occur in the contraction events. However, the lack of BDNF can lead to muscle atrophy due to the reduced capacity to produce proteins. It is also associated with the reduction of myogenic regulatory factors. Herein, it was observed that the synthesis and release of BDNF in the brain can increase with physical activity, delaying the onset of sarcopenia
[20][21][22][23].
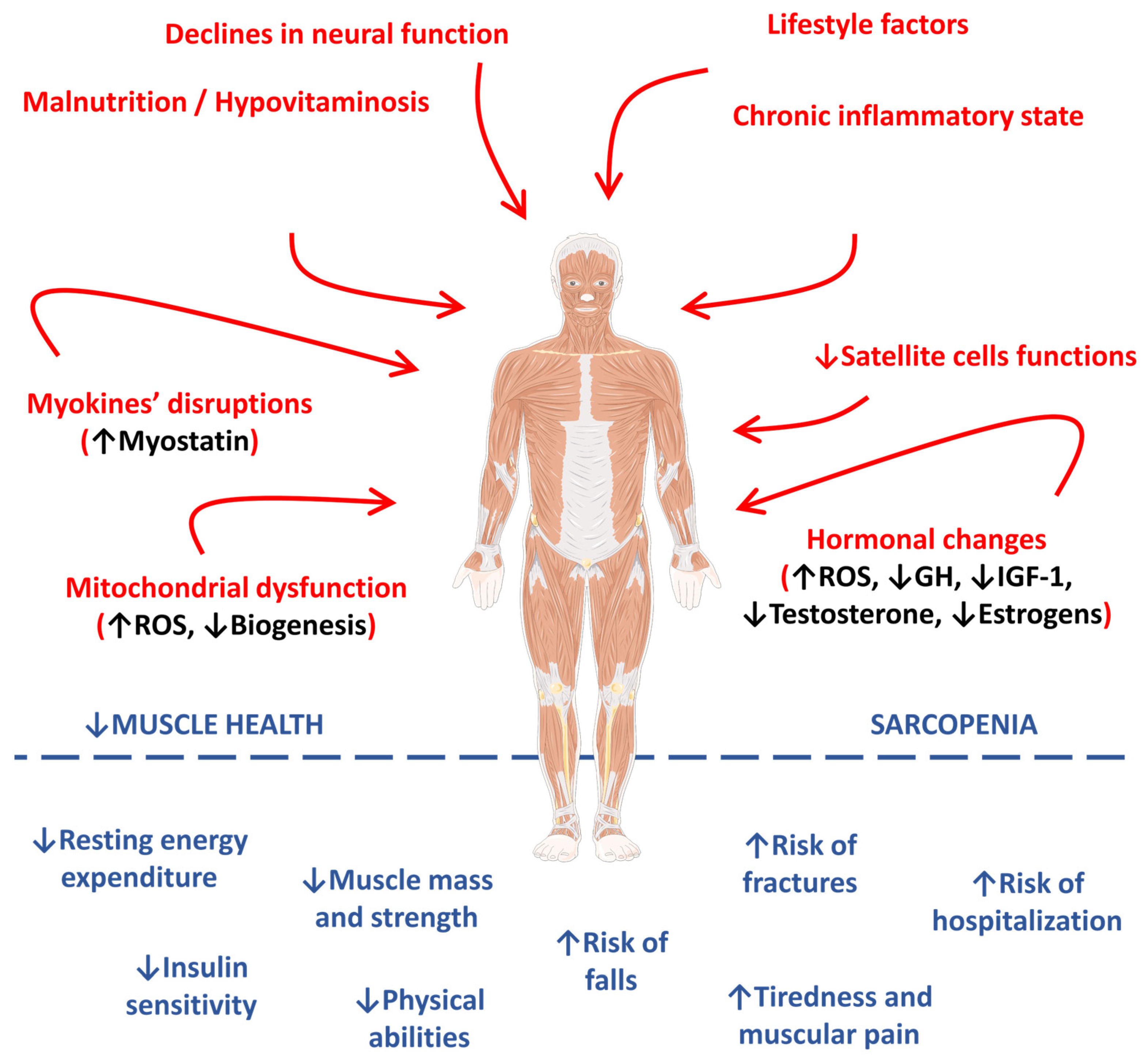
Figure 1 represents the main pathophysiological pathways observed in the occurrence and progression of sarcopenia.
Figure 1. Main pathophysiological pathways involved in the occurrence and progression of sarcopenia. ↑, increase; ↓, decrease; GH, growth hormone; IGF-1, insulin-like growth factor 1; IL-1β, interleukin 1 beta; IL-6, interleukin 6; ROS, reactive oxygen species; TNF-α, tumor factor necrosis alpha.
2. Sarcopenia and Diabetes
In sarcopenia, a phenomenon occurs in which the loss of skeletal mass and changes in fat deposition can promote metabolic complications since the accumulation of visceral and intramuscular fat can be observed at an increased risk of cardiovascular disease, insulin resistance, and diabetes
[24]. Insulin resistance, especially in skeletal muscle and the liver, and defective insulin secretion by the pancreas are the two main pathophysiological mechanisms observed in T2DM. However, there is a set of diverse factors that involve T2DM resulting from an interaction between genes and the environment, as there is growing evidence that the risk of T2DM is strongly influenced by genetic factors
[25][26][27].
Insulin resistance in diabetes is associated with chronic inflammation through the release of pro-inflammatory cytokines and microparticles and can trigger the activation of innate immune cells, inducing a vicious cycle of inflammation in various tissues and insulin resistance
[28][29].
The pro-inflammatory scenario observed in T2DM patients results in systemic inflammation directly related to sarcopenia, as they share chemical mediators related to the pathophysiology of the two conditions. Park et al.
[30] demonstrated that older adults with T2DM had a higher loss of muscle mass and leg strength over three years than non-diabetic controls. These associations were partially attenuated after adjustment for cytokines, including IL-6 and TNF-α. Moreover, Visser et al.
[31] assessed 2746 older adults in good general condition aged between 70 and 79 years, and they noted that there was a decrease in handgrip strength between 1.1 and 2.4 kg in the presence of an increase in the concentration of IL-6, revealing a decline in muscle strength in these conditions. In the English Longitudinal Ageing Study, increased CRP was negatively associated with handgrip strength only in women and lower body strength in both sexes. Therefore, it is concluded that the inflammation, in vivo, associated with T2DM can affect muscle mass and strength
[32].
Chronic complications of T2DM, such as neuropathy, retinopathy, nephropathy, other microvascular complications, and innervations that end up reaching the muscles, will result in the reduction of muscle contractility and, consequently, loss of muscle strength. OS, together with the inflammatory environment, promotes endothelial dysfunction, which can lead to peripheral arterial disease (PAD). This extra macrovascular complication affects up to a quarter of patients with T2DM. The blood pressure reduction can lead to ischemia, decreasing the results of strength, mass, and muscle performance. Furthermore, due to the pain associated with this disorder, PAD can lead to a decrease of physical activity and exercise, further contributing to the reduction of muscle health
[32][33][34][35].
In summary, the available pieces of evidence reviewed show that sarcopenia occurrence in patients with T2DM is increased. As pointed out previously, numerous direct and indirect links between T2DM and sarcopenia involve insulin resistance, inflammatory factors, AGE accumulation, oxidative stress, and macro- and microvascular complications that can affect metabolism, regeneration, and muscle strength in several ways. Moreover, the existence of one condition can increase the risk of developing the other
[35].
3. Sarcopenia and Obesity
When approaching aging and obesity, it is understood that there is an increase in life expectancy along with a lifestyle change. The aging process is associated with decreased muscle mass and strength and increased body fat mass, leading to disability, frailty, falls, social isolation, and possible hospitalization. Therefore, the designation of sarcopenic obesity (SO) appears to also represent the presence of sarcopenia and obesity
[36].
Several factors cause OS. The age events could be sited, reduced physical activity, unhealthy nutrition, low-grade inflammatory processes, resistance to insulin, and hormonal changes which lead to changes in body composition. Aging reduces basal metabolic rates leading to weight gain and a decrease in muscle mass, as shown by in vivo studies
[37][38]. Older people typically reduce physical activity, contributing to muscle strength loss and resulting in atrophic muscles that further aggravate the lack of physical performance. Furthermore, in the elderly, there is a significant imbalance between energy intake and expenditure, which is also related to OS and an increase in inflammatory processes in in vivo studies. Adipocytes promote macrophage recruitment, so there is an augmented organokine secretion by adipocytes and immune cells (leptin, resistin, and chemerin) and other pro-inflammatory markers (TNF-α, interleukins, and interferon-γ), creating a circumstance of low-grade inflammation which plays a significant role in OS progression. In addition to being related to low-grade chronic inflammation, insulin resistance is also correlated with mitochondrial dysfunction, decreasing muscle strength and increasing fat accumulation in muscle and the liver. Lastly, the disruption in the release of organokines and reduced concentrations of testosterone and estrogen are important factors related to the aging process
[39][40][41].
In addition, in sarcopenia, there is an increase in IL-6, which is produced by macrophages and stimulated by the stimulation of the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB). NF-κB is capable of reducing IRS1 (insulin receptor substrate 1) and GLUT-4 (glucose transporter type 4) expression in tissues such as adipocytes, promoting a pro-inflammatory environment. Furthermore, a reduction of adiponectin in plasma levels contributes to the increase of insulin resistance and the inflammatory pattern of adipocytes
[33][42][43].
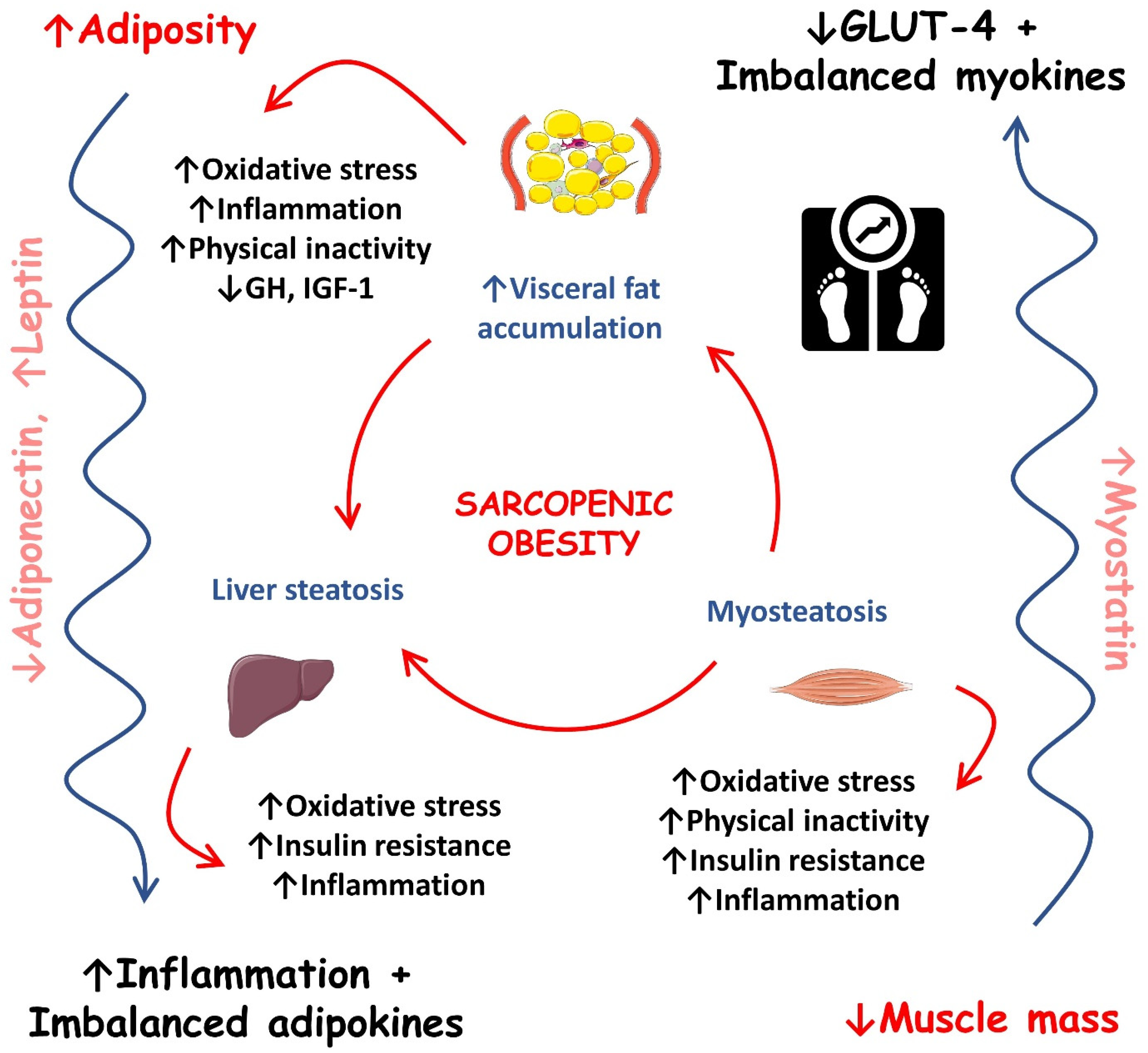
Figure 2 shows the most important events that occur during sarcopenic obesity development.
Figure 2. Most important events during sarcopenic obesity development. ↑, increase; ↓, decrease; GH, growth hormone; GLUT-4, solute carrier family 2/facilitated glucose transporter member 4; IGF-1, insulin-like growth factor 1.
4. Sarcopenia and Dyslipidemia
Dyslipidemia and diabetes are closely interlinked mainly due to their pathogenic mechanisms; therefore, they are also interlinked with obesity and sarcopenia. In recent decades, several mechanisms have been shown to contribute to the worsening of atherosclerosis in patients with insulin resistance, which increases endothelial cell dysfunction and thus decreases the bioavailability of nitric oxide, which is a potent vasodilator. In addition, a review suggested that hyperglycemia contributes to glucotoxicity and exerts a synergistic pro-atherogenic effect in the vascular bed alongside dyslipidemia and hypertension
[44].
As mentioned previously, with aging, there is a decrease in hormone production. In menopause, for example, there is an increase in cardiovascular risk due to estrogen deficiency and unregulated lipid metabolism. Estrogens play a protective role in the cardiovascular system and can be produced in the ovaries using LDL-c. However, circulatory LDL-c is not used to synthesize estrogen during menopause, leading to a decrease of estrogen production. Therefore, some reviews suggested that menopause is associated with high levels of LDL-c and reduced estrogen and testosterone levels, leading to a reduction in muscle mass and muscle strength
[39][45].
Insulin resistance promotes the increase of glycogenesis, increase of the expression of sterol regulatory element-binding protein 1c (SREBP-1c), inhibition of β-oxidation, increase of free fatty acids supply, and alters the transport of triglycerides, resulting in the accumulation of triglycerides in skeletal muscle and the liver
[4][5]. Habib et al.
[5] analyzed a population of 288 adult male individuals to conclude that total cholesterol and triglycerides levels were significantly higher, and HDL-c was significantly lower in sarcopenic obese subjects compared to non-sarcopenic obese subjects, showing that sarcopenia aggravates dyslipidemia.
Finally, a Korean study reported that regardless of abdominal obesity, people with a lower skeletal muscle mass index were significantly associated with a higher risk of dyslipidemia. The authors conclude that preventing muscle wasting may be an interesting strategy to manage LDL-c levels, which further proves the need to intervene in sarcopenia as a whole, since it is directly associated with classic features of metabolic dysfunction that may reflect pathologies at vascular levels in the long term
[45][46].
5. Organokines and the Relations with Sarcopenia, DM, Sarcopenic Obesity, and Dyslipidemia
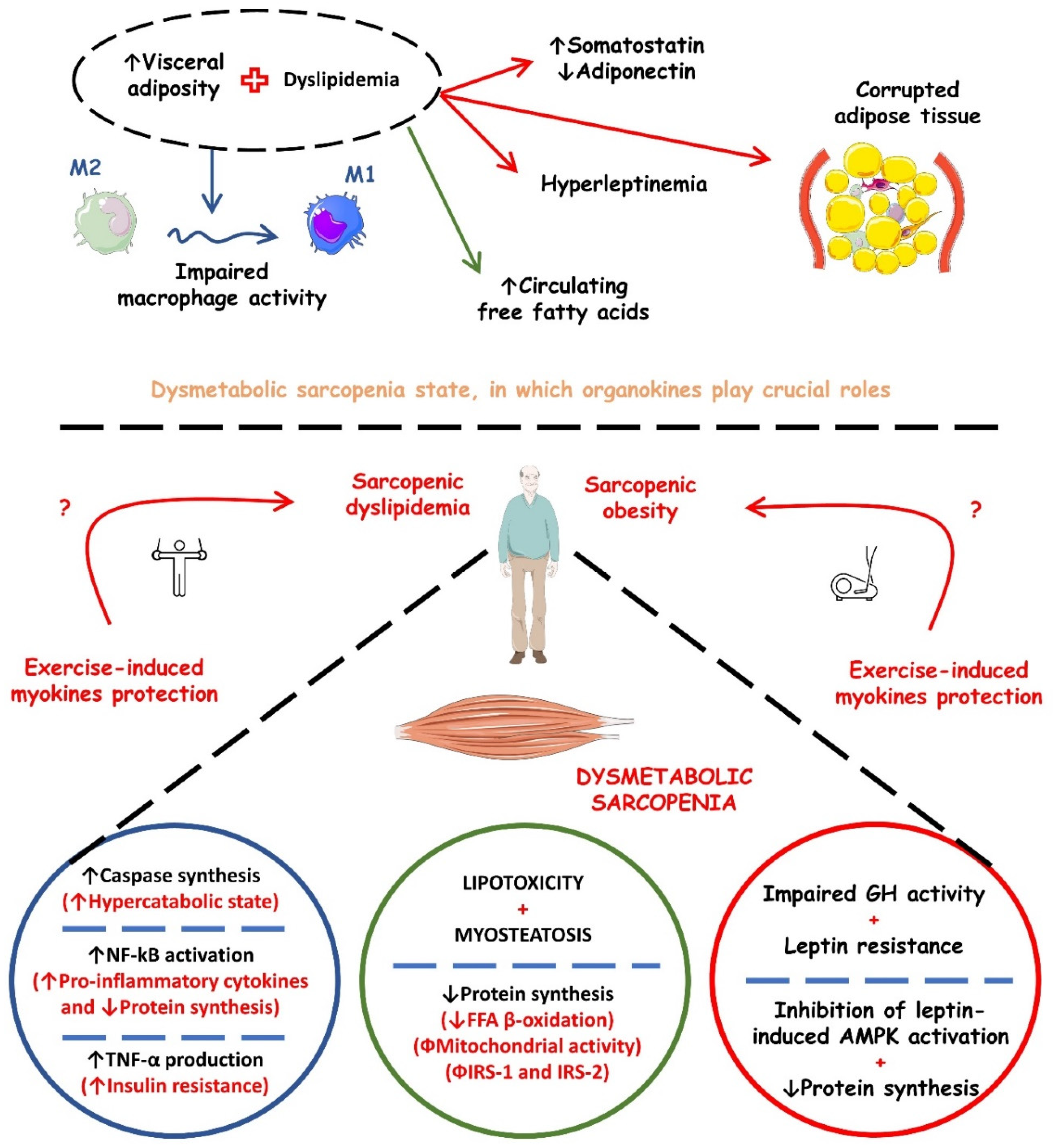
Sarcopenia and its metabolic implications may have a pathophysiological mechanism at the molecular level, which brings us to organokines, as they are involved in mechanisms such as insulin resistance, T2DM, obesity, metabolic syndrome, and CVD. These molecules are being increasingly investigated and can be found in skeletal, adipose, and liver cells that, respectively, release myokines, adipokines, and hepatokines. Thus, it is noted that organokines can act as showing benefits or harmful effects to the organism and even perform the known crosstalk when it acts in endocrine, paracrine, and autocrine pathways. Table 1 shows the most studied organokines involved in sarcopenia and its metabolic repercussions. In turn, Figure 3 shows the possible mechanistic associations between organokines secretion and the occurrence of dysmetabolic sarcopenia.
Figure 3. Possible mechanistic associations between organokines secretion and the occurrence of dysmetabolic sarcopenia. ↑, increase; ↓, decrease; AMPK, AMP (adenosine monophosphate)-activated protein kinase; FFA, free fatty acids; GH, growth hormone; IRS-1, insulin receptor substrate 1; IRS-2, insulin receptor substrate 2; NF-kB, nuclear factor kappa b; TNF-α, tumor factor necrosis alpha.
Table 1. Main characteristics of the organokines involved in the pathophysiology of sarcopenia, diabetes, sarcopenic obesity, and dyslipidemia.
| Classification |
Organokine |
Role in Sarcopenia and Metabolic Repercussions |
Expression in Sarcopenia |
General Actions |
Roles in Sarcopenia |
References |
| MYOKINES |
Irisin |
Dyslipidemia, sarcopenic obesity, and diabetes. |
↓ |
In ↑ concentrations: ↓ body weight and↑ insulin sensitivity. |
In ↓ concentrations, it predicts sarcopenic obesity and ↑ insulin resistance. It is inversely correlated with the occurrence of dyslipidemia. |
[47][48] |
| Myostatin |
Sarcopenic obesity and diabetes. |
↑, it is inversely proportional to skeletal muscle mass. |
Generates muscle waste and correlates positively with a pro-inflammatory. |
In ↑ concentrations, sarcopenic obesity and its driven diabetes can be predicted by myostatin levels. Additionally, it impairs muscle growth and contributes to muscle insulin resistance. |
[49][50][51] |
| Sclerostin |
Sarcopenia and diabetes. |
↓ |
Formation and regulation of bone mass and decrease in bone mass in diabetic patients. |
In ↑ concentrations, it is significantly associated with a lower risk of sarcopenia. Hyperglycemia directly increases sclerostin production. |
[52][53][54][55] |
| |
BDNF |
Dyslipidemia, sarcopenia, obesity, and diabetes. |
↓ |
↑ Memory, ↑ insulin sensitivity, regulates lipid metabolism, and acts in myogenesis process. |
In ↓ concentrations, it predicts sarcopenia, obesity, ↑ insulin resistance, and promotes dyslipidemia. |
[56][57][58][59] |
| IL-6 |
Sarcopenia, sarcopenic obesity, metabolic syndrome, and diabetes. |
↑ |
↑ Insulin resistance, ↑ inflammation, ↑ muscle volume, and strength. |
In ↑ concentrations, sarcopenic obesity, metabolic syndrome, and diabetes, as long as IL-6 is a pro-inflammatory factor. |
[33][60][61][62] |
| IL-15 |
Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. |
↓ |
↓ Inflammation, ↓ oxidative stress, ↓ obesity, and ↓ glucose levels. |
Prevents the reduction of muscle mass, increases glucose uptake by the skeletal muscle, promotes muscle hypertrophy, and reduces subcutaneous fat and adipose tissue mass. |
[59][60][63][64][65] |
| ADIPOKINES |
Leptin |
Sarcopenic obesity. |
↑ |
↑ Lipolysis and ↑ insulin sensitivity by skeletal muscles. |
Risk predictor for sarcopenic obesity. In ↓ concentrations, leptin can induce insulin resistance by decreasing glucose consumption by muscles. |
[66][67]. |
| LCN2 |
Sarcopenic obesity and diabetes. |
↑ |
↑ Insulin resistance, ↑ inflammation, and ↑ oxidative stress. |
In ↑ concentrations, sarcopenic obesity involves metabolic disorders such as obesity, insulin resistance, and type 2 diabetes. |
[33][68][69][70] |
| IL-6 |
Sarcopenia, sarcopenic obesity, metabolic syndrome, and diabetes. |
↑ |
↑ Inflammation and ↑ insulin resistance by inhibiting the expression of IRS1 and GLUT4 in adipocytes. |
In ↑ concentrations, sarcopenic obesity, metabolic syndrome, and diabetes. |
[58][60][61][71] |
| IL-10 |
Sarcopenia, sarcopenic obesity, obesity, oxidative stress, and autoimmune diabetes. |
↓ |
↓ Inflammation, oxidative stress, and obesity in skeletal muscle. |
In spite of an increase in the levels of IL-10 in older humans, in ↑ concentrations it promotes an anti-inflammatory environment and induces protein synthesis in individuals with sarcopenic obesity. |
[51][60][72] |
| IL-15 |
Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. |
↓ |
↓ Inflammation, ↓ oxidative stress, ↓ obesity, and ↓ glucose levels. |
Prevents the reduction of muscle mass, promotes muscle hypertrophy, and reduces glucose levels, subcutaneous fat, and adipose tissue mass. |
[59][60][65][73] |
| Apelin |
Sarcopenia, obesity, and diabetes. |
↓ |
↓ Inflammation, ↓ glucose levels, and ↓ insulin resistance index. |
Improves muscle function in an anti-inflammatory way, increases the regenerative abilities of muscles, and also improves metabolism in obesity and DM2. |
[59][60][74] |
| IGF-1 |
Sarcopenia, sarcopenic obesity, and oxidative stress. |
↓ |
↑ Muscle hypertrophy and ↓ atrophy. |
In ↓ concentrations, it promotes sarcopenia through ↓ protein synthesis, autophagy, and ↓ muscle regeneration. It also promotes sarcopenic obesity. |
[75][76][77] (Naranjo et al., 2017) |
| FGF-21 |
Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. |
↓ |
Regulates glucose and lipid metabolism, antioxidant, ↓ obesity, and ↓ sarcopenia. |
In ↓ concentrations, it promotes sarcopenia and obesity since it is directly linked with muscle mass along with irisin. |
[59][60][63][78] |
| Adiponectin |
Sarcopenia, sarcopenic obesity, obesity, and oxidative stress. |
↑ |
↓ Inflammation, ↓ atherosclerosis, ↓ oxidative stress, and ↑ insulin resistance. |
In ↑ concentrations, it promotes muscle hypertrophy and ↓ sarcopenic obesity. At the muscle level, ↑free fatty acid oxidation and glucose uptake. At the liver level, ↓ gluconeogenesis. |
[60][79] |
| HEPATOKINES |
Fetuin A |
Sarcopenic obesity and diabetes. |
↑ |
↑ Insulin resistance, ↑ inflammation, and ↑ oxidative stress. |
In ↑ concentrations, it contributes to a state of obesity, sarcopenia, sarcopenic obesity, and insulin resistance through a pro-inflammatory scenario. |
[33][80][81][82] |
| SHBG |
Sarcopenia, sarcopenic obesity, diabetes, and CVD. |
↓ |
↑ Inflammation, ↑ oxidative stress, ↑ obesity, ↑ fatty liver disease, and ↑ CVD. |
↑ Levels of SHBG are related to sarcopenia in both sexes of older individuals. |
[33][83][84] |
| LECT-2 |
Insulin resistance and obesity. |
Probably ↑ |
In ↑ concentrations: ↑ insulin resistance, obesity, and NAFLD. |
Although LECT-2 has no known implications for the mechanism of sarcopenia, it acts directly on skeletal muscle by positively correlating with insulin resistance and obesity. |
[79][85][86] |
| |
IGF-1 |
Sarcopenia, sarcopenic obesity, and oxidative stress. |
↓ |
↑ Muscle hypertrophy, ↓ atrophy, and acts on oxidative stress. |
In ↓ concentrations, ↓ protein synthesis, autophagy, and ↓ muscle regeneration, and it promotes sarcopenia and also sarcopenic obesity. |
[60][75][87] |
| OSTEOKINES |
Osteocalcin |
Sarcopenia, sarcopenic obesity, and diabetes. |
↓ |
In ↑ concentrations: ↑ insulin sensitivity, uptake of glucose, anti-inflammatory, and ↑ muscle mass. |
In ↑ concentrations, it contributes to the survival and function of pancreatic β cells, thereby increasing insulin secretion. It also contributes to muscle hypertrophy and strength. Consequently, its reduction would promote the opposite effect. |
[88][89] |
| Irisin |
Dyslipidemia, sarcopenic obesity, and diabetes. |
↓ |
In ↑ concentrations: ↓ body weight and↑insulin sensitivity. |
In ↓ concentrations, it predicts sarcopenic obesity and ↑ insulin resistance. |
[11][88][90][91]. |
| Sclerostin |
Sarcopenia and diabetes. |
↓ |
Formation and regulation of bone mass and decreased bone mass in diabetic patients. |
In ↑ concentrations, it is prospectively associated with a lower risk of sarcopenia. |
[52][54][55][92] |